Abstract
Background
The Triglyceride and Glucose (TyG) index has been found to have a strong correlation with the recurrence of acute ischemic stroke (AIS) and poor patient outcomes. Nevertheless, the relationship between the TyG index and early neurological deterioration (END) has not been fully explored. Therefore, the present study aims to investigate the potential association between the TyG index and END.
Methods
A retrospective analysis of 2129 patients diagnosed with AIS between January 2019 and December 2022 at the Second Affiliated Hospital of Harbin Medical University. Patients were divided into END and non-END groups based on changes in National Institutes of Health Stroke Scale scores within 7 days of admission, and the differences in the indicators between the two groups were examined using univariate analysis. The patients were then divided into three groups based on the tertile of the TyG index (T1: TyG index < 8.662; T2: 8.662 ≤ TyG index < 9.401; T3: TyG index ≥ 9.401), and logistic regression analysis was used to examine the association between the TyG index and END. Finally, the predictive ability of the TyG index was evaluated using the receiver operating characteristic (ROC) curve.
Results
A total of 724 patients experienced END. The results of the analysis showed that the TyG index was significantly higher in the END group compared to the non-END group. Furthermore, the TyG index was found to be an independent risk factor for the development of END (OR, 1.561; 95% 1.166–2.090, P = 0.003). After adjusting for confounders, the risk of END was 3.953 (95% CI 2.793–5.595; P < 0.001) and 5.906 (95% CI 3.676–9.488; P < 0.001) times higher in the T2 and T3 groups, respectively, in contrast to the T1 group. The area under the ROC curve of the TyG index was 0.711 (0.688–0.733), indicating an excellent predictive indicator.
Conclusions
Our study uncovered that higher TyG index levels were associated with END development in AIS patients.
Similar content being viewed by others
Introduction
Ischemic stroke (IS) is the most prevalent type of stroke [1], characterized by the impaired blood supply to the brain tissue due to various causes, leading to ischemic and hypoxic necrosis and consequent brain dysfunction [2]. Early neurological deterioration (END) is a common complication occurring in approximately 8.1–28.1% of IS patients, worsening the patient’s condition [3], prolonging hospitalization, and imposing a substantial burden on society and families [4]. Identifying early indicators that can predict the occurrence of END is therefore urgently needed.
Insulin resistance (IR) refers to a condition in which target organs that insulin acts upon are less sensitive to insulin’s action, resulting in less than normal biological effects despite a normal dose of insulin [5]. IR is not only at the root of the pathogenesis of type II diabetes but is also a common pathophysiological basis of many metabolic diseases [6]. Previous studies have indicated that IR is positively associated with the risk of ischemic stroke and can promote stroke progression, affecting patient prognosis [7]. However, no standardised clinical measure of IR exists to guide clinical practice [8]. The triglyceride glucose (TyG) index can be a reliable marker of IR[9]. Accumulating evidence suggests that the triglyceride and glucose (TyG) index can serve as a predictor of cardiovascular disease [10], atherosclerosis [11], IS [12], and metabolic disease [13]. A recent survey found that the TyG index was associated with END in single subcortical infarcts [14]. However, only one study has examined the relationship between the TyG index and END in IS patients. This study was limited by its sample size, analyzing only 305 patients following screening. Therefore, the present study aimed to investigate the association between the TyG index and END in a larger clinical cohort based on real-world data.
Methods
Recruitment
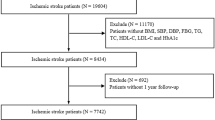
In this large-scale retrospective study, we examined a cohort of 2129 patients diagnosed with acute ischemic stroke (AIS) who received treatment at the Second Affiliated Hospital of Harbin Medical University between January 2019 and December 2022. The inclusion criteria were as follows: The patient was over 18 and under 80 years of age, and the diagnosis of AIS was confirmed by head magnetic resonance imaging. Also, patients with severe impairment of consciousness, patients who could not cooperate with the test, patients who had undergone thrombolysis or mechanical thrombectomy, and patients with tumours, trauma, surgery, bleeding or incomplete data were excluded from the study. The patient accrual process is detailed in Fig. 1.
Data collection
This study collected baseline information such as age, sex, history of hypertension, diabetes mellitus (DM), coronary heart disease (CHD), smoking, and drinking status. In addition, examination and laboratory indices were assessed, including initial systolic blood pressure (SBP), initial diastolic blood pressure (DBP), initial National Institutes of Health Stroke Scale (NIHSS), triglycerides (TG), total cholesterol (TC), fasting plasma glucose (FPG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), C-reactive protein (CRP), and homocysteine (HCY). Blood samples were collected from all patients by a professional nurse on an empty stomach in the morning of the second day after admission.
Definitions
Patients were grouped according to the change in NIHSS score. END was defined as a deterioration of ≥ 2 points in the patient’s NIHSS score within 7 days of admission compared with the admission [15]. TyG was defined using the formula: Ln [TG (mg/dL)×FPG (mg/dL) ÷ 2] [16].
Statistical analysis
For continuous variables, the Shapiro-Wilk test was used to assess the normality of the data. Student’s t-tests were used to analyze normal distribution data, which were expressed as mean ± standard deviation. For non-normally distributed data, P50 (P25, P75) was used and analyzed with the Mann-Whitney U test. Meanwhile, the chi-square test was used for categorical data analysis. The predictive value of TyG indicators for END was evaluated using the receiver operating characteristic curve (ROC). All statistical tests were two-tailed, and significance was set at 0.05 for all data.
Results
Patient baseline characteristics
The cohort comprised of 724 patients diagnosed with END and 1405 patients with non-END. Statistical analysis indicated that the END group had a significantly higher proportion of older male patients with smoking, hypertension, and diabetes mellitus history compared to the non-END group (P < 0.05). Moreover, the END group also exhibited higher initial SBP, initial DBP, initial NIHSS, TC, TG, FPG, CRP, HCY, and TyG indexes in comparison to the non-END group (P < 0.05). Meanwhile, the remaining indicators showed no significant differences between the two groups. The findings are detailed in Table 1.
Associations between END and risk factors
Next, we conducted a univariate logistic regression analysis, including all relevant patient characteristics, to investigate the relationship between these factors and END. This model used END as the dependent variable, with non-END as a reference. Our results indicate that several factors, including age, sex, hypertension, DM, smoking status, initial SBP and DBP measurements, initial NIHSS scores, TC, TG, FPG, HDL-C, CRP, HCY, and TyG indexes, were significantly associated with the occurrence of END, as shown in Table 2.
Multivariate analysis of risk factors associated with END is shown in Table 3. Both continuous and categorical variables were used to investigate the correlation between the TyG index and END. According to our results, when the TyG index was analyzed as a continuous variable, it was significantly associated with END after adjusting for different risk factors (P < 0.05). Using TyG as a categorical variable, the T2 and T3 groups showed higher END rates compared to the T1 group. After adjusting for confounding factors, the risk of END remained higher in the T2 and T3 groups in contrast to the T1 group, as seen in models 2 and 3. In model 3, the risk of END in the T3 group was 5.906 (3.676–9.488) times higher than in the T1 group.
In addition, we utilized the area under the ROC curve to evaluate the predictive value of the TyG index. As shown in Fig. 2, the AUC of TyG was 0.711 (0.688–0.733), indicating a good predictive ability. Meanwhile, the sensitivity, specificity, and accuracy of the TyG index were also superior (Table 4).
Discussion
To our knowledge, this is the first and largest sample-size study to explore the relationship between the TyG index and the development of END in patients with acute ischemic stroke (AIS). In this study, the TyG index was found to be positively associated with the development of END in AIS patients. Our results were consistent even after adjusting for potential confounding factors, providing further evidence of this correlation.
Approximately 16.9 million people suffer a stroke each year [17] and the IS accounts for 85% of all strokes [18]. END occurs in a large proportion of patients despite timely and regular treatment, and symptoms progressively exacerbate, increasing the risk of death and disability [19]. IR plays an important role in the progression and prognosis of IS by inducing a variety of metabolic disorders, thereby promoting the rupture of atherosclerotic plaques leading to thrombosis [20]. Normoglycaemic clamp testing, the “gold standard” for the diagnosis of IR, is complicated, time-consuming, expensive and requires frequent blood sampling, which leads to poor clinical application. In recent years, the TyG index has been shown to be a novel alternative index for IR. It can be used to predict stroke recurrence [21],all-cause mortality, poor prognosis [22]. However, the relationship between the TyG index and END is unknown to date. In this study, to provide new ways of predicting END and thus improving patient prognosis, we investigated whether the IR-related index TyG could predict the occurrence of END.
Our study found that the TyG index is a significant risk factor for END. Patients in the END group had significantly higher TyG index values than those in the non-END group, and even after adjusting for confounding factors, the TyG index remained an independent risk factor for END. Furthermore, we divided patients into tertiles based on their TyG index values and found a significant association between TyG index tertiles and END occurrence, with the highest two tertiles having a greater risk compared to the lowest tertile. Our analysis of the overall discriminatory power of the index yielded an AUC of 0.711 (0.688–0.733), indicating its reliable predictive power. Therefore, the TyG index has the potential to serve as a promising indicator for predicting the occurrence of END.
Possible reasons for the TyG Index affecting END are as follows: Firstly, insulin resistance may exacerbate endothelial dysfunction, excessive platelet activation [23], and biochemical imbalances that can contribute to the development of atherosclerotic thrombotic disease [24]. Recent research has shown that higher TyG index values are linked to carotid atherosclerosis in patients with ischemic stroke [25]. Secondly, IR can intensify oxidative stress, leading to the accumulation of reactive oxygen species that can result in mitochondrial dysfunction. IR can also elevate the activity of matrix metalloproteinase 9, exacerbating the inflammatory response and ischemia-reperfusion injury [26, 27]. In addition, IR can contribute to the worsening of patients’ condition by affecting sympathetic activation, ion transport across membranes and inhibition of lipolysis [28, 29].
Moreover, our study identified several risk factors significantly associated with END, including age, sex, SBP, DBP, DM, hypertension, smoking, NIHSS, HDL, CRP, and HCY. Similarly, Liu et al. demonstrated that age, SBP, DM, TG, and baseline NIHSS were independent risk factors for END [30]. Meanwhile, Tan et al. also reported that age and higher NIHSS scores were risk factors for END [31]. Additionally, another study found that after adjusting for factors related to hypertension, DM, NIHSS score at admission, and various blood laboratory indicators, END was associated with CRP and HCY levels [32]. Therefore, our results are in agreement with these earlier findings.
Despite the notable findings in our study, there are several limitations that should be acknowledged. First, our study may have limited population selection because it was a retrospective analysis conducted at a single centre. Further validation of our findings is required through prospective studies involving multiple centers. Second, the TyG index may be influenced by various factors such as recent use of glucose-lowering and lipid-lowering medications, dietary intake, and other confounding factors that were not adequately controlled in this study. Therefore, caution is warranted when interpreting our results. Lastly, our study did not distinguish between patients with anterior and posterior circulation. Therefore, a further in-depth research is warranted.
Conclusion
In conclusion, our study provides evidence that elevated TyG index levels are associated with the occurrence of END and may serve as an independent predictor of END development. The TyG index has also been shown to have a high predictive value in our study. This suggests that monitoring changes in the index may be useful for clinicians to assess changes in patients’ conditions and take timely action.
Data Availability
On reasonable request, the corresponding author will allow access to the raw data of all of the patients who participated in this investigation.
Abbreviations
- IS:
-
Ischemic stroke
- END:
-
Early neurological deterioration
- IR:
-
Insulin resistance
- TyG:
-
Triglyceride and glucose index
- AIS:
-
Acute ischemic stroke
- DWI:
-
Diffusion-weighted imaging
- DM:
-
Diabetes mellitus
- CHD:
-
Coronary heart disease
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- NIHSS:
-
National Institutes of Health Stroke Scale
- TG:
-
Triglycerides
- TC:
-
Total cholesterol cholesterol
- FPG:
-
Fasting plasma glucose
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- CRP:
-
C-reactive protein
- HCY:
-
Homocysteine
- ROC:
-
Receiver operating characteristic curve
- OR:
-
Odds ratios
- CI:
-
Confdence interval
- Β:
-
Regression coefcient
- AUC:
-
Area under the curve
References
Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 2017, 390(10100):1151–1210.
Paul S, Candelario-Jalil E: Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Experimental neurology 2021, 335:113518.
Sabir Rashid A, Huang-Link Y, Johnsson M, Wetterhäll S, Gauffin H: Predictors of Early Neurological Deterioration and Functional Outcome in Acute Ischemic Stroke: The Importance of Large Artery Disease, Hyperglycemia and Inflammatory Blood Biomarkers. Neuropsychiatric disease and treatment 2022, 18:1993–2002.
Zhang YB, Su YY, He YB, Liu YF, Liu G, Fan LL. Early neurological deterioration after recanalization treatment in patients with Acute ischemic stroke: a retrospective study. Chin Med J. 2018;131(2):137–43.
Lebovitz HE: Insulin resistance: definition and consequences. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association 2001, 109 Suppl 2:S135-148.
Cai X, Hu J, Zhu Q, Wang M, Liu S, Dang Y, Hong J, Li N. Relationship of the metabolic score for insulin resistance and the risk of stroke in patients with hypertension: a cohort study. Front Endocrinol. 2022;13:1049211.
Miao M, Bi Y, Hao L, Bao A, Sun Y, Du H, Song L, You S, Zhong C: Triglyceride-glucose index and short-term functional outcome and in-hospital mortality in patients with ischemic stroke. Nutrition, metabolism, and cardiovascular diseases: NMCD 2023, 33(2):399–407.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F: The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metabolic syndrome and related disorders 2008, 6(4):299–304.
Cho YK, Han KD, Kim HS, Jung CH, Park JY, Lee WJ: Triglyceride-Glucose Index Is a Useful Marker for Predicting Future Cardiovascular Disease and Mortality in Young Korean Adults: A Nationwide Population-Based Cohort Study. Journal of lipid and atherosclerosis 2022, 11(2):178–186.
Guo X, Shen R, Yan S, Su Y, Ma L: Triglyceride-glucose index for predicting repeat revascularization and in-stent restenosis in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovascular diabetology 2023, 22(1):43.
Tang X, Zhang L, Li Y, Zhou Y, Cai X, Yao Y, Fang Q: Relationship between triglyceride-glucose index and carotid plaques in a high-stroke-risk population in southeast china: A population-based cross-sectional survey. Frontiers in endocrinology 2022, 13:1023867.
Hoshino T, Mizuno T, Ishizuka K, Takahashi S, Arai S, Toi S, Kitagawa K: Triglyceride-glucose index as a prognostic marker after ischemic stroke or transient ischemic attack: a prospective observational study. Cardiovascular diabetology 2022, 21(1):264.
Zhang Y, Liu C, Xu Y, Wang Y, Dai F, Hu H, Jiang T, Lu Y, Zhang Q: The management correlation between metabolic index, cardiovascular health, and diabetes combined with cardiovascular disease. Frontiers in endocrinology 2022, 13:1036146.
Nam KW, Kang MK, Jeong HY, Kim TJ, Lee EJ, Bae J, Jeon K, Jung KH, Ko SB, Yoon BW: Triglyceride-glucose index is associated with early neurological deterioration in single subcortical infarction: Early prognosis in single subcortical infarctions. Int J Stroke 2021, 16(8):944–952.
Kim JM, Bae JH, Park KY, Lee WJ, Byun JS, Ahn SW, Shin HW, Han SH, Yoo IH: Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. Journal of neurology 2019, 266(3):609–615.
Han Y, Hu H, Li Q, Deng Z, Liu D: Triglyceride glucose-body mass index and the risk of progression to diabetes from prediabetes: A 5-year cohort study in Chinese adults. Frontiers in public health 2023, 11:1028461.
Boldsen JK, Engedal TS, Pedraza S, Cho TH, Thomalla G, Nighoghossian N, Baron JC, Fiehler J, Østergaard L, Mouridsen K: Better Diffusion Segmentation in Acute Ischemic Stroke Through Automatic Tree Learning Anomaly Segmentation. Frontiers in neuroinformatics 2018, 12:21.
Xu Q, Deng F, Xing Z, Wu Z, Cen B, Xu S, Zhao Z, Nepomuceno R, Bhuiyan MI, Sun D et al: Long non-coding RNA C2dat1 regulates CaMKIIδ expression to promote neuronal survival through the NF-κB signaling pathway following cerebral ischemia. Cell death & disease 2016, 7(3):e2173.
Han L, Hou Z, Ma M, Ding D, Wang D, Fang Q: Impact of glycosylated hemoglobin on early neurological deterioration in acute mild ischemic stroke patients treated with intravenous thrombolysis. Frontiers in aging neuroscience 2022, 14:1073267.
Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, Jiang S, Wang J, Yang Y, Pan G et al: The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovascular diabetology 2023, 22(1):2.
Wu L, Zhu J, Li C, Zhu J, Dai Z, Jiang Y: Association of triglyceride-glucose index with ischemic stroke recurrence in nondiabetic patients with small vessel occlusion: a multicenter hospital-based prospective cohort study. Cardiovascular diabetology 2022, 21(1):250.
Zhou Y, Pan Y, Yan H, Wang Y, Li Z, Zhao X, Li H, Meng X, Wang C, Liu L et al: Triglyceride Glucose Index and Prognosis of Patients With Ischemic Stroke. Frontiers in neurology 2020, 11:456.
Barale C, Russo I: Influence of Cardiometabolic Risk Factors on Platelet Function. International journal of molecular sciences 2020, 21(2).
Hill MA, Jaisser F, Sowers JR: Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovascular research 2022, 118(1):130–140.
Miao M, Zhou G, Bao A, Sun Y, Du H, Song L, Cao Y, You S, Zhong C: Triglyceride-glucose index and common carotid artery intima-media thickness in patients with ischemic stroke. Cardiovascular diabetology 2022, 21(1):43.
Oh J, Riek AE, Darwech I, Funai K, Shao J, Chin K, Sierra OL, Carmeliet G, Ostlund RE, Jr., Bernal-Mizrachi C: Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell reports 2015, 10(11):1872–1886.
Chen W, Wang S, Lv W, Pan Y: Causal associations of insulin resistance with coronary artery disease and ischemic stroke: a Mendelian randomization analysis. BMJ open diabetes research & care 2020, 8(1).
Watanabe K, Sekiya M, Tsuruoka T, Funada J, Kameoka H, Miyagawa M, Kohara K: Relationship between insulin resistance and cardiac sympathetic nervous function in essential hypertension. Journal of hypertension 1999, 17(8):1161–1168.
Salvetti A, Brogi G, Di Legge V, Bernini GP: The inter-relationship between insulin resistance and hypertension. Drugs 1993, 46 Suppl 2:149–159.
Liu H, Liu K, Zhang K, Zong C, Yang H, Li Y, Li S, Wang X, Zhao J, Xia Z et al: Early neurological deterioration in patients with acute ischemic stroke: a prospective multicenter cohort study. Therapeutic advances in neurological disorders 2023, 16:17562864221147743.
Tan C, Zhao L, Dai C, Liang Y, Liu H, Zhong Y, Liu G, Mo L, Den F, Liu X et al: Risk factors related to early neurological deterioration in lacunar stroke and its influence on functional outcome. Int J Stroke 2022:17474930221145259.
Geng HH, Wang Q, Li B, Cui BB, Jin YP, Fu RL, Zhang Q, Wang JJ, Wang PX: Early neurological deterioration during the acute phase as a predictor of long-term outcome after first-ever ischemic stroke. Medicine 2017, 96(51):e9068.
Acknowledgements
We would like to express our sincere gratitude to the subjects who participated in this study. We thank Home for Researchers editorial team (www.home-forresearchers.com) for language editing service.
Funding
This study was supported by the First-class disciplines and specialties project of the Second Affiliated Hospital of HarbinMedical University (No. YLXK202007).
Author information
Authors and Affiliations
Contributions
JW, HT, XW, JW and YW participated in the study design and statistical analysis. JW, HT, XW and YW analyzed the data and drafted the manuscript. JG and SD participated in data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee of the Second Affiliated Hospital of Harbin Medical University. Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Tang, H., Wang, X. et al. Association of triglyceride-glucose index with early neurological deterioration events in patients with acute ischemic stroke. Diabetol Metab Syndr 15, 112 (2023). https://doi.org/10.1186/s13098-023-01091-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01091-0