Abstract
Background
Metabolic syndrome (MetS) is a group of metabolic abnormalities characterised by hypertension, central obesity, dyslipidaemia and dysregulation of blood glucose, associated with the risk of diabetes, cardiovascular disease and overall mortality. The presence of elevated liver enzymes may precede the development of MetS, with alterations of the liver being observed that are directly related to metabolic problems. The study aims to provide the best evidence on the association between liver enzymes (ALT, AST, GGT) and MetS by determining the effect size of these biomarkers.
Methods
A systematic review and meta-analysis of studies indexed in PubMed and Scopus databases were performed. Study quality was assessed using the STROBE tool. The Grade Pro tool was used to evaluate the evidence, and the quantitative synthesis was performed using RevMan (Cochrane Collaboration).
Results
Seventeen articles comparing liver enzyme concentrations between 76,686 with MetS (MetS+) and 201,855 without MetS (MetS-) subjects were included. The concentration of ALT, AST and GGT in the MetS + subjects was significantly higher than in the control group 7.13 IU/L (CI95% 5.73–8.54; p < 0.00001; I2 = 96%), 2.68 IU/L (CI95% 1.82–3.54; p < 0.00001; I2 = 96%) and 11.20 IU/L (CI95% 7.11–15.29; p < 0.00001; I2 = 96%), respectively.
Conclusions
The evaluation of the relationship of liver enzymes in the pathophysiological process of MetS could lead to new insights into early diagnosis.
Similar content being viewed by others
Background
Metabolic syndrome (MetS) encompasses several cardiovascular risk factors, including insulin resistance, atherogenic dyslipidaemia, central obesity and hypertension [1]. It is a multifactorial non-communicable disease that significantly contributes to morbidity and mortality and is considered a public health burden worldwide [2].
In addition to increasing the risk of cardiovascular disease (CVD), MetS and its risk factors, including obesity and diabetes mellitus (DM), are associated with liver disease. Liver function is essential for glucose and fatty acid metabolism. Hepatic glucose homeostasis influences insulin sensitivity, while peripheral insulin resistance and lipolysis contribute to fat accumulation in the liver (hepatic steatosis) [3].
In this regard, MetS has a direct relationship with non-alcoholic fatty liver disease (NAFLD) [4], both being predictors of the development of fibrosis and hepatocellular carcinogenesis [5].
NAFLD affects approximately 25% of the world’s population and is a leading cause of cirrhosis, hepatocellular carcinoma and liver transplantation [6]. This disorder, characterised by lipid deposition in hepatocytes, encompasses a group of liver diseases that resemble alcoholic liver disease, ranging from simple steatosis to steatohepatitis and cirrhosis [7]. These liver diseases have become the leading causes of liver-related morbidity and mortality and a risk factor for DM, chronic kidney disease, hypertension, MetS and CVD [8].
In this context, early liver impairment detection would help prevent or diagnose other metabolic disorders. According to recent studies, liver function tests, including serum alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT), can be valuable parameters in the assessment of metabolic status, especially in the investigation of cardio-metabolic disorders [9]. Specifically, several authors have explored the associations between liver enzymes, MetS, and CVD in different populations [10, 11]. In this regard, elevated ALT levels have been shown to help predict CVD in prospective studies [12, 13], and MetS and its components [14]. Although GGT is considered an indicator of the degree of liver disease and alcohol consumption, several studies have shown that the level of this enzyme is also associated with diabetes, hypertension and cardiovascular mortality independently of liver damage or alcohol consumption [15, 16]. One of the advantages of these parameters is that they are commonly measured in liver function tests and are well-known markers of liver damage [17].
Therefore, this possible relationship between serum liver enzymes and MetS has recently attracted much attention. Therefore, the main objective of the systematic review and meta-analysis is to provide the best degree of evidence on the association between liver enzymes (ALT, AST, GGT) and MetS, determining the effect size of these biomarkers.
Methods
Search strategy and eligibility criteria
This systematic review and meta-analysis were conducted according to the criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18] (Supplementary file). The search was carried out in the PubMed and Scopus databases. The search strategy was developed by combining the following Medical Subject Headings (MeSH) descriptors: (“aspartate aminotransferase” OR “alanine aminotransferase” OR “gamma-glutamyltransferase”) AND (“metabolic syndrome”) (Supplementary file). In addition, we included cross-sectional and longitudinal studies published between January 2017 and July 2022 that investigated the association between liver enzymes (ALT, AST, GGT) and MetS. In addition, the results had to include the mean and standard deviation. Only papers written in English and Spanish, and those that collected data from subjects over 18 years of age, were considered. The systematic review was registered in PROSPERO with ID CRD42023366810.
Selection of papers
Two researchers (E.R.C and M.R.S) reviewed titles, abstracts and full texts. In addition, three researchers independently extracted data for studies that met the inclusion criteria (R.J.M, R.M.L. and G.M.R.). Finally, a fourth author (M.V.A.) acted as a judge in case of discrepancy. After sensitivity analysis, two articles [19, 20] were eliminated from the qualitative synthesis due to the heterogeneity of the reported data.
Data extraction
One researcher (E.R.C.) was responsible for extracting the data verified by a second researcher (R.J.M.). A third researcher (M.R.S.) resolved the disagreement in case of a tie. Cohen’s Kappa index was used to assess the degree of agreement. The following data were extracted from each study: citation, details of the study population (including age and sex), study design, follow-up period, sample size, and mean and standard deviation of ALT, AST, and GGT in those subjects with Metabolic Syndrome (MetS+) and without Metabolic Syndrome (MetS-). In addition, for articles collecting ALT, AST and GGT data, the mean and standard deviation were extracted.
Evaluation of the qualitative synthesis
The evaluation of the qualitative synthesis was carried out through a triple analysis, and four authors were responsible (R.M.L., R.J.M., E.R.C. and GMR):
-
a)
Assessment of methodological quality. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement [21] was used for observational studies.
-
b)
Risk of bias assessment. Using the Cochrane Collaboration tool [22] included in the REVMAN 5.4.2 software, the risks of selection, conduct, detection, attrition and reporting were analysed.
-
c)
Assessment of the quality of evidence. With the help of the Grade Pro tool, the evidence profile table was developed, establishing the following levels [23]:
-
High: high confidence in the match between the actual and estimated effect.
-
Moderate: Moderate confidence in the effect estimate. There is a possibility that the actual effect is far from the estimated effect.
-
Low: limited confidence in the estimate of the effect. The actual effect may be far from the estimated effect.
-
Very low: low confidence in the estimated effect. The actual effect is very likely to be different from the estimated effect.
-
Statistical analysis (evaluation of quantitative synthesis or meta-analysis)
The Cochrane Review Manager software (RevMan 5.4.2) was used for the meta-analysis to perform the statistical calculation and create the forest and funnel plots. Due to the difference in effect size of the included studies, a meta-analysis was performed using the Mantel-Haenszel random-effects method according to the DerSimonian and Laird model. The difference between arithmetic means with a 95% confidence interval was used to measure effect size. Liver enzyme counts were considered in IU/L. The risk of publication bias was assessed using the funnel plot. Heterogeneity was analysed using the Chi-square test and the inconsistency index (I2). According to the Cochrane Collaboration tool, heterogeneity was classified as follows: unimportant (0–40%), moderate (30–60%), substantial (50–90%) and considerable (75–100%).
Results
Characteristics of the studies
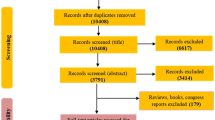
The search yielded 2,687 records, of which 205 were identified for full-text review (Fig. 1).
Of these, 17 met the inclusion criteria and were selected for systematic review and meta-analysis. Cohen’s Kappa clinical concordance index between the two authors (E.R.C and M.R.S.) who conducted the search was 82.8% (95% CI 70.3–95.3).
The detailed characteristics of the selected studies are shown in Table 1.
In total, 17 articles compared liver enzyme concentrations between 76,686 MetS + and 201,855 MetS- subjects. The age of the participants ranged from 22 to 78 years. Most papers (82.35%) [25, 26, 29,30,31,32,33,34,35,36,37,38,39,40] included participants of both sexes but analysed the data globally; 3 studies (17.65%) [24, 27, 28] collected data from men and women separately. Concerning origin, 5 articles were developed in China [27, 31, 37, 38, 40], 5 in Japan [26, 32, 34, 35, 37], three articles in Taiwan [25, 30, 39], 1 in Italy [24], 1 in Poland [33], Korea [28] and Iran [29]. Data were extracted from 17 reports from ALT [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], 15 studies from AST [25, 26, 28,29,30,31,32,33,34,35,36,37,38,39,40], and five from GGT [25, 26, 29, 31, 32].
In seven of the manuscripts [24, 26, 27, 34, 35, 38, 39], MetS was defined according to the criteria of the third report of the National Cholesterol Education Program (NCEP - ATP III) [41]; 5 studies [28,29,30,31, 37] assessed MetS using the harmonised criteria [42]; 2 papers [25, 32] using the International Diabetes Federation (IDF) definition [43]; and one article [40] used the Chinese diabetes Society criteria [44]. Finally, Sumiyoshi et al. [36] used the Japanese standards [45] and Osadnik [33], those defined by Buscemi et al. [46].
Methodological quality assessment
According to the STROBE reporting guidelines [21], all reports scored 18 points or more out of the 22 items included (highest tercile). No articles were excluded for poor methodological quality. The score for each of the papers is shown in Table 1.
Bias risk analysis
Overall (Fig. 2), it can be seen that the main biases were: random sequential generation, concealment of allocation and blinding of outcome evaluation (related to participants and staff). Figure 3 represents the individual assessment of the included studies.
Quantitative analysis. Meta-analysis
Figure 4 includes the results for both sexes from the 17 reviewed papers. MetS + subjects showed a higher mean ALT, with the difference reaching 7.13 IU/L (95% CI 5.73–8.54); compared to MetS- subjects. Furthermore, this analysis had a low risk of publication bias (Fig. 5). On the other hand, MetS + subjects showed a higher mean AST, namely, the mean difference was 2.68 IU/L (95% CI 1.82–3.54); compared to MetS- subjects (Fig. 6). Concerning GGT (Fig. 7), the mean difference reached 11.20 IU/L (95% CI 7.11–15.26), being higher among MetS + subjects. All results showed considerable heterogeneity (> 95%). Annex I shows a low risk of publication bias in the AST and GGT analysis.
Quality of evidence
Using the Grade Pro tool, the quality of the evidence in this meta-analysis was assessed, and a very low degree of certainty was obtained due to the high inconsistency and risk of bias in the included studies (Table 2).
Discussion
This systematic review with meta-analysis was conducted to analyse the most recent evidence on the relationship between MetS and liver enzymes (ALT, AST and GGT). Seventeen articles were selected in which the effect size was quantified and the limitations that have conditioned the results of the different studies. All demonstrated sufficient reliability and methodological quality regarding the association between ALT, AST, GGT and MetS.
The present meta-analysis has shown the relationship between the levels of different liver enzymes studied and MetS. The concentration of the liver enzymes studied in the 76,686 MetS + subjects was significantly higher than in the group of 201,855 controls (MetS-).
The presence of elevated liver enzymes may precede the development of MetS, with alterations of the liver being observed that are directly related to metabolic problems, such as NAFLD. Recently, it was considered a manifestation of metabolic diseases. However, it has been suggested that NAFLD temporarily precedes DM and that hepatic steatosis may cause insulin resistance [47] and may be an early sign of the development of metabolic diseases [48]. In addition, when fat is deposited in insulin-sensitive organs such as the liver, muscle and visceral compartments, free fatty acids and inflammatory cytokines increase while adiponectin levels decrease [49, 50]. These changes can lead to peripheral insulin resistance, early atherogenesis, impaired glucose metabolism and MetS [51, 52].
Previous studies have reported that NAFLD predates MetS components such as impaired fasting glucose and hypertension [53,54,55]. The study in young adults by Yoo et al. [56] concludes that the degree of hepatic steatosis can predict the future occurrence of MetS. Several studies have reported that NAFLD contributes to the development of DM2 and is associated with increased cardiovascular risk [57, 58]. The meta-analysis of prospective studies by Ballestri et al. [59] concluded that NAFLD significantly increases the incident risk of DM2 and MetS. This fact is highly relevant given that NAFLD is associated with elevated liver enzymes, such as ALT, AST and GGT, so early detection can help in interventions to prevent metabolic diseases such as MetS.
Concerning MetS, studies have shown that liver enzymes could be new candidate biomarkers for its early diagnosis. Our results are consistent with the associations reported between liver enzymes and MetS by other authors. The cross-sectional study by Chen et al. [17] concludes that serum ALT levels, even within the reference range, are significantly associated with MetS. The study by Sattar et al. [60] informs that serum ALT levels, but not AST levels, increased progressively as the number of MetS components increased. The meta-analysis of 10 prospective cohort studies by Kunutsor et al. [61] reported a dose-response relationship between GGT level and the risk of MetS. The meta-analysis by Liu et al. [62], involving 9 cohort studies, evidenced a positive association between GGT levels and the risk of MetS independent of alcohol intake.
In addition, there are significant gender differences, with males having higher levels than females, and the reference ranges established by the laboratories also vary. The study by Cheng et al. [24] reveals that male subjects had a higher prevalence of MetS and higher ALT levels; these results are in line with studies by Huang et al. [27] and Kim et al. [28].
However, further epidemiological investigations using longitudinal designs are needed to understand the associations between serum ALT, AST, and GGT levels and MetS.
These findings have important clinical implications regarding the optimal strategies to be adopted to prevent the development of MetS. In addition, monitoring liver enzyme values to detect their gradual elevation could alert to future metabolic problems.
Limitations and strengths
At the methodological level, the assessment of risks of bias in studies is a major issue in this type of research, in line with PRISMA recommendations. Studies with similar methodologies but with discrepancies in quality may have biased results. The quality of the evidence obtained is “very low” because observational studies have been analysed where there is a high risk of bias and, in addition, present a very high inconsistency (heterogeneity).
The authors were unable to fully examine the impact of adjustment for all known and potential risk factors, due to the varying degree of adjustment for confounding factors across individual studies.
One of the main strengths of this review is the comprehensive search covering a wide geographical area. In addition, a large sample size of subjects with and without MetS was included, which increased the study’s statistical power. However, considering some limitations, interpreting the findings in this systematic review and meta-analysis should be done cautiously. Firstly, non-randomised comparisons in observational studies may suffer from bias, which could affect the findings and thus weaken the strength of the evidence. Secondly, the included studies used different definitions to diagnose MetS, which may alter the findings. Also, the heterogeneity of the analyses was very high, which makes the results less robust. Finally, another limitation was that no additional strategies were used in the current search to locate unpublished reviews (grey literature).
Conclusions
The results have shown that MetS + subjects have higher levels of all liver enzymes tested than MetS- subjects. These findings provide a rationale for further evaluation of the relationship of liver enzymes in the pathophysiological process of MetS and could lead to new perspectives in early diagnosis.
The relevance of these findings has several implications for clinical practice, such as early diagnosis of MetS, early prevention of associated liver damage, better understanding of the pathophysiology, as well as the management and direction of effective care strategies for these patients.
However, primary studies with higher methodological quality should be performed to provide more robustness to the collected findings. Also, regarding this severe health problem, more research is needed in different populations to identify the importance of liver enzymes in MetS or other cardiovascular diseases.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004; 109(3):433-8. 10.1161/01. cir.0000111245.75752.c6.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. https://doi.org/10.1007/s11906-018-0812-z
Wree A, Schlattjan M, Bechmann LP, Claudel T, Sowa JP, Stojakovic T, et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metab Clin Exp. 2014;63(12):1542–52. https://doi.org/10.1016/j.metabol.2014.09.001
Kuo YH, Kee KM, Wang JH, Hsu NT, Hsiao CC, et al. Association between chronic viral hepatitis and metabolic syndrome in southern Taiwan: a large population-based study. Aliment Pharmacol Ther. 2018;48(9):993–1002. https://doi.org/10.1111/apt.14960
Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(4):64–70. https://doi.org/10.1111/jgh.12271
Schreiner AD, Zhang J, Durkalski V, Livingston S, Marsden J, Bian J, et al. Advanced liver fibrosis and the metabolic syndrome in a primary care setting. Diabetes Metab Res Rev. 2021;37(8):e3452. https://doi.org/10.1002/dmrr.3452
Kopec KL, Burns D. Nonalcoholic fatty Liver Disease: a review of the spectrum of Disease, diagnosis, and therapy. Nutr Clin Practice: Official Publication Am Soc Parenter Enter Nutr. 2011;26(5):565–76. https://doi.org/10.1177/0884533611419668
Fukuda Y, Hashimoto Y, Hamaguchi M, Fukuda T, Nakamura N, et al. Triglycerides to high-density lipoprotein cholesterol ratio is an Independent predictor of incident fatty Liver Disease; a population-based cohort study. Liver Int. 2016;36(5):713–20. https://doi.org/10.1111/liv.12977
Kim HR, Han MA. Association between serum liver enzymes and metabolic syndrome in Korean adults. Int J Environ Res Public Health. 2018;15(8):1658. https://doi.org/10.3390/ijerph15081658
Koskinen J, Magnussen CG, Kähönen M, Loo B, Marniemi J, Jula A, et al. Association of liver enzymes with metabolic syndrome and carotid Atherosclerosis in young adults. The Cardiovascular Risk in Young finns Study. Ann Med. 2012;44(2):187–95. https://doi.org/10.3109/07853890.2010.532152
Villegas R, Xiang Y, Elasy T, Cai Q, Xu W, Li H, et al. Liver enzymes, type 2 Diabetes, and metabolic syndrome in middle-aged, urban Chinese men. Metab Syndr Relat Disord. 2011;9(4):305–11. https://doi.org/10.1089/met.2011.0016
Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, et al. Gamma Glutamyl transferase and metabolic syndrome, Cardiovascular Disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(1):127–33. https://doi.org/10.1161/01.ATV.0000251993.20372.40
Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ et al. Alanine aminotransferase predicts coronary Heart Disease events: a 10-year follow-up of the Hoorn Study. 2007; 191(2):391–6. https://doi.org/10.1016/j.atherosclerosis.2006.04.006
Kälsch J, Bechmann LP, Heider D, Best J, Manka P, Kälsch H, et al. Normal liver enzymes are correlated with severity of metabolic syndrome in a large population based cohort. Sci Rep. 2015;5:13058. https://doi.org/10.1038/srep13058
Lee MY, Hyon DS, Huh JH, Kim HK, Han SK, Kim JY, et al. Endocrinol metabolism. 2019;34(4):390–7. https://doi.org/10.3803/EnM.2019.34.4.390. Association between Serum Gamma-Glutamyltransferase and Prevalence of Metabolic Syndrome Using Data from the Korean Genome and Epidemiology Study.
Liu CF, Zhou W, Fang N. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract. 2012;66(7):692–8. https://doi.org/10.1111/j.1742-1241.2012.02959.x
Chen S, Guo X, Yu S, Zhou Y, Li Z, Sun Y. Metabolic syndrome and serum liver enzymes in the General Chinese Population. Int J Environ Res Public Health. 2016;13(2):223. https://doi.org/10.3390/ijerph13020223
Urrutia G, Bonfill X. PRISMA statement: a proposal to improve the publication of systematic reviews and meta-analyses. Med Clin. 2010;135(11):507–11. https://doi.org/10.1016/j.medcli.2010.01.015
Xie QY, Wang MW, Hu ZY, Cao C, Wang C, Kang J, et al. Screening the influence of biomarkers for metabolic syndrome in Occupational Population based on the Lasso Algorithm. Front Public Health. 2021;9:743731. https://doi.org/10.3389/fpubh.2021.743731
Lan Q, Zhang Y, Lin F, Meng Q, Buys NJ, Fan H, et al. Association between serum aminotransferases and risk of new-onset cardiometabolic Disease in a healthy Chinese Population: a cohort study. Front Public Health. 2022;10:902393. https://doi.org/10.3389/fpubh.2022.902393
Von EE, Altman DG, Egger M, Pocock Stuart J, Gøtzsche C, Vandenbroucke P. STROBE (strengthening the reporting of observational studies in Epidemiology) Initiative Statement: guidelines for reporting observational studies. Gac Sanit. 2008;22:144–50.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman A, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Cheng YY, Kao TW, Chang YW, Wu CJ, Peng TC, Wu L, et al. Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. PLoS ONE. 2017;12(7):e0180687. https://doi.org/10.1371/journal.pone.0180687
Cheng YL, Wang YJ, Lan KH, Huo TI, Huang YH, Su CW, et al. Fatty liver index and lipid Accumulation Product can predict metabolic syndrome in subjects without fatty Liver Disease. Gastroent Res Pract. 2017;9279836. https://doi.org/10.1155/2017/9279836
Choi ES, Cho SH, Kim JH. Relationship between Rectus Abdominis muscle thickness and metabolic syndrome in middle-aged men. PLoS ONE. 2017;12(9):e0185040. https://doi.org/10.1371/journal.pone.018504
Huang HH, Chen YL, Chen JS, Lin JD, Hsieh CH, Pei D, Wu CZ, Relationships Among C-R, Protein. Alanine aminotransferase, and metabolic syndrome in apparently healthy Chinese subjects. Metab Syndr Relat Disord. 2018;16(5):232–9. https://doi.org/10.1089/met.2017.0059
Kim S. Association between Cardiorespiratory Fitness and metabolic syndrome in Korean older adults. Int J Environ Res Public Health. 2022;19(6):3671. https://doi.org/10.3390/ijerph19063671
Kohsari M, Moradinazar M, Rahimi Z, Pasdar Y, Shakiba E. Liver enzymes and their association with some Cardiometabolic Diseases: evidence from a large kurdish cohort. BioMed Res Int. 2021;5584452. https://doi.org/10.1155/2021/5584452
Kuo YH, Kee KM, Wang JH, et al. Association between chronic viral hepatitis and metabolic syndrome in southern Taiwan: a large population-based study. Aliment Pharmacol Ther. 2018;00:1–10. https://doi.org/10.1111/apt.14960
Liu CF, Zhou WN, Lu Z, Wang XT, Qiu ZH. The associations between liver enzymes and the risk of metabolic syndrome in the elderly. Exp Gerontol. 2018;106:132–6. https://doi.org/10.1016/j.exger.2018.02.026
Mitsuhashi K, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukuda T, et al. Impact of fatty Liver Disease and metabolic syndrome on incident type 2 Diabetes; a population based cohort study. Endocr J. 2017;64(11):1105–14. https://doi.org/10.1507/endocrj.EJ17-0245
Osadnik K, Osadnik T, Delijewski M, Lejawa M, Fronczek M, Reguła R, et al. Calcium and phosphate levels are among other factors Associated with metabolic syndrome in patients with Normal Weight. Diabetes Metb Syndr Obes. 2020;13:1281–8. https://doi.org/10.2147/DMSO.S232497
Sakane N, Kotani K, Suganuma A, et al. Effects of obesity, metabolic syndrome, and non-alcoholic or alcoholic elevated liver enzymes on incidence of Diabetes following lifestyle intervention: a subanalysis of the J-DOIT1. J Occup Health. 2020;62:e12109. 1002/1348-9585.12109.
Sogabe M, Okahisa T, Kurihara T, Takehara M, Kagemoto K, Okazaki J, et al. Differences among patients with and without nonalcoholic fatty Liver Disease having elevated alanine aminotransferase levels at various stages of metabolic syndrome. PLoS ONE. 2020;15(8):e0238388. https://doi.org/10.1371/journal.pone.0238388
Sumiyoshi H, Ohyama Y, Imai K, Kurabayashi M, Saito Y, Nakamura T. Association of Uric Acid with Incident Metabolic Syndrome in a Japanese General Population. Int Heart J. 2019;60(4):830–5. https://doi.org/10.1536/ihj.18-444
Wang H, Shi L, Liu C, Liu S, Shi S. Association between uric acid and metabolic syndrome in elderly women. Open Med. 2018;13(1):172–7. https://doi.org/10.1515/med-2018-0027
Wang J, Wang Y, Chen F, Ma G, Wang D. Measurement of the combined levels of serum uric acid and alanine aminotransferase and the risk of metabolic syndrome in a Population aged 60 years or more in Northeastern China. Med Sci Monit. 2020;20(26):e916459. https://doi.org/10.12659/MSM.916459
Wu YS, Tzeng WC, Chu CM, Wang WY. Metabolic syndrome and its related factors among Hospital employees: a Population-based Cohort Study. Int J Environ Res Public Health. 2021;18(18):9826. https://doi.org/10.3390/ijerph18189826
Yang T, Pei D. Association of cystatin C levels with metabolic syndrome incidence: a nested case-control study with propensity score matching. J Int Med Res. 2021;49(1):300060520986311. https://doi.org/10.1177/030006060520986311
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285(19): 2486-97. PMID: 1136870.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association. Harmonizing the metabolic syndrome: a Joint Interim Statement of. Circulation. 2009;120(16):1640–5. https://doi.org/10.1161/circulationaha.109.192644. World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity.
Alberti G, Zimmet P, Shaw J. The metabolic syndrome a new worldwide definition. IDF epidemiology task force consensus group. Lancet. 2005;366:1059e62.
Chinese Medical Association. The suggestion on Chinese metabolic syndrome. Shanghai, China. J Clin Med Assoc. 2004.
Matsuzawa Y. Definition and the diagnostic standard for metabolic syndrome. Committee to Evaluate Diagnostic standards for metabolic syndrome. J Jpn Soc Intern Med. 2005;94:794–809.
Buscemi S, Chiarello P, Buscemi C, Corleo D, Massenti MF, Barile AM, et al. Characterization of metabolically healthy obese people and metabolically unhealthy normal-weight people in a general population cohort of the ABCD study. J Diabetes Res. 2017;1–9. https://doi.org/10.1155/2017/
Gastaldelli A. Fatty Liver Disease: the hepatic manifestation of metabolic syndrome. Hypertens Res. 2010;33:546–7. https://doi.org/10.1038/hr.2010.60
Stefan N, Haring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60(8):2011–7. https://doi.org/10.2337/db11-0231
Lara Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin N Am. 2008;37(4):841–56. https://doi.org/10.1016/j.ecl.2008.09.002
Chait A, den Hartigh L. Adipose tissue distribution, inflammation and its metabolic consequences, including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22. https://doi.org/10.3389/fcvm.2020.00022
Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 Diabetes Mellitus. World J Diabetes. 2015;6(3):456–80. https://doi.org/10.4239/wjd.v6.i3.456
Ormazabal V, Nair S, Elfeky O, Aguayo C, Solomon C, Zuñiga F. Association between insulin resistance and the development of Cardiovascular Disease. Cardiovasc Diabetol. 2018;17:122. https://doi.org/10.1186/s12933-018-0762-4
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty Liver Disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47(3):181–90. https://doi.org/10.1016/j.dld.2014.09.020
Ryoo J, Choi J, Moon SY, Suh Y, Shin JY, Shin H. The clinical availability of non alcoholic fatty Liver Disease as an early predictor of the metabolic syndrome in Korean men: 5-year’s prospective cohort study. Atherosclerosis. 2013;227(2):398–403. https://doi.org/10.1016/j.atherosclerosis.2013.01.002
Jung K, Cho S, Kim H, Kim S, Song H. Nonalcoholic Steatohepatitis Associated with metabolic syndrome. J Clin Gastroenterol. 2014;48(10):883–8. https://doi.org/10.1097/MCG.00000000000000000065
Yoo JJ, Cho E, Chung G, Chang Y, Cho Y, Park S, et al. Nonalcoholic fatty Liver Disease is a precursor of New-Onset metabolic syndrome in metabolically healthy young adults. J Clin Med. 2022;11(4):935. https://doi.org/10.3390/jcm11040935
Bae J, Kim S, Han J, Kwon S, Lee D, Kim J, et al. Additive effect of non-alcoholic fatty Liver Disease on the development of Diabetes in individuals with metabolic syndrome. Diabetes Res Clin Pract. 2017;129:136–43. https://doi.org/10.1016/j.diabres.2017.03.037
Mantovani A, Byrne C, Bonora E, Targher G. Nonalcoholic fatty Liver Disease and risk of Incident Type 2 Diabetes: a Meta-analysis. Diabetes Care. 2018;41(2):372–82. https://doi.org/10.2337/dc17-1902
Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty Liver Disease is associated with an almost twofold increased risk of incident type 2 Diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–44. https://doi.org/10.1111/jgh.13264
Sattar N, Scherbakova O, Ford I, O’Reilly D, Stanley A, Forrest E, et al. Elevated alanine aminotransferase predicts new-onset type 2 Diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes. 2004;53(11):2855–60. https://doi.org/10.2337/diabetes.53.11.2855
Kunutsor S, Apekey T, Seddoh D. Gamma glutamyltransferase and metabolic syndrome risk: a systematic review and dose-response meta-analysis. Int J Clin Pract. 2015;69(1):136–44. https://doi.org/10.1111/ijcp.12507
Liu CF, Zhou W, Fang N. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract. 2012;66(7). https://doi.org/10.1111/j.1742-1241.2012.02959.x
Acknowledgements
None.
Funding
The authors received no financial support for this article’s research, authorship, and/or publication.
Author information
Authors and Affiliations
Contributions
Selection of papers. Two researchers (E.R.C and M.R.S) reviewed titles, abstracts and full texts. In addition, three researchers independently extracted data for studies that met the inclusion criteria (R.J.M, R.M.L. and G.M.R.). Finally, a fourth author (M.V.A.) acted as a judge in case of discrepancy. Data extractionOne researcher (E.R.C.) was responsible for extracting the data verified by a second researcher (R.J.M.). A third researcher (M.R.S.) resolved the disagreement in case of a tie. Cohen’s Kappa index was used to assess the degree of agreement. The following data were extracted from each study: citation, details of the study population (including age and sex), study design, follow-up period, sample size, and mean and standard deviation of ALT, AST, and GGT in those subjects with Metabolic Syndrome (MetS+) and without Metabolic Syndrome (MetS-). In addition, for articles collecting ALT, AST and GGT data, the mean and standard deviation were extracted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Raya-Cano, E., Molina-Luque, R., Vaquero-Abellán, M. et al. Metabolic syndrome and transaminases: systematic review and meta-analysis. Diabetol Metab Syndr 15, 220 (2023). https://doi.org/10.1186/s13098-023-01200-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01200-z