Abstract
Background
The prognostic nutritional index (PNI) and different glucose metabolisms have been separately reported to be correlated with long-term prognosis in patients with acute myocardial infarction (AMI) undergoing percutaneous coronary intervention (PCI). However, PNI application in patients with an impaired glucose metabolism has not been well validated, especially in pre-diabetic patients. This study evaluated whether PNI influences a long-term risk of mortality along different glucose metabolism statuses.
Methods
A total of 17,697 patients with AMI and a history of PCI were enrolled in this retrospective observational cohort study from January 2007 to December 2020. Three subgroups with different glucose metabolism statuses, including normal glucose regulation (NGR), pre-diabetes mellitus (pre-DM), and diabetes mellitus (DM), were divided into three groups according to the tertiles of PNI, respectively.
Results
All-cause mortality occurred in 2613 (14.8%) patients within a median of 4.1 years of follow-up. Upon analyzing the Kaplan–Meier plots for the NGR, pre-DM, and DM groups, the incidence of all-cause or cardiovascular mortality in the low PNI (PNI-L, ≤ 42.7) subgroup was significantly higher than that in the median PNI (PNI-M, > 42.7 and ≤ 48.2) and high PNI (PNI-H, > 48.2) subgroups (all, P < 0.001). After adjusting for confounding factors, the hazard ratio (HR) for all-cause mortality in the PNI-L group significantly increased compared to that in the PNI-H subgroups of the NGR group (HR, 1.35; 95% CI 1.14–1.66; P < 0.001), pre-DM group (HR, 1.29; 95% CI 1.02–1.62; P < 0.001), and DM group (HR, 1.36; 95% CI 1.13–1.63; P < 0.001). Given that there was evidence of interactions between PNI and different glucose statuses (P for interaction < 0.001), patients were divided into nine subgroups, and we found that DM patients with PNI-L statuses had the highest risk of all-cause mortality compared to NGR patients with PNI-H statuses (HR, 1.69; 95% CI 1.42–2.01; P < 0.001).
Conclusion
Lower PNI is a significant and independent risk factor for all-cause mortality in AMI patients undergoing PCI with different glucose metabolism statuses, and this risk further increases with DM compared to NGR or pre-DM statuses.
Similar content being viewed by others
Introduction
Early revascularization in patients with acute myocardial infarction (AMI) can save the dying myocardium, but reperfusion myocardium can cause myocardial ischemia–reperfusion injury (MIRI) due to endothelial cell dysfunction, inflammatory reactions, oxygen free radical injury, and calcium overload [1]. MIRI is associated with more than 50% of postoperative adverse cardiovascular events, which seriously affects the quality of life and the prognosis of patients [2]. The disorder of glucose metabolism can further aggravate MIRI. Studies have suggested that admission hyperglycemia measurements in patients with AMI undergoing percutaneous coronary intervention (PCI) can cause inflammatory reactions and oxidative stress injury, aggravate endothelial dysfunction, block coronary artery blood flow, and increase the infarct size, which are significantly related to the long-term adverse event outcome [3]. Using our Cardiorenal ImprovemeNt-II (CIN-II) study (ClinicalTrials.gov identifier NCT05050877) database, a total of 23,162 patients with AMI undergoing PCI were divided into normal glucose regulation (NGR) (n = 10,456), pre-diabetes (pre-DM) (n = 4929), and diabetes mellitus (DM) (n = 7777) groups. Assessments during a median follow-up of 1681 days revealed that the cardiac mortality rates in the NGR, pre-DM, and DM groups were 4.9%, 5.4%, and 7.6%, respectively (P < 0.001) (Additional file 1: Fig. S1). The above research shows that AMI patients with glucose metabolism disorders can experience a high long-term risk of cardiac death, whereas there is no or very low risk of cardiac death among people without diabetes, indicating that patients with AMI undergoing PCI still have a large cardiovascular residual risk to further explore.
Malnutrition is common in people with coronary heart disease (CAD) with impaired glucose metabolism. It was demonstrated that obese yet malnourished [high BMI, low serum albumin (SA)] individuals not only often have diabetes but also carry the highest comorbidity burden, the most adverse cardiac remodeling, and the least favorable composite outcome [4]. Previous studies reported that more than 50% of CAD patients with diabetes had malnutrition, and the mortality rate of those patients with severe malnutrition was nearly doubled [5]. The prognostic nutritional index (PNI) is an important tool for objective malnutrition screening of the cardiovascular disease population and may become a new biomarker to predict long-term outcomes of CAD patients after PCI [6]. Another study showed that a low level of PNI was closely associated with increased acute kidney injury and mortality after PCI [7]. Additionally, it was demonstrated that a lower PNI is associated with all-cause mortality and heart failure hospitalizations in outpatients with preserved ejection fractions [8].
The level of impaired glucose metabolism and malnutrition could reflect the poor long-term prognosis of CAD patients undergoing PCI. Nevertheless, there are few studies investigating PNI in relation to the prognosis of AMI patients undergoing PCI with different glucose states. Our objective was to examine the relationship between PNI and long-term outcomes in patients with AMI undergoing PCI with different glucose metabolisms.
Methods
Study population
The Cardiorenal Improvement II (CIN-II) study was a multi-center, retrospective, observational cohort study. This database is based on the population of coronary angiography, and we enrolled 25,020 consecutive AMI patients who underwent PCI in Guangdong Provincial People’s Hospital, Guangdong, China, between January 2007 and December 2020 (ClinicalTrials.gov identifier NCT05050877). Study exclusion criteria included ① age < 18 years (n = 245), ② missing admission albumin or lymphocyte measurements (n = 6896), ③ lost to follow-up (n = 146), and ④ removal of maxima and minima (n = 72). Eventually, a total of 17,661 patients were included (Fig. 1). AMI concluded acute ST-elevation myocardial infarction and acute non–ST-elevation myocardial infarction, and the diagnosis, indication for PCI, and drug treatment were determined following standard clinical practice guidelines [9].
Collection of clinical and demographic characteristics
From January 2007 to December 2020, data were extracted from the CIN-II study database. The baseline information included demographic characteristics, coexisting conditions, laboratory examinations, and medications at discharge. Patients were advised to fast for at least 12 h before fasting plasma glucose (FPG) and blood lipid profile measurements were collected, and other values were all collected at admission or before PCI. The laboratory data included plasma and biochemical parameters, such as levels of hemoglobin A1c (HbA1c), serum creatinine, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum albumin (ALB), fibrinogen, total lymphocyte count (LYM), absolute neutrophil count, platelet count, and hemoglobin. The ratio of neutrophils to lymphocytes was calculated by dividing the absolute neutrophil count by the LYM. Medical history was assessed using International Classification of Diseases, 10th revision, codes, and 54.6% of patients underwent trans-thoracic echocardiography before or after PCI. The left atrial diameter, left ventricular end-diastolic dimension, and left ventricular end-systolic dimension were obtained in the parasternal long-axis view.
Clinical definition
DM patients were defined by ① a previous history of diabetes or use of antidiabetic agents and ② FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% at admission. Pre-DM patients were defined by an FPG of 5.6 to < 7.0 mmol/L or an HbA1c concentration of 5.7% to < 6.5% at admission, while NGR patients were defined as those without DM or pre-DM [10]. Anemia was defined by a hematocrit concentration of ≤ 39% (male) or ≤ 36% (female). The PNI score was calculated using the formula: 10 × ALB (g/dL) + 0.005 × LYM (per mm3). According to the tertiles of PNI, the whole study group was divided into the following three subgroups: PNI-L, PNI-M, and PNI-H. The three groups of NGR, pre-DM, and DM adopted a unified classification standard. CKD was defined by an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [11]. Congestive heart failure (CHF) was defined by Killip class > 1 or New York Heart Association functional classification > 2. Definitions of other variables, such as valvular heart disease, atrial fibrillation (AF), hypertension (HT), and stroke, were made using ICD-10 codes.
Study endpoints
All-cause mortality and cardiovascular mortality events were obtained from the official department for disease control and prevention. Other non-mortality events were adjudicated by trained nurses and research assistants through outpatient interviews and telephones according to pre-specified definitions [12]. The primary endpoint was long-term all-cause mortality. The secondary endpoint was long-term cardiovascular mortality.
Statistical analysis
Baseline characteristics are presented as mean ± standard deviation values for continuous variables and proportions for categorical variables. The differences in baseline characteristics between groups were compared using Student’s t-test for continuous variables and chi-squared tests for categorical variables. The differences in characteristics in a box plot were compared using the Kruskal–Wallis test. Time-to-event data among groups are presented graphically using Kaplan–Meier curves and compared by the log-rank test. Cox proportional hazards models were used to analyze all-cause mortality and calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Baseline variables exhibiting a P-value<0.0001, or those with clinical significance were incorporated into Cox regression models. The final model with adjustment for major covariables, including age, sex, HT, CHF, CKD, AF, stroke, and anemia, was made by stepwise selection method based on P < 0.05; then, it was refined to select clinically meaningful covariates.Competing risk model analyses were used to investigate the prognosis impact of cardiovascular mortality. Interactions between treatment and subgroups were examined using a test for heterogeneity, using P <0.05 as signifiacant. Presented tests were 2-tailed for all, and P < 0.05 was considered statistically significant. All statistical analyses were performed using R (ver. 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of the study population
A total of 17,661 AMI patients who underwent PCI were included in the analysis. Table 1 presents baseline characteristics for patients according to their baseline PNI level and glucose statuses. Patients with lower PNI levels were older, more likely to be female, and more often had DM or pre-DM. Additionally, these patients tended to have greater incidence rates of HT, CHF, CKD, AF, stroke, and anemia than those with higher PNI levels. Moreover, the PNI-L group had lower LVEF and eGFR values but higher hs-cTnT, NT-proBNP, FPG, hs-CRP, and HbA1c levels than the PNI-H group. Patients with DM were oldest and had the highest incidence rates of HT, CHF, CKD, AF, stroke, and anemia compared to those with pre-DM or NGR statuses. In addition, values of LVEF, PNI, and eGFR were lowest and those of hs-cTnT, NT-proBNP, FPG, and hs-CRP were highest in patients with DM (Table 1).
Ability of PNI to predict all-cause mortality and cardiovascular mortality
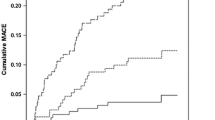
Restricted cubic spline models showed that a J-shaped association existed between PNI and long-term all-cause or cardiovascular mortality, and the relationship of both was non-linear (non-linear P = 0.001 or P < 0.001) (Fig. 2). A PNI index of 45.1 was selected as the reference level represented by the vertical dotted lines. Lower levels of PNI were associated with increased risks of all-cause and cardiovascular mortality. All-cause mortality occurred in 2613 (14.8%) patients and cardiovascular mortality occurred in 1222 (6.9%) patients during a median of 4.1 years of follow-up. The multivariable analysis showed that the PNI-L subgroup among NGR, pre-DM, and DM patients had a similar heightened risk of al-cause mortality compared to the PNI-H subgroup (adjusted HR, 1.35; 95% CI 1.2–1.51; P < 0.001) (Table 2). PNI-L increased the risk of cardiovascular mortality in DM patients (HR, 1.316; 95% CI 1.009–1.717; P = 0.043) and pre-DM patients HR, 1.765; 95% CI 1.147–2.716; P = 0.01), while no significant difference was found in the NGR group (P > 0.05) (Table 3). Moreover, there was no significant difference between the PNI-M and PNI-H subgroups among NGR, pre-DM, and DM patients. The Kaplan–Meier survival curve showed the same trends for the relationship between PNI and all-cause mortality of different glucose statuses. The cumulative hazard in the PNI-L group was significantly higher than those of other groups (log-rank P < 0.001) (Fig. 3A, C, E, and G). Similarly, the competing risk analysis showed that PNI-L statuses was associated with cardiac death after adjusting for a competing event (non-cardiac death) (Fine–Gray test P < 0.001) (Fig. 3B, D, F, and H).
Cumulative incidences of all-cause mortality (Kaplan-–Meier curve) and cardiac mortality (cumulative incidence function curve) risks for patients with different glucose statuses. Cumulative hazard of all‑cause mortality A and cardiovascular mortality B across the whole group with different PNI index; Cumulative hazard of all‑cause mortality C and cardiovascular mortality D across the NGR group with different PNI index; Cumulative hazard of all‑cause mortality E and cardiovascular mortality F across the pre-DM group with different PNI index; Cumulative hazard of all‑cause mortality G and cardiovascular mortality H across the DM group with different PNI index. PNI, prognostic nutritional index; DM, diabetes mellitus; pre-DM, pre-diabetes mellitus; NGR, normal glucose regulation
Subgroup analysis
The results of subgroup analysis were in line with those of the overall analysis, and formal testing for interactions showed that the relative risks of 4.1-year all-cause mortality in a comparison between the high PNI and low PNI groups were consistent across almost all subgroups among patients with DM, pre-DM, or NGR. The relationship between PNI and a risk of all-cause mortality was modified by glucose metabolism statuses (P for interaction < 0.001) (Fig. 4A). Different glucose metabolism also had interaction with PNI with respect to cardiovascular mortality (P for interaction < 0.001) (Fig. 4B). Therefore, we further assessed for mortality risk in the nine subgroups due to the interaction between PNI and glucose metabolism statuses, which was independently associated with the risk of all-cause and cardiovascular mortality (Fig. 4C, D). Especially, patients with PNI-L and DM were 1.69 times more at risk for all-cause mortality than patients with PNI-H or NGR statuses (adjusted HR, 1.69; 95% CI 1.42–2.01).
Hazard ratios for long-term all-cause A and cardiovascular mortality B in different glucose metabolism statuses. Hazard ratios for long-term all-cause C and cardiovascular mortality D in different subgroups. PNI, prognostic nutritional index; DM, diabetes mellitus; pre-DM, pre-diabetes mellitus; NGR, normal glucose regulation; Ref., Reference; CI, confidence interval; PNI-H: PNI>48.2; PNI-M: 42.7
Discussion
To our knowledge, this is the first large real-world cohort study to explore the relationship between nutritional statuses and glucose statuses among patients with AMI undergoing PCI in China. The main finding of our study is prevalent among patients with glucose metabolism disorder, even in DM patients, and is strongly associated with increased mortality.
As the most serious state of blood glucose metabolism disorder, DM is closely related to the progression of coronary heart disease and a poor prognosis [13]. The progression of diabetes to coronary artery disease is mainly related to hyperglycemia, dyslipidemia, changes in the secretion of hormones other than insulin, and a pro-inflammatory statuses, and oxidative stress and inflammation interact to promote an abnormal glucose metabolism statuses and accelerate atherosclerosis [14]. Cardiovascular disease is the most common cause of death for DM patients, and AMI patients are more likely to be associated with blood glucose metabolism disorder. Studies show that the annual incidence of insulin resistance in patients with an AMI history is twice that in patients without an AMI history [15]. Several factors may explain the worse long-term outcome of AMI patients with DM, including a higher coronary atherosclerotic burden, more vulnerable plaques, and enhanced platelet reactivity [16, 17]. Although pre-DM patients tend to have a mild glucose metabolism disorder, more and more studies have confirmed that it is significantly related to cardiovascular events. Only 5–10% of pre-DM patients develop DM every year, while the same proportion will be converted into an NGR statuses. The division of these three blood glucose states is mainly based on the level of HbA1c, and it was found that every 1% increase in HbA1c was associated with a greater risk of cardiovascular events, with an HR of 1.07 [18]. The increase in the HbAlc level can increase the viscosity of red blood cells, heighten the occurrence of thrombotic events, and change the affinity of red blood cells for oxygen, leading to hypoxia of myocardial cells, aggravating the pathological changes of cardiac microvessels, and causing an abnormal myocardial metabolism. Therefore, grouping research based on blood glucose statuses is a preferable approach for evaluating the disease progression and prognosis of AMI patients who underwent PCI.
Although AMI patients with PCI have undergone timely blood supply reconstruction, they still experience high long-term all-cause and cardiovascular mortality rates, suggesting that there are many residual cardiovascular risks still to be identified. Therefore, in addition to focusing on traditional cardiovascular risk factors, the identification and intervention of non-traditional factors will help to play an important role in understanding the disease progression and prognosis of the AMI with PCI history population. Recent studies have shown that non-traditional cardiovascular risk factors represented by malnutrition are closely related to a poor prognosis of AMI patients. At present, the scoring indicators of malnutrition mainly include PNI; the Geriatric Nutritional Risk Index (GNRI); and triglyceride, total cholesterol, and body weight index scores [19]. PNI can provide nutritional, inflammatory, and immunological information, is more and more widely used to evaluate the prognosis of the AMI population, can be used as an important indicator of the prognosis of patients with congestive heart failure [20], and has a high predictive value for long-term cardiovascular and cerebrovascular events in the CAD intervention population [6]. To our knowledge, there are no specific large-scale studies considering the prognostic value of PNI in different glucose metabolism statuses groups, and glucose metabolism disorder leads to high cardiovascular risks and should be paid more attention. In our cohort, the all-cause mortality of NGR patients was 12.9%, that of pre-DM patients was 14.5%, and that of DM patients was as high as 17.1%, with all cases significantly associated with malnutrition. In contrast, DM patients with PNI-L had the highest risk of all-cause mortality, which could be up to 1.36 times higher than that of DM patients with PNI-H and 1.69 times higher than that of NGR patients with PNI-H. Due to disorders of glucose and lipid metabolism, diet control, and the influence of hypoglycemic drugs, DM patients are more likely to suffer from malnutrition. After the occurrence of AMI, gastrointestinal absorption dysfunction leads to a nutrient intake decline, reducing the stability of the internal environment and the reserve function of organs, leaving the nutritional needs of the body unsatisfied; moreover, patients with nutritional risks also have poor drug resistance and experience obstacles in food intake and nutrient absorption, which can worsen the nutritional statuses of AMI patients. Evidence shows that the blood glucose level has a significant impact on the nutritional statuses of patients with CAD and is more likely to lead to the aggravation of coronary artery lesions and cardiovascular events [21]. Additionally, malnutrition associated with low serum albumin at admission is independently associated with a greater risk for bleeding events in patients with AMI undergoing PCI [22]. DM can aggravate the inflammatory reaction process of coronary atherosclerosis, while long-term chronic inflammation aggravates the occurrence of malnutrition; subsequently, malnutrition and inflammation form a vicious circle, increasing the risk of long-term cardiovascular death in patients with AMI. Therefore, AMI combined with malnutrition can further aggravate myocardial injury and inflammatory reactions, which is consistent with the findings that troponin, BNP, and CRP significantly increased and LVEF significantly decreased in the PNI-L subgroup at the baseline during our study. Additionally, DM patients with malnutrition could also show an association with carcinogenesis, cancer progression, and death [23].
In the AMI population without a DM history, more than 50% of patients were diagnosed with pre-DM [24]. A disorder of glucose metabolism exists in the pre-DM state, but clinical interventions for this population are fewer in number and more easily ignored. Previous studies have reported that AMI patients with pre-DM are older, had higher BMIs and worse cardiac function, and were more likely to be associated with multiple cardiovascular risk factors, which became an independent predictor of cardiovascular events in AMI [25]. Additionally, pre-DM has a similar impact as DM on the long-term prognosis of major adverse cardiovascular events in patients with myocardial infarction with non-obstructive coronary arteries [26]. In this study, we found that the long-term all-cause mortality risk of the PNI-L subgroup was significantly different in the pre-DM population. Interestingly, there was a greater risk of cardiovascular mortality in the PNI-L subgroup than the total DM population. In addition, it was found that LYMs from the pre-DM group were lower than those from the DM and NGR groups. Coronary atherosclerosis rupture, acute thrombosis, and systemic inflammatory reactions are important mechanisms of AMI. MIRI after revascularization can further aggravate the damage caused by cardiac inflammatory reactions [1]. Lymphocytes, as a core component of the adaptive immune response, participate in inflammatory reactions in the process of atherosclerosis and play anti-inflammatory and anti-atherosclerosis roles [27]. The study confirmed that the level of Tregs in patients with AMI decreased, which is closely related to cardiovascular events [28]. In the acute phase of coronary events, the increase in corticosteroid hormones causes a stress reaction, leading to a large trend of lymphocyte apoptosis, weakening the anti-inflammatory effect, which can lead to an increased risk of cardiovascular events [29]. Leukocytes are an independent predictor of inflammation and the immune response. Neutrophils can cause vascular endothelial dysfunction and vascular wall degradation by secreting inflammatory cytokines. The ratio of neutrophils to lymphocytes has gradually been considered as a reliable indicator of systemic inflammation and the immune response [30]. Our study shows that the highest ratio of neutrophils to lymphocytes in the PNI-L subgroup indicates the most serious inflammatory state. Moreover, DM patients show inhibition of the immune response, while pre-DM patients start to show upregulation of inflammatory markers and immune activation [31]. Therefore, PNI has a significant value in predicting the risk of cardiac death in the pre-DM population.
Our study had several limitations. First, despite including a large sample size, this was a single-center study; thus, generalization of the findings should be done cautiously. Second, laboratory parameters were are not all checked at admission, which might cause potential bias due to measurement errors in different time periods. Third, DM was defined according to the ICD-10 code recorded at admission. Although there are some undetected DM patients that might have been enrolled in our study, this real-world study can also reflect the current situation of DM diagnosis. In addition, prospective cohort studies are required to confirm our findings.
Conclusion
At present, malnutrition is common in patients with AMI undergoing PCI in different glucose states, especially DM patients, which can significantly increase their long-term mortality. Therefore, it is particularly important to conduct a hierarchical assessment of nutritional statuses, and early intervention for malnutrition will help improve the prognosis of patients with different glucose metabolism statuses. Our study firstly indicated that a lower PNI is a significant and independent risk factor for all-cause mortality in AMI patients undergoing PCI with different glucose metabolism statuses. Moreover, this risk was further increased in the DM group compared to the NGR and pre-DM groups. Pre-DM patients also had a worse risk of cardiovascular mortality. Further study is required to explore whether effective PNI increases can improve the prognosis in AMI patients undergoing PCI.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the institution policy but are available from the corresponding author on reasonable request.
Abbreviations
- PNI:
-
Prognostic nutritional index
- DM:
-
Diabetes mellitus
- pre-DM:
-
Pre-diabetes mellitus
- NGR:
-
Normal glucose regulation
- Prior PCI:
-
Prior percutaneous coronary intervention
- Prior MI:
-
Prior myocardial infarction
- Prior CABG:
-
Prior coronary artery bypass graft
- HbA1C:
-
Hemoglobin A1c
- FPG:
-
Fasting plasma glucose
- eGFR:
-
Estimated glomerular filtration rate
- LDL-C:
-
Low density lipoprotein cholesterol
- HDL-C:
-
High density lipoprotein cholesterol
- ALB:
-
Serum albumin
- FIB:
-
Fibrinogen
- LYM:
-
Total lymphocyte count
- NEU:
-
Absolute neutrophil count
- PLT:
-
Platelet count
- NLR:
-
Neutrophil-to-lymphocyte ratio
- LVEF:
-
Left ventricular ejection fraction
- LVEDD:
-
Left ventricular end-systolic dimension
- LVESD:
-
Left ventricular end-systolic dimension
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin receptor blocker
- CCB:
-
Calcium block
- Ref.:
-
Reference
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- RCS:
-
Restricted cubic spline
References
Algoet M, Janssens S, Himmelreich U, Gsell W, Pusovnik M, Van den Eynde J, et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2022;33(6):357–66.
Hu M, Chen G, Yang H, Gao X, Yang J, Xu H, et al. Short- and long-term outcomes in patients with right ventricular infarction according to modalities of reperfusion strategies in China: data from China acute myocardial infarction registry. Front Cardiovasc Med. 2022;9: 741110.
Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–7.
Chien SC, Chandramouli C, Lo CI, Lin CF, Sung KT, Huang WH, et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in Asian adults: a cohort study. PLoS Med. 2021;18(6): e1003661.
Wei W, Zhang L, Li G, Huang Z, Liu J, Wu Z, et al. Prevalence and prognostic significance of malnutrition in diabetic patients with coronary artery disease: a cohort study. Nutr Metab. 2021;18(1):102.
Liu TD, Zheng YY, Tang JN, Wang W, Dai XY, Zhang JC, et al. Prognostic nutritional index as a novel predictor of long-term prognosis in patients with coronary artery disease after percutaneous coronary intervention. Clin Appl Thromb Hemost. 2022;28:10760296221103272.
Han M, Lee HW, Lee HC, Kim HJ, Seong EY, Song SH. Impact of nutritional index on contrast-associated acute kidney injury and mortality after percutaneous coronary intervention. Sci Rep. 2021;11(1):7123.
ZencirkiranAgus H, Kahraman S. Prognostic nutritional index predicts one-year outcome in heart failure with preserved ejection fraction. Acta Cardiol. 2020;75(5):450–5.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123–55.
American Diabetes Association Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–38.
Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current statuses and future directions. Nat Rev Nephrol. 2020;16(1):51–64.
Huang H, Li Q, Liu J, Qiao L, Chen S, Lai W, et al. Association between triglyceride glucose index and worsening heart failure in significant secondary mitral regurgitation following percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21(1):260.
Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Admission blood glucose and 2-year mortality after acute myocardial infarction in patients with different glucose metabolism statuses: a prospective, nationwide, and multicenter registry. Front Endocrinol. 2022;13: 898384.
Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–60.
Mozaffarian D, Marfisi R, Levantesi G, Silletta MG, Tavazzi L, et al. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet. 2007;370(9588):667–75.
Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126(4):296–306.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–81.
Gerstein HC, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the hope study: a prospective epidemiological analysis. Diabetologia. 2005;48(9):1749–55.
Kim HR, Kang MG, Kim K, Koh JS, Park JR, Hwang SJ, et al. Comparative analysis of three nutrition scores in predicting mortality after acute myocardial infarction. Nutrition. 2021;90: 111243.
Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6(6): e004876.
Abu-Lebdeh HS, Hodge DO, Nguyen TT. Predictors of macrovascular disease in patients with type 2 diabetes mellitus. Mayo Clin Proc. 2001;76(7):707–12.
Yoshioka G, Natsuaki M, Goriki Y, Shinzato K, Nishihira K, Kuriyama N, et al. Serum albumin and bleeding events after percutaneous coronary intervention in patients with acute myocardial infarction (from the HAGAKURE-ACS registry). Am J Cardiol. 2022;165:19–26.
Supabphol S, Seubwai W, Wongkham S, Saengboonmee C. High glucose: an emerging association between diabetes mellitus and cancer progression. J Mol Med. 2021;99(9):1175–93.
Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of glycated hemoglobin for assessment of glucose metabolism in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2016;117(5):749–53.
Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370: m2297.
Gao S, Ma W, Huang S, Lin X, Yu M. Impact of prediabetes on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Diabetol Metab Syndr. 2021;13(1):103.
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–27.
Wigren M, Bjorkbacka H, Andersson L, Ljungcrantz I, Fredrikson GN, Persson M. Low levels of circulating CD4+FOXP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol. 2012;32(8):2000–4.
Nunez J, Sanchis J, Bodi V, Nunez E, Mainar L, Heatta AM, et al. Relationship between low lymphocyte count and major cardiac events in patients with acute chest pain, a non-diagnostic electrocardiogram and normal troponin levels. Atherosclerosis. 2009;206(1):251–7.
Paolisso P, Foa A, Bergamaschi L, Donati F, Fabrizio M, Chiti C, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. 2021;20(1):33.
Mzimela NC, Ngubane PS, Khathi A. The changes in immune cell concentration during the progression of pre-diabetes to type 2 diabetes in a high-fat high-carbohydrate diet-induced pre-diabetic rat model. Autoimmunity. 2019;52(1):27–36.
Acknowledgements
We are grateful to all the subjects who participated in the study.
Funding
National Natural Science Foundation of China (82170440, U220A20270); Hainan Province Science and Technology special fund (ZDYF2020122); Natural Science Foundation of Hainan Provincial (821QN0986); Hainan Provincial Health Foundation (20A200369, 22A200130); National Science Fund for Distinguished Young Scholars (JBGS202104); the Cardiovascular Disease Research Science Innovation Group of Hainan Medical University; the Project of Hainan Province Clinical Medical Center; Guangdong Provincial science and technology project (2020B1111170011, KJ022021049).Open Fund for Key Laboratory of Emergency and Trauma Research, Ministry of Education(KLET-202214);China Scholarship Scheme (China Scholarship Council [CSC]) from simply China Scholarship Council, Grant/Award Number(CSCNO.202287).
Author information
Authors and Affiliations
Contributions
XL, YL, SC and JG designed the research. XL, CL and JL analyzed the data and wrote the manuscript. YH, YY, NL and WJ collected the data and performed statistical analysis. YL, SC and JG critically revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All traceable personal identifiers were removed from the analytic dataset to protect patients’ privacy. The study protocol was approved by Guangdong Provincial People’s Hospital ethics committee and the study was performed according to the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Cumulative incidences of cardiac mortality (cumulative incidence function curve) risks for AMI patients with different glucose statuses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ling, X., Lin, C., Liu, J. et al. Prognostic value of the prognostic nutritional index for patients with acute myocardial infarction undergoing percutaneous coronary intervention with variable glucose metabolism statuses: a retrospective cohort study. Diabetol Metab Syndr 15, 207 (2023). https://doi.org/10.1186/s13098-023-01160-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01160-4