Abstract
Background
Several studies have evaluated the effects of zinc supplementation on glycemic biomarkers in humans and have demonstrated varying results. We systematically evaluated the literature and performed an umbrella meta-analysis of the effects of zinc supplementation on type 2 diabetes biomarkers.
Methods
A comprehensive literature search was conducted in the following databases; PubMed, Embase, Embase, Cochrane Library, Scopus, and Web of Science for studies published up to March 10, 2024.
Results
Zinc supplementation was effective in reducing serum FBS (WMD: − 13.58, 95% CI: − 17.38, − 9.77; p < 0.001; SMD: − 0.52, 95% CI − 0.79, − 0.25; p = < 0.001), insulin (SMD: − 0.67, 95% CI − 0.96, − 0.38; p < 0.001), HOMA-IR levels (WMD − 0.52, 95% CI − 0.66, − 0.38; p < 0.001; SMD: − 0.78, 95% CI − 1.02, − 0.42; p < 0.001), and HbA1c (WMD: − 0.35, 95% CI − 0.43, − 0.27; p < 0.001).
Conclusion
Zinc supplementation significantly reduced FBS, HOMA-IR, insulin and HbA1c. These findings suggest that zinc is potentially an effective complementary intervention to improve type 2 diabetes biomarkers.
Similar content being viewed by others
Introduction
Considering the increasing financial burden caused by type 2 diabetes and its associated comorbidities on the health system ($306.6 billion in direct medical costs in the US), attention to more effective treatments for glycemic disorders is increasing [1]. The effect of different micronutrients on type 2 diabetes as one of the most common non-communicable diseases [6.28% of the world’s population [2]] has been investigated in various studies [3]. Different nutritional strategies have been proposed to manage type 2 diabetes including body weight loss, proper macronutrient distribution, choosing food sources from a low glycemic index, consuming fiber intake > 40 g/d, and moderate intake of free sugars [4]. Among the myriad of dietary components, zinc has emerged as a micronutrient of paramount importance, playing a pivotal role in various physiological processes, including immune function, DNA synthesis, and cellular metabolism [5]. Zinc, an essential trace element, is indispensable for the proper functioning of numerous enzymes, transcription factors, and signaling pathways within the human body. Its involvement in insulin metabolism and pancreatic beta-cell function places it at the epicenter of glucose homeostasis. As an integral cofactor for insulin, zinc facilitates insulin hexamer formation and storage in pancreatic beta cells, influencing the release of insulin in response to fluctuating blood glucose levels [6]. Furthermore, zinc is intricately linked to the regulation of glucose transporter proteins, impacting glucose uptake and utilization in peripheral tissues [7]. The perturbation of these processes due to inadequate zinc levels and zinc deficiency has been implicated in the development of insulin resistance and impaired glucose tolerance [8].
Glycemic biomarkers, encompassing a spectrum of indicators reflecting glucose metabolism, provide invaluable insights into an individual's metabolic health. Fasting blood sugar (FBS), glycosylated hemoglobin (HbA1c), insulin levels, and homeostasis model assessment-estimated insulin resistance (HOMA-IR) stand as sentinel markers, offering clinicians a panoramic view of an individual's glycemic status. Elevations in these biomarkers are not only indicative of impaired glucose metabolism but are also key predictors of diabetes onset and its associated complications. The intricate relationship between zinc status and glycemic biomarkers has prompted a surge of interest in elucidating whether optimizing zinc intake could serve as a modifiable factor in glycemic control and diabetes prevention [9].
To synthesize the existing body of evidence on the relationship between zinc intake and glycemic biomarkers, we employ an umbrella systematic review and meta-analysis methodology. This comprehensive approach enables us to encapsulate a wide array of studies, including systematic reviews and meta-analyses, providing a more nuanced understanding of the topic. By amalgamating data from diverse sources, we aim to discern patterns, identify potential sources of heterogeneity, and derive robust conclusions that transcend individual study limitations. Several meta-analysis studies with conflicting results have been performed [10,11,12,13,14,15,16,17]. Some studies showed beneficial effects of zinc on all studied biomarkers [10, 11]; on the other hand, and non-significant results were shown on FBS [12], HbA1c [12], HOMA-IR [12,13,14,15], and insulin [14,15,16,17]. Also, different statistical methods have been used in different meta-analyses, which have led to different results. As a result, conducting this study using a single statistical method can lead to a definite conclusion about the anti-hyperglycemic effects of zinc.
Methods
This research adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure thorough reporting. Our protocol was registered in PROSPERO database (CRD42024543516).
Literature search
To perform this umbrella meta-analysis investigating the impact of zinc supplementation on glycemic index, a systematic search method was applied. Various databases such as PubMed, Embase, Embase, Cochrane Library, Scopus, and Web of Science were scrutinized up to March 10, 2024 using search terms like ((Zinc OR Zn) AND (insulin OR glucose OR glycemic OR HbA1c OR diabet) combined with Meta-analysis. The search terms employed are included in Supplementary Table 1. Additional research was discovered through manual review of reference lists. This study considered only articles in the English language for inclusion.
Inclusion and exclusion criteria
The PICOS criteria utilized in this umbrella meta-analysis were outlined as follows: Population/Patients (P) consisted of adults aged 18 years and above who had undergone zinc treatment in any health condition; Intervention (I) specifically focused on the administration of zinc; Comparison (C) involved a control group or placebo; Outcome (O) measured the glycemic biomarkers, which included factors like FBS, HbA1c, insulin, and HOMA-IR; (S) meta-analyses of RCTs. Only meta-analysis studies published in English that investigated the impact of zinc supplementation on the type 2 diabetes biomarkers and provided effect sizes (ES) with corresponding confidence intervals (CI) were considered for inclusion. Moreover, only moderate-to-high quality studies scored by AMSTAR method were included. Original studies, editorials, letters to the editor, studies involving children, as well as those involving pregnant or lactating women, were excluded from consideration.
Evaluating methodology and quality of evidence
Two researchers independently assessed the methodological quality of the incorporated studies utilizing the AMSTAR2 evaluation tool [18], comprising 16 criteria with response categories such as ‘‘yes,’’ ‘‘partial yes,’’ ‘‘no,’’ or ‘‘no meta-analysis.’’ Any inconsistencies were resolved through mutual consensus or with the assistance of a third author if required. The AMSTAR 2 checklist developed four classifications: ‘‘Critical low quality,’’ ‘‘low quality,’’ ‘‘moderate quality,’’ and ‘‘high quality’’. The GRADE approach was utilized to evaluate the studies, which consisted of five factors including, risk of bias, consistency of results, directness, precision, and potential for publication bias. The evidence is classified into four categories: high, moderate, low, or very low [19].
Study selection and data extraction
Two reviewers independently conducted the screening of studies and extracted data from the identified ones using predetermined criteria. This involved details such as the author's name, year of publication, sample size, study location, dosage and duration of zinc supplementation, as well as, effect sizes (ESs) ((weighted mean difference (WMD), and standardized mean difference (SMD)) and confidence intervals (CI) for glycemic biomarkers.
Data synthesis and statistical analysis
ESs and CIs were employed to calculate the overall effect. The analysis was conducted separately for SMD and WMD due to their natural differences. Heterogeneity was assessed using Cochran’s Q test and I2 statistics. If the I2-value exceeded 50% or the Q-test resulted in a p-value below 0.1, significant between-study heterogeneity was acknowledged. To address this, the random-effects model was utilized. Subgroup analysis based on predetermined variables such as intervention duration, health condition, supplementation dosage, and mean age helped identify potential sources of heterogeneity. Sensitivity analysis was performed to assess the impact of excluding a single study on the combined effect size. Egger’s and Begg’s tests were utilized to examine small-study effects. Publication bias was evaluated through visual examination of funnel plots, and if detected, a trim and fill test was subsequently conducted. The meta-analysis was conducted using STATA (version 16, Stata Corporation, College Station, TX, United States). A significance level of p < 0.05 was set for this study.
Result
Selected studies and systematic review
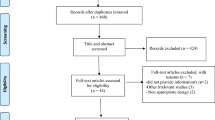
The PRISMA flowchart illustrating the process of literature search is presented in Fig. 1. Initially, 1146 articles were identified through electronic database searches, with 441 duplicates. Upon reviewing the titles and abstracts of the remaining 705 studies, 693 articles did not meet the inclusion criteria and were consequently excluded from further analysis. Ultimately, 10 meta-analyses (13 ESs) published between 2012 and 2023 met the requirements for inclusion in the umbrella review. Table 1 outlines the characteristics of these included meta-analyses. The average administered dosage of zinc across the studies ranged between 18.04 and 210 mg/day. The duration of zinc supplementation varied from 6.6 to 18 weeks. The studies were conducted across different locations: two in China [10, 20], four in Iran [14,15,16, 21], one in UK [17], one in USA [12], one in Taiwan [22], and one in Sri Lanka [23]. One included study in the meta-analysis by Jayawardena et al. reported that two cases of mild abdominal pain in patients receiving Zinc sulfate 660 mg/day for 12 weeks [24].
Methodological quality and quality of evidence
Table 2 displays results from the AMSTAR2 questionnaire, revealing that eight articles were evaluated as high quality, and while two was categorized as moderate quality. The GRADE approach showed that HbA1c had high-quality evidence, but insulin, HOMA-IR, and FBS having moderate-quality evidence (Table 3).
The effects of zinc on FBS levels
Zinc supplementation resulted in a significant reduction in FBS levels, according to the SMD analysis (SMD: − 0.52, 95% CI − 0.79, − 0.25; p = < 0.001, I2 = 0.0%, p = 0.999) (Fig. 2A) [10, 14]. The supplementation of zinc resulted in a significant decrease in FBS levels, according to the WMD analysis (WMD: − 13.58, 95% CI − 17.38, − 9.77; p < 0.001, I2 = 69.8%, p < 0.001) (Fig. 2B) [12, 15,16,17, 20,21,22,23]. Additionally, the results from subgroup analysis suggested that participant age, dosage, and health conditions were identified as the origins of heterogeneity (Table 4).
The effects of zinc on HbA1C levels
The administration of zinc supplements led to a notable reduction in HbA1c levels, according to the WMD analysis (WMD: − 0.35, 95% CI − 0.43, − 0.27; p < 0.001, I2 = 0.0%, p = 0.57) (Fig. 3) [12, 15,16,17, 20,21,22,23]. The subgroup analysis indicated that the impact of consuming zinc in reducing HbA1c is more noticeable in individuals diagnosed with diabetes (Table 4).
The effects of zinc on HOMA-IR levels
Zinc supplementation led to a significant decrease in HOMA-IR, according to the SMD analysis (SMD: − 0.78, 95% CI − 1.02, − 0.42; p < 0.001, I2 = 0.0%, p = 0.53) (Fig. 4A) [10, 14]. The addition of zinc resulted in a noteworthy reduction in HOMA-IR, according to the WMD analysis (WMD: − 0.52, 95% CI − 0.66, − 0.38; p < 0.001, I2 = 15%, p = 0.30) (Fig. 4B) [12, 15,16,17, 20,21,22]. Subgroup analysis showed that the effect of zinc supplementation in reducing A1c is more significant in people with diabetes, age < 50 years, and intervention duration ≤ 10 weeks (Table 4).
The effects of zinc on insulin levels
Zinc supplementation significantly decreased serum insulin levels, according to the SMD analysis (SMD: − 0.67, 95% CI − 0.96, − 0.38; p < 0.001, I2 = 0.0%, p = 0.82) (Fig. 5A) [10, 14]. Administration of zinc supplementation did not lead to a significant decrease in serum insulin levels, according to the WMD analysis (WMD: − 0.16, 95% CI − 0.74, 0.43; p = 0.59, I2 = 26.3%, p = 0.24) (Fig. 5B) [15,16,17, 20, 21]. The lack of a significant result in subgroup analyses remained consistent and did not show any significant findings (Table 4).
Sensitivity analysis and publication bias
There were no notable variations detected in the sensitivity analysis concerning each of the examined factors.
Neither Egger’s nor Begg’s tests indicated a small study effect (P˃0.05). The trim and fill method was performed following the uneven distribution of the funnel plots (Supplementary Figs. 1–4), and no imputed study was included for glycemic biomarkers.
Discussion
The current umbrella systematic review and meta-analysis was conducted with the aim of investigating the effect of zinc supplementation on glycemic biomarkers and the findings showed that zinc supplementation has an improving effect on these biomarkers. Therefore, zinc can be considered as an adjuvant therapy in improving glycemic parameters like other adjuvant therapy agents such as okra [25], probiotic [26], purslane [27], curcumin [28], flaxseed [29], cinnamon [30], and cumin [31]. The rationale of the present study is the existence of meta-analysis studies with conflicting results. Some studies showed beneficial effects of zinc on all studied biomarkers [10, 11]; on the other hand, non-significant results were shown on FBS [12], HbA1c [12], HOMA-IR [12,13,14,15], and insulin [14,15,16,17]. The reason for the difference in the results of the studies can be related to the study population, the dosage and duration of the supplementation, and the statistical methods used (random effects vs. fixed effects model or WMD vs. SMD calculation). Although the population of the included studies had diseases of different natures, they were similar in one aspect and that is having insulin resistance as one of the causes of their pathogenesis. As a result, the results of these studies were comparable from this point of view. Also, subgroup analysis was performed based on the study population to obtain more accurate results. For a more detailed analysis, we analyzed the studies that reported SMD and the studies that reported WMD separately. Since SMD adjusts results based on SD, in some analyzes results from WMD differed from SMD, which could be due to wide SD range [32]. For example, zinc does not have a positive effect on insulin levels when considering WMD, whereas zinc leads to a significant improvement in insulin when considering SMD. There are different methods and kits with different sensitivities and specificities for measuring the level of biomarkers, which can have different results on same biomarker. Therefore, the SMD report can be more accurate than the WMD, which reports a raw mean difference. However, the results on the effect of zinc on insulin levels should be interpreted with caution. The quality of most of the included studies was acceptable, which makes the results more valid.
Subgroup analysis showed that zinc has an improving effect on glycemic indices in both subgroups of age (< 50 and ≥ 50 years), dose (< 100 and ≥ 100 mg/day), duration of supplementation (≤ 10 and > 10 weeks). It must be noted that the Tolerable Upper Intake Level (UL) of zinc for adults is 40 mg/day [33], therefore, high doses should be taken with caution. The side effects of high zinc intake are digestive problems, headaches, decreased concentrations of HDL cholesterol, reduced copper and iron status, and impairment in immunological response [33]. The greatest effect of this supplement was shown in hyperglycemic status in diabetic patients, which seems reasonable. The mechanisms of zinc's anti-hyperglycemic actions are not fully understood, but several potential mechanisms have been proposed. Zinc may activate insulin receptor tyrosine kinase, enhancing insulin sensitivity [34]. This activation can lead to increased glucose uptake by cells, particularly in skeletal muscle and adipose tissue. Moreover, zinc may modulate key components of the insulin signaling pathway, such as phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt), promoting glucose uptake and utilization [35]. Zinc is concentrated in pancreatic β-cells, where it is co-released with insulin. It has been proposed that zinc may play a role in insulin granule formation and insulin secretion [36]. In addition, zinc has antioxidant properties [37, 38], and by protecting β-cells from oxidative stress, it may help maintain their function and prevent apoptosis. Regarding gluconeogenesis, zinc may inhibit the enzymes involved in gluconeogenesis [39], the process by which the liver produces glucose. By limiting glucose production, zinc can contribute to maintaining normal blood glucose levels. In addition, it has been found that zinc may facilitate glucose uptake by cells, similar to its insulin-sensitizing effects [40]. This could contribute to lowering blood glucose levels. Another anti-hyperglycemic effect of zinc is related to its anti-inflammatory [41, 42] and immunomodulatory effects [43, 44]. Chronic inflammation and immune imbalance are associated with insulin resistance [45]. Apart from its effect on insulin, some studies have suggested that zinc may enhance GLP-1 (Glucagon-Like Peptide-1) secretion, contributing to improved glucose homeostasis [46]. GLP-1 is an incretin hormone that stimulates insulin secretion and inhibits glucagon release. It's important to note that while these mechanisms are supported by some experimental and clinical studies, the exact role of zinc in glucose metabolism is complex and may involve interactions with other factors (Fig. 6). Moreover, the optimal dosage of zinc for anti-hyperglycemic effects and potential side effects need further investigation. Three included studies performed dose–response analysis. Two of three studies found that the association between zinc and glycemic parameters was in a duration dependent manner not dose dependent [15, 16]. Ghaedi et al. reported that the association between zinc and HbA1c and FBS was dose-dependent [13]; so that, the more improving effect was shown in 40 mg/day dose. This study was conducted on diabetic subjects; therefore, dose–response studies in other populations are also needed.
Some limitations of our study should be mentioned. First, studies that reported their effect size as SMD were limited. As a result, subgroup analysis was not performed for these studies. Second, the results of all analyzes showed that zinc has a positive effect on glycemic biomarkers in diabetic patients. However, its results on other diseases that have insulin resistance conditions are not certain and need more studies. Third, our results on FBS should be interpreted with caution due to high heterogeneity. However, it was tried to check the sources of heterogeneity using subgroup analysis. This statistical heterogeneity was due to variability in the intervention effects caused by methodological differences between studies (dose, duration, and mean age of participants). Fourth, the first is the repetition of some studies in different meta-analyses, which can affect the final result. Further evaluations indicated that the repeated studies did not have a significant impact on the final outcome. Along with the limitations, we should also mention the strengths. All systematic reviews of RCTs that investigated the effects of zinc supplementation on glycemic indices were included in the current umbrella review. Most of the included studies were of high quality and low risk of bias. In addition, potential sources of bias were assessed. Also, most subgroup analyzes had low heterogeneity, which indicates acceptable validity of the results. Regarding the overall results, except for FBS-WMD, the rest of the results had low heterogeneity.
Conclusion
Zinc supplementation has an improving effect on FBS, HbA1c, and HOMA-IR. The significant effect of zinc on insulin was confirmed in SMD analysis. Subgroup analysis based on health condition revealed that diabetic patients benefited more from zinc supplementation to reduce glycemic parameters compared to other health conditions. According to evidence-based recommendations, nutritional supplements are not supported for diabetic patients and instead it is recommended that micronutrients be acquired from a well-balanced diet. However, diabetic patients often suffer from micronutrient deficiencies and may also need supplementation [4]. The results are valid due to the high quality of the included studies and the low heterogeneity of the results.
Availability of data and materials
Not applicable.
References
Parker ED, Lin J, Mahoney T, Ume N, Yang G, Gabbay RA, et al. Economic costs of diabetes in the US in 2022. Diabetes Care. 2024;47(1):26–43.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–11.
Kaur B, Henry J. Micronutrient status in type 2 diabetes: a review. Adv Food Nutr Res. 2014;71:55–100.
Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007.
Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: an integrative review. J Res Med Sci. 2013;18(2):144–57.
Li YV. Zinc and insulin in pancreatic beta-cells. Endocrine. 2014;45(2):178–89.
Sun Z, Shao Y, Yan K, Yao T, Liu L, Sun F, et al. The link between trace metal elements and glucose metabolism: evidence from zinc, copper, iron, and manganese-mediated metabolic regulation. Metabolites. 2023;13(10):1048.
Farooq DM, Alamri AF, Alwhahabi BK, Metwally AM, Kareem KA. The status of zinc in type 2 diabetic patients and its association with glycemic control. J Family Community Med. 2020;27(1):29–36.
Bandeira VDS, Pires LV, Hashimoto LL, Alencar LL, Almondes KGS, Lottenberg SA, et al. Association of reduced zinc status with poor glycemic control in individuals with type 2 diabetes mellitus. J Trace Elem Med Biol. 2017;44:132–6.
Li X, Zhao J. The influence of zinc supplementation on metabolic status in gestational diabetes: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2021;34(13):2140–5.
Yang HY, Hung KC, Chuang MH, Chang R, Chen RY, Wang FW, et al. Effect of zinc supplementation on blood sugar control in the overweight and obese population: a systematic review and meta-analysis of randomized controlled trials. Obes Res Clin Pract. 2023;17(4):308–17.
Pompano LM, Boy E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta-analysis. Adv Nutr. 2021;12(1):141–60.
Ghaedi K, Ghasempour D, Jowshan M, Zheng M, Ghobadi S, Jafari A. Effect of zinc supplementation in the management of type 2 diabetes: a grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2023. https://doi.org/10.1080/10408398.2023.2209802.
Khazdouz M, Djalalinia S, SarrafiZadeh S, Hasani M, Shidfar F, Ataie-Jafari A, et al. Effects of zinc supplementation on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Biol Trace Elem Res. 2020;195(2):373–98.
Nazari M, Nikbaf-Shandiz M, Pashayee-Khamene F, Bagheri R, Goudarzi K, Hosseinnia NV, et al. Zinc supplementation in individuals with prediabetes and type 2 diabetes: a GRADE-assessed systematic review and dose-response meta-analysis. Biol Trace Elem Res. 2023. https://doi.org/10.1007/s12011-023-03895-7.
Nazari M, Ashtary-Larky D, Nikbaf-Shandiz M, Goudarzi K, Bagheri R, Dolatshahi S, et al. Zinc supplementation and cardiovascular disease risk factors: a GRADE-assessed systematic review and dose-response meta-analysis. J Trace Elem Med Biol. 2023;79: 127244.
Wang Z, Ronsmans C, Woolf B. Triangulating evidence for the causal impact of single-intervention zinc supplement on glycaemic control for type 2 diabetes: systematic review and meta-analysis of randomised controlled trial and two-sample Mendelian randomisation. Br J Nutr. 2023;129(11):1929–44.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Wang X, Wu W, Zheng W, Fang X, Chen L, Rink L, et al. Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;110(1):76–90.
Ghaedi K, Ghasempour D, Jowshan M, Zheng MB, Ghobadi S, Jafari A. Effect of zinc supplementation in the management of type 2 diabetes: a grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of randomized controlled trials. Critical Rev Food Sci Nutr. 2023. https://doi.org/10.1080/10408398.2023.2209802.
Yang HY, Hung KC, Chuang MH, Chang RN, Chen RY, Wang FW, et al. Effect of zinc supplementation on blood sugar control in the overweight and obese population: a systematic review and meta-analysis of randomized controlled trials. Obes Res Clin Pract. 2023;17(4):308–17.
Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine GR, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndrome. 2012. https://doi.org/10.1186/1758-5996-4-13.
Afkhami-Ardekani M, Karimi M, Mohammadi SM, Nourani F. Effect of zinc sulfate supplementation on lipid and glucose in type 2 diabetic patients. Pak J Nutr. 2008;7(4):550–3.
Mokgalaboni K, Lebelo SL, Modjadji P, Ghaffary S. Okra ameliorates hyperglycaemia in pre-diabetic and type 2 diabetic patients: a systematic review and meta-analysis of the clinical evidence. Front Pharmacol. 2023. https://doi.org/10.3389/fphar.2023.1132650.
Zarezadeh M, Musazadeh V, Faghfouri AH, Sarmadi B, Jamilian P, Jamilian P, et al. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: an umbrella meta-analysis. Pharmacol Res. 2022;183: 106397.
Abbasi S, Mashatan N, Farmani E, Khodashenas M, Musazadeh V, Ahrabi SS, et al. The effects of purslane (Portulaca oleracea) on glycemic indices: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2023;37(12):5529–40.
Musazadeh V, Golandam F, Faghfouri AH, AbdoliShadbad M, Keramati M, Moridpour AH, et al. Curcumin supplementation contributes to relieving anthropometric and glycemic indices, as an adjunct therapy: a meta-research review of meta-analyses. J Functional Foods. 2022;99: 105357.
Kavyani Z, Pourfarziani P, Mohamad JafariKakhki A, SedghAhrabi S, HosseinMoridpour A, Mollaghasemi N, et al. The effect of flaxseed supplementation on glycemic control in adults: an updated systematic review and meta-analysis of randomized controlled trials. J Functional Foods. 2023;110:105816.
Moridpour AH, Kavyani Z, Khosravi S, Farmani E, Daneshvar M, Musazadeh V, et al. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes mellitus: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Phytother Res. 2024;38(1):117–30.
Tavakoli-Rouzbehani OM, Faghfouri AH, Anbari M, Papi S, Shojaei FS, Ghaffari M, et al. The effects of cuminum cyminum on glycemic parameters: a systematic review and meta-analysis of controlled clinical trials. J Ethnopharmacol. 2021;281: 114510.
Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. 2020. https://doi.org/10.4088/JCP.20f13681.
Institute of Medicine. 2001. Panel on micronutrients. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press. Washington.
Norouzi S, Adulcikas J, Sohal SS, Myers S. Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS ONE. 2018;13(1): e0191727.
Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2009;297(2):H569–75.
Fukunaka A, Fujitani Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J Mol Sci. 2018;19(2):476.
Faghfouri AH, Zarezadeh M, Aghapour B, Izadi A, Rostamkhani H, Majnouni A, et al. Clinical efficacy of zinc supplementation in improving antioxidant defense system: a comprehensive systematic review and time-response meta-analysis of controlled clinical trials. Eur J Pharmacol. 2021;907: 174243.
Zarezadeh M, Faghfouri AH, Aghapour B, Rostamkhani H, Malekahmadi M, NaemiKermanshahi M, et al. Investigation of the clinical efficacy of Zn supplementation in improvement of oxidative stress parameters: a systematic review and dose-response meta-analysis of controlled clinical trials. Int J Clin Pract. 2021;75(12): e14777.
Dharmalingam M, Sam JE. Zinc and glycemic control. Indian J Endocrinol Metab. 2019;23(2):173–4.
Sun W, Yang J, Wang W, Hou J, Cheng Y, Fu Y, et al. The beneficial effects of Zn on Akt-mediated insulin and cell survival signaling pathways in diabetes. J Trace Elem Med Biol. 2018;46:117–27.
Faghfouri AH, Baradaran B, Khabbazi A, Bishak YK, Zarezadeh M, Tavakoli-Rouzbehani OM, et al. Profiling inflammatory cytokines following zinc supplementation: a systematic review and meta-analysis of controlled trials. Br J Nutr. 2021;126(10):1441–50.
Faghfouri AH, Baradaran B, Khabbazi A, Shadbad MA, Papi S, Faghfuri E, et al. Regulation of NLRP3 inflammasome by zinc supplementation in Behçet’s disease patients: a double-blind, randomized placebo-controlled clinical trial. Int Immunopharmacol. 2022;109: 108825.
Faghfouri AH, Khabbazi A, Baradaran B, Khajebishak Y, Baghbani E, Noorolyai S, et al. Immunomodulatory and clinical responses to zinc gluconate supplementation in patients with Behçet’s disease: a double-blind, randomized placebo-controlled clinical trial. Clin Nutr. 2022;41(5):1083–92.
Faghfouri AH, Zarrin R, Maleki V, Payahoo L, Khajebishak Y. A comprehensive mechanistic review insight into the effects of micronutrients on toll-like receptors functions. Pharmacol Res. 2020;152: 104619.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801.
Moran BM, Miskelly MG, Abdel-Wahab YH, Flatt PR, McKillop AM. Zinc-induced activation of GPR39 regulates glucose homeostasis through glucose-dependent insulinotropic polypeptide secretion from enteroendocrine K-cells. Biol Chem. 2019;400(8):1023–33.
Acknowledgements
Not applicable.
Funding
The authors reported no funding received for this study.
Author information
Authors and Affiliations
Contributions
MD, and MA designed research; MGH and DS conducted systematic search, FK, PAH, and AH screened articles, extracted data and drew tables; MD analyzed and interpreted data; MD, MGH and DS wrote the paper. MA had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Daneshvar, M., Ghaheri, M., Safarzadeh, D. et al. Effect of zinc supplementation on glycemic biomarkers: an umbrella of interventional meta-analyses. Diabetol Metab Syndr 16, 124 (2024). https://doi.org/10.1186/s13098-024-01366-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01366-0