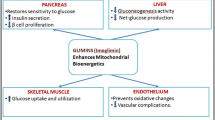
Abstract
Future targets are a promising prospect to overcome the limitation of conventional and current approaches by providing secure and effective treatment without compromising patient compliance. Diabetes mellitus is a fast-growing problem that has been raised worldwide, from 4% to 6.4% (around 285 million people) in past 30 years. This number may increase to 430 million people in the coming years if there is no better treatment or cure is available. Ageing, obesity and sedentary lifestyle are the key reasons for the worsening of this disease. It always had been a vital challenge, to explore new treatment which could safely and effectively manage diabetes mellitus without compromising patient compliance. Researchers are regularly trying to find out the permanent treatment of this chronic and life threatening disease. In this journey, there are various treatments available in market to manage diabetes mellitus such as insulin, GLP-1 agonist, biguanides, sulphonyl ureas, glinides, thiazolidinediones targeting the receptors which are discovered decade before. PPAR, GIP, FFA1, melatonin are the recent targets that already in the focus for developing new therapies in the treatment of diabetes. Inspite of numerous preclinical studies very few clinical data available due to which this process is in its initial phase. The review also focuses on the receptors like GPCR 119, GPER, Vaspin, Metrnl, Fetuin-A that have role in insulin regulation and have potential to become future targets in treatment for diabetes that may be effective and safer as compared to the conventional and current treatment approaches.
Graphical Abstract

Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a group of metabolic illnesses characterized by a constant increase in blood sugar concentration in which the pancreas is not able to produce enough insulin from β-cells or the insulin is unable to bind to its receptors due to which there is an increase in the amount of blood glucose level [1]. Recent studies predict that the prevalence of diabetes in adults will rise from 4% in 1995 to 6.4 per cent by 2025, data was collected from recent surveys [2]. Currently DM is being treated by using anti-diabetic drugs like metformin, sulfonylurea, thiazolidinedione or DPP-4 inhibitors [3]. However, these medications are unable to control diabetes completely, and research is ongoing to develop a better treatment. Receptors are chemical structures made up of proteins that receive and transmit signals in biological systems [4]. These are some of the receptors and drugs that are now being employed in the treatment of diabetes for e.g. Insulin [5], GLP-1 [6], PPAR’s [7], Biguanides [8], Sulphonylureas [5], Glinides [5], Thiazolidinediones [5], Gliptins [5], α- Glucosidase inhibitors [5], Amylin analogues [5], SGLT-2 [9], Dopamine D-2 agonists [10].
When blood glucose levels reach high, β cells of the pancreas are participating actively and release the insulin which subsequently attaches to its receptor to activate it. Exo protease carboxypeptidase and pro-hormone convertases (PC I and PC 2) synthesize insulin from pro-insulin. These enzymes are accountable for the generation of insulin and C-peptide [11]. Insulin allows the (GLUT4) to be translocated to the cell, due to which body cells (adipose/skeletal muscle cells) consume some extra glucose. This functions in the regularization of blood glucose levels [12].
There are also other novel targets for diabetes mellitus control that could be exploited, such as GPCR 119 [13], GPER [14], 11β-hydroxysteroid dehydrogenase 1 [15], Vaspin [16], Metrnl [17], PEDF [18], Fetuin-A [19], ACRP 30(AdipoQ) [20], Visfatin, Melatonin [21], GIP [22], GPCR [23]. These targets could be the future of the diabetes treatment.
Conventional targets in diabetes
Conventional targets are the agents that are being used in the market for a long time for the treatment of diabetes but they are limited in number and have several disadvantages like weight gain, hypoglycemia, etc. also they only can manage the condition and delay the complications. They work by maintaining blood glucose levels, such as Biguanides which decreases glucose output and increases glucose utilization in skeletal muscles and liver. SGLT-2 inhibitors that increases the glucose excretion from the kidney. Α- Glucosidase inhibitors helps in decreasing the glucose and free fatty acids absorption from intestine. Sulphonyl ureas increases the insulin release and sensititvity from pancreas. 2,4- thiazolidinediones decreases the secretion of FFA from the fats cells (Fig. 1).
Recent targets in diabetes
Recent targets are the receptors and mediators that are recently being targeted in the discovery of new agents for diabetes treatment. Lots of in-silico, in-vitro, in-vivo and clinical studies have been already done by targeting these receptors.
PPAR (Peroxisome proliferator-activated receptors)
Peroxisomes are the sub cellular organelles that are found in animal or human cells and play an emerging role in metabolic procedures like the metabolism of free fatty acids, cholesterol [24] and lipids to improve insulin sensitivity in the body. Peroxisome proliferator-activated receptors or PPARs functions as the transcription factors regulating the expression of genes which is divided into the three types; PPAR α, PPAR-γ, and PPARβ/δ [25]. PPAR- γ agonists (Thiazolidinediones) activates the receptor and improve overall insulin sensitivity in the body. After activating, they reduce free fatty acid levels in the blood also changes the adipokines levels, which is facilitated by lowering glucose synthesis in the liver, improving glucose intake in skeletal muscle & adipose tissues, & increasing insulin release from the pancreas (Fig. 2) [26].
GIP (Glucose-dependent insulinotropic polypeptide)
GIP is one of the incretin hormones, located in the β-cells, adipose tissue & in brain [27] where it plays an important role in the type-2 diabetes mellitus and other metabolic disorders (Fig. 3) [28, 29] by boosting the insulin response which is triggered by the post-prandial rise in glycemia [28].
Mechanism
GIP performs its insulinotropic action by attaching to the GIP receptor (GIPR) which increases the intracellular (cAMP) levels. Increased levels of cAMP activate the Protein kinase-A (PKA) & exchange protein activated cAMP2 (EPAC2). Depolarization of the voltage-gated Ca+ channels allows the rise of intracellular Ca2+ concentration that activates the Ca2+ from intracellular stores by PKA and EPAC2 mechanisms. The increase in Ca2+ concentration promotes the transcription of the proinsulin gene, thus help in rising the insulin secretion from β-cells (Fig. 4) [30].
G-Protein coupled receptor (GPCR 119)
GPR119 is a G-protein coupled receptor of Class-I [31], found in the muscles, liver [32] along with the β-cells of the pancreas [33]. The activation of GPR119 may similarly enhance insulin production just like incretin hormones [34, 35] and show the positive effects in insulin secretion when the agonists attached to its binding site [36, 37]. GPR119 acts in two different ways to improve glucose homeostasis, one is the direct effect on glucose-activated insulin release in β-cells & an indirect effect on the release of GLP-1 and GIP in enteroendocrine cells (Fig. 5) [38,39,40].
FFA 1 (Free fatty receptor-1)
FFA1 are the receptors that belong to the Class-A G-protein coupled receptor, also known as G- protein coupled receptor-40 [41]. Basically, FFA1 (Table 1) are found in the pancreatic cells, intestinal cells also found in the taste buds and central nervous system cells in mammals (Fig. 6) [42].
In an ex-vivo study, using beta-cell lines of mouse islets, it is found that the FFA1 receptor affects the lipid and glucose metabolism [43] and increases the insulin secretion from beta-cell of the pancreas [44]. FFA1 affects the blood glucose level by two pathways: By Indirectly increasing incretin hormones as well as directly promoting insulin release from pancreatic β-cells (Fig. 7) [44, 45].
Incretin release is stimulated by the glucose present in the small intestine, then incretins are passed to their target tissue is the pancreas, to stimulate the β- cells lead them to release additional insulin in action to the equal volume of blood glucose [47]
There is an increased production of Melatonin in T1DM (Type-1 diabetes mellitus) due to the activation of enzyme cascade which causes reduced β- cell function which then reduces the formation of insulin and rises the amount of glucagon in cells resulting in high blood glucose. Then in T2DM, the decreased production of Melatonin causes the increase in mRNA expression of melatonin membrane receptor which leads to the impaired insulin signaling that causes a upsurge in the insulin level leading to beta-cell exhaustion with high glucagon concentration leading to hyperglycemia
Melatonin
Melatonin is a neuroendocrine hormone, released from the pineal gland at night [48]. It is found that the melatonin is also responsible for glucose regulation and insulin release from pancreas [49, 50] which turns it into a possible goal for the management of the diabetes mellitus. It performs its pharmacological actions by interacting with the melatonin receptors MT1 and MT2 [50] that are present at the extracellular membrane present in several cells throughout the body [51]. It is found in the recent studies that melatonin MT1 receptor knockout-mouse [52] has shown the results with increased insulin resistance and glucose tolerance [52, 53] in them which makes the MT1 receptor of melatonin an essential target for maintaining blood glucose in the body. Also it is revealed from a clinical study that administering melatonin as treatment to the diabetic patients who have low levels of melatonin in their circulation [48], can improve their glucose levels by increasing insulin secretion [48, 54]in their body (Fig. 8).
Future targets
Future targets, as the name suggest are the potential receptors or targets that can be new site for the developing new lead compounds in the diabetes treatment. Today, there is very little information available regarding their role in diabetes but these targets have potential to play a vital role in the treatment of diabetes (Table 2).
11β Hydroxysteroid dehydrogenase
It is an enzyme that converts the cortisone which is a glucocorticoid [72] to its active form named cortisol [73]. It is currently available in these two isoforms which are 11-hydroxysteroid dehydrogenase type 1 (11β-HSD1) & 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) [74]. It is stated that the high levels of glucocorticoid in the blood may cause glucose intolerance to the person, so by maintaining the levels of 11β-HSD1 enzyme it naturally improves insulin sensitivity [55]. Reported studies suggesting that in many diabetic and obese animal studies [75] or when the specific 11β-HSD1 knockout mouse is used then there is a decrease in blood glucose levels, improved insulin sensitivity [76], have a better glucose tolerance [77] and also the regeneration of glucocorticoids in the body was absent in them. So, it is concluded that inhibiting the 11β-HSD1 may work in reducing insulin resistance and thus increasing insulin sensitivity by regulating the insulin signaling transduction system (Fig. 9) [78]. By taking all into consideration presented above 11β-HSD1 is a novel molecular target for the treatment of diabetes mellitus.
ACRP-30
Adipose tissue is long known for its ability to store fats but now the studies reveal that they also serve as a source of hormones like resistin, adipsin, leptin, TNF-α, adiponectin or Acrp30 [79]. It is discovered that serum protein Acrp30 performs a major part in the management of diabetes mellitus [79], TNF-α is one of the main pro-inflammatory mediators responsible for the insulin resistance [58]. It is also revealed from a study that Acrp30 levels are found to be decreased in many models of obesity and diabetes [80] due to high levels of TNF-α [56], which shows that this protein is negatively linked with diabetes (Fig. 10) [57], also showed that the mice lacking Acrp30 shows insulin resistance [58] leading to the development of diabetes mellitus. So, if the levels of Acrp30 will be increased in the circulation then the insulin sensitivity can be increased and blood glucose levels (Table 3) can be easily managed which will make Acrp30 a potential novel target for the treatment of diabetes mellitus.
FETUIN-A
It is a glycoprotein produced primarily from the liver & releases into the circulation [82]. Fetuin-A is the major protein required for carrying free fatty acids (FFA) to the circulation [83] and involved in the inflammation of the β-cells [59] and can leads to β-cell deterioration in the pancreas thus causing insulin resistance and some other metabolic disorders (Fig. 11). Along with the insulin, Fetuin-A is a major protein that can bind with the outer region of the insulin receptor [84]. Fetuin-A inhibit the autophosphorylation of the tyrosine kinase which is one of the main enzymes for the insulin signaling [85], that is totally opposite to the insulin action. There is the major interface among insulin and tyrosine kinase to balance the blood glucose in the system and if the concentration of the Fetuin-A will increase in the blood then the insulin resistance may occur in the body (Fig. 12) [59] and ultimately diabetes. Studies revealed that there is an increase in the insulin sensitivity in mice which are having Fetuin-A knockout genes in them [86] which shows the negative relation of the Fetuin-A with insulin sensitivity in diabetes [87]. These above listed factors indicate that Fetuin-A have potential to become a innovative aim for the management of diabetes mellitus in the future.
Visfatin
Visfatin, a multifunctional protein also known as Nicotinamide phosphoribosyl-transferase [88] discovered in 2005 having different types (Table 4) [60]. It is found in number of tissues & organs like but mostly articulated in the visceral adipose tissue [60]. Previously it is also known as the PBEF(Pre-B colony Enhancing Factor) and has insulin-like features[89]which means it supports to recover insulin sensitivity[89]that indicates it may have a role in diabetes also and makes it a novel approach for the treatment of diabetes mellitus. It has been shown that the serum visfatin concentration are increased along-with the worsening of T2DM [90, 91] which creates a relation between visfatin and T2DM. Recent studies showed that visfatin attaches to the insulin receptor at a location other than that of insulin which shows that it has the insulin-mimetic action and enhances cell proliferation (Fig. 13) [60].
However, till now it is not clear how the Visfatin is completely related to diabetes but there are some stimulators and inhibitors of visfatin (Table 5), although scientists are working on the mechanism of visfatin in diabetes. With these evidences, it can be concluded that there is a correlation between diabetes and visfatin in the body which turns it into a possible target for the management of diabetes mellitus.
Metrnl
Metrnl is derived as an adipokine obtained from the adipose tissues which are abundantly present in the subcutaneous white fat in the body [101] which play an important role in maintaining glucose homeostasis (Table 6), Metrnl also plays a major role in maintaining energy metabolism, lipid metabolism, cardiovascular function, immunological inflammation and also in insulin sensitivity [62, 63]. In a study, researchers found that it works through the up regulation of the PPARγ pathway due to which there is an increase in the insulin sensitivity in Mice model [61]. Concurrently it is also found that, it promotes adipose tissue browning due to which there is an increase in energy expenditure and improved glucose tolerance (Fig. 14) [102].
Metrnl is involved in various pharmacological pathways through intracellular signalling between the cells. In nerve cells, it promotes the neurite outgrowth via the JAKSTAT3 and MEK-ERK signalling pathway. In fat cells due to upregulation of the Metrnl increases the lipid metabolism, relieves from the high-fat diet-induced inflammation and improves adipose remodeling through upregulation of PPARγ, due to which the insulin resistance is also improved. In muscle cells or myocytes it increases the PPARγ signalling which increases the phosphorylation of AMPK due to increased intracellular calcium and also encourages the phosphorylation of TBC1D1, HDAC5, and p38 MAPK in an AMPK-mediated manner, then promotes the expression and translocation of GLUT4, which thus improves the insulin sensitivity and reduces the inflammation [103]
PEDF (Pigment epithelium-derived factor)
It is a 50 kDa secreted glycoprotein released from the adipose tissue and human retinal pigment cells which belongs to the family of serine protease inhibitors [112]. It works in hydrolyzing the lipid triglycerides into glycerol and free fatty acids and thus moving the free fatty acids to the systemic circulation leading to inflammation in the cells. It gives rise to the kinase-mediated Serine/Threonine phosphorylation cascade of IRS (insulin receptor substrate), due to this process the insulin signaling is reduced which causes insulin resistance in the body cells [64]. Along with this, it also releases inflammatory mediators like TNF-α and IL-1(Interleukin-1) in the system due to that insulin insensitivity occurs in the body [112]. In a study it is investigated that after the administration of PEDF in animals there is a decrease in the insulin sensitivity which restores after the anti-PEDF given to them [113]. In children and adults, PEDF shows a positive correlation with insulin resistance [114]. So, if we can decrease the amount of PEDF in the circulation that may help to increase the insulin sensitivity, this makes PEDF a potential novel approach for the treatment of diabetes mellitus and other metabolic syndromes in the body. PEDF show its action by targeting the insulin receptor substrate (IRS) given in (Fig. 15) where it blocks the insulin signaling that further stops the process of glucose uptake by the cells, protein synthesis, glycogen synthesis which shows an increase in the amount of blood glucose levels in the body. The other factors which are also activated by the PEDF are the free fatty acid (FFA), toll-like receptor4(TLR4), nuclear factor kappa B (NFκB), suppressor of cytokine signalling (SOCS3),Janus kinase (JAK2) which also blocks the insulin receptor substrate which together contributes in the decreased insulin sensitivity and ultimately diabetes mellitus (Fig. 15).
Vaspin (Serpin A12)
Vaspin or Serpin A12 is a serum glycoprotein that comes under the serpin superfamily [115]. It is derived from the fat cells [116], plays an important role in modifying insulin activity [117]. It has been studied that, When diabetes severity increases, the serum levels of the vaspin start decreasing [118], this creates an idea that if the levels of vaspin start increasing in the circulation then it could be helpful in the management of diabetes mellitus. In animal studies, it has been also observed that the administration of the vaspin into the rats shows the improvement in insulin sensitivity along with increased glucose tolerance [116]. These evidences make it a potential target for the treatment of diabetes mellitus and other metabolic disorders like obesity (Fig. 16). Vaspin performs its action by inhibiting the KLK7 (kallikrein 7) which is an insulin-degrading enzyme that degrades the insulin and decreases the insulin half-life [65]. Due to the inhibition of KLK7, the insulin signalling is improved and also the half-life of insulin is increased that helps in decreasing the blood glucose levels [66]. It also performs some other actions which indirectly reduce the blood glucose from the body like it reduces the food intake that ultimately reduces the hepatic glucose production (HGP) via the hepatic branch of the vagus nerve by reducing hepatic lipid accumulation and increasing insulin signalling in the liver. In white adipose tissue (WAT) and Brown adipose tissue (BAT), it reduces inflammation and increases insulin signaling and in CNS central nervous system it decreases food intake by triggering the vagus nerve (Fig. 16) [119].
GPER (G protein-coupled estrogen receptor)
GPER is an orphan 7-transmembrane G-protein coupled estrogen receptor [120, 121] that involved in estrogen signalling [122]. They are located in the intracellular membrane of cells [123] and plays an important role in the regulation of glucose homeostasis, inflammation [124], vascular tone and cell growth [122] by binding to both Gi/o and Gs proteins in the body [67]. In a GPER deficient female mouse model, it was found that there is an insufficient amount of insulin [68, 69] is producing in them that lead to the development of diabetes mellitus. It also has been shown in a study that in premenopausal women estrogen levels are high which shows the positive effects on maintaining the blood pressure, lipid metabolism, glucose homeostasis, as well as reducing inflammation [125] but after menopause when the estrogen levels start declining which makes the women more prone to the insulin resistance and multiple metabolic disorders all of them contributes to the diabetes mellitus [126]. These evidences suggest that GPER could play a crucial role in management diabetes and could become an interesting drug target for diabetes and related disorders (Fig. 17).
Gene therapy
Gene therapy is an emerging method for the treatment of diabetes mellitus that act by correcting or repairing the defective genes [71] which are responsible for diabetes mellitus. In this technique, transfer of genes can be done by the viral vector and non-viral transduction method to get the effect by the suppression of auto reactive T cells to stop islet cells destruction as a preventive method of treatment or the replacement of the insulin gene [70]. It is found in a study that the stem cells may be used for the treatment of diabetes serving as the surrogate β-cells [127] as they can multiply in the culture easily. It has also been studied that when the modified stem cells are transplanted into the mice by intrahepatic injection the level of blood glucose was found to be low (Fig. 18). When the mice are sacrificed for the histopathological studies, the distribution of stem cells shows green fluorescence under fluorescent microscope and insulin presence was identified by brown stain after staining with anti-human insulin polyclonal antibody. It showed that mesenchymal stem cells successfully expressed human insulin and was able to maintain normal blood glucose at the end of 42 days study [70] This was compared to the mice not treated by gene therapy. So, there is a scope in gene therapy as an evolving new technology that can used for the treatment of diabetes mellitus (Fig. 18).
Systemic representation of the characteristics of cell-based gene therapy procedures in diabetes treatment [128]
Conclusion and future perspectives
Diabetes is a worldwide epidemic and vulnerable disease from which large number of patient are suffering currently. The primary goal of every therapy in the treatment of diabetes mellitus is to attain near-normal blood glucose levels in the body. Treatments available for diabetes are only able to manage its symptoms and delay its progression but not able to cure it properly, along with there are also various side effects associated with their uses. Researchers are continuously working in search of new lead compounds for the proper cure for the diabetes mellitus and its complications and trying to make an approach in which the side effects should be minimal. The conventional approaches which has been used for a long time for the treatment of diabetes mellitus includes the Insulin therapy [5], Biguanides [8], Sulphonylureas [5], Glinides [5], Thiazolidinediones [5], Gliptins [5], α- Glucosidase inhibitors [5], Amylin analogues [5], SGLT-2 [9], Dopamine D-2 agonists [10]. As a primary targets they can only manage the symptoms and delay the progression and also consist of many side effects like weight gain, hypoglycemia, diarrhoea, nausea, mitogenic effect, bladder cancer etc., [5, 129, 130] which is not good for the patients who are dealing with these metabolic diseases. To overcome these side effects researchers are continuously searching for new targets for diabetic therapy, in last decade targets like PPAR’s are the primary focus of researchers but despite of enormous pre-clinical studies very few leads are in clinical studies and in market, Because of these facts we can’t rely upon the current approaches to diabetes treatment and should explore some new innovative pharmacological targets. In this view, receptor like GPCR 119 [13], GPER [14], 11β-hydroxysteroid dehydrogenase 1 [15], Vaspin [16], Metrnl [17], PEDF [18], Fetuin-A [19], ACRP 30 [20], Visfatin, Melatonin [21], GIP [22], GPCR [23] having direct or indirect role in insulin regulation as suggested by studies done. These receptors have potential to become targets in the treatment of diabetes and can become the landmark to find the permanent cure for diabetes and related complications. It is also suggested that in future there are possibilities in gene therapy or stem cells to become a therapeutic agent with better potential with lesser side effects.
Availability of data and materials
Not applicable.
Abbreviations
- ACRP:
-
Adipocyte complement related protein
- AMP:
-
Adenosine monophosphate
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- BAT:
-
Brown adipose tissue
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- cAMP:
-
Cyclic adenosine monophosphate
- CAD:
-
Coronary artery disease
- CCK:
-
Cholecystokinin
- CKD:
-
Chronic kidney disease
- CNS:
-
Central nervous system
- CVD:
-
Cardiovascular disease
- CVS:
-
Cardiovascular system
- DKA:
-
Diabetic ketoacidosis
- DPP:
-
Dipeptidyl peptidase
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPAC:
-
Exchange protein directly activated by cAMP
- ERK:
-
Extracellular-signal-regulated kinase
- FFA:
-
Free fatty acids
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP:
-
Glucagon-like peptide 1
- GLUT:
-
Glucose transporters
- GPCR:
-
G-Protein coupled receptors
- GPER:
-
G protein-coupled estrogen receptor
- HAART:
-
Highly active antiretroviral therapy
- HDAC:
-
Histone deacetylase
- HDL:
-
High-density lipoprotein
- HGP:
-
Hepatic glucose production
- IL:
-
Interleukin
- INSR:
-
Insulin receptor
- IRS:
-
Insulin receptor substrate
- JAK:
-
Janus activated kinase
- JAKSTAT:
-
Janus kinase-signal transducers and activators of transcription
- KATP:
-
ATP-sensitive potassium
- KLK:
-
Kallikrein 7
- LCFAs:
-
Long chain fatty acids
- LDL:
-
Low-density lipoprotein
- WAT:
-
White adipose tissue
- UTI:
-
Urinary tract infection
- UKPDS:
-
UK prospective diabetes study
- TNF:
-
Tumor necrosis factor
- TLR:
-
Toll-like receptor
- STAT:
-
Signal transducers and activators of transcription
- SOCS:
-
Suppressors of cytokine signaling
- SGLT-2:
-
Sodium-glucose cotransporter-2
- SCFAs:
-
Short-chain fatty acids
- RNA:
-
Ribonucleic acid
- RIA:
-
Radioimmunoassay
- PYY:
-
Peptide YY
- PPAR:
-
Peroxisome proliferator-activated receptor
- PKA:
-
Protein kinase-A
- PEDF:
-
Pigment epithelium derived factor
- PCOS:
-
Polycystic ovary syndrome
- PBEF:
-
Pre-B cell colony-enhancing factor
- NPH:
-
Neutral protamine Hagedorn
- MT:
-
Melatonin receptors
- MEK:
-
Mitogen-activated protein kinase
- MCFAs:
-
Medium-chain fatty acids
- MAPK:
-
Mitogen-activated protein kinase
References
Amin N. An overview of diabetes mellitus; types, complications, and management. Int J Nurs Sci Pract Res. 2018;4(1):119–24.
King H, Aubert RE, Herman WHJDc. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31.
Berkowitz SA, et al. Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med. 2014;174(12):1955–62.
Pasquier EK, Andersson E. Diseases Pulmonary recruitment maneuver reduces pain after laparoscopic bariatric surgery: a randomized controlled clinical trial. Surg Obes Relat. 2018;14(3):386–92.
Hollander P. Anti-diabetes and anti-obesity medications: effects on weight in people with diabetes. Diabetes Spectr. 2007;20(3):159–65.
Müller TD, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130.
Holm LJ, et al. PPARs and the development of type 1 diabetes. PPAR Res. 2020;2020:1.
Madaan T, Akhtar M, Najmi AK. Sodium glucose CoTransporter 2 (SGLT2) inhibitors: current status and future perspective. Eur J Pharm Sci. 2016;93:244–52.
Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9(7):551–9.
Mihailova S, Tsvetkova A, Todorova A. Pharmacological trends in the treatment of Diabetes type 2–New classes of antidiabetic drugs. 2015.
Pessin JE, Saltiel ARJTJoci. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106(2):165–9.
Zimmerman M. The diabetic nerve studies on outcome after open carpal tunnel release and the development of autonomic neuropathy. Hand Microsurg. 2018. https://doi.org/10.5455/handmicrosurg.25681.
Pola S, et al. Discovery of a potent G-protein-coupled receptor 119 agonist for the treatment of type 2 diabetes. Bioorganic Med Chem. 2021;35:116071.
Ferreira NS, et al. Diabetes impairs the vascular effects of aldosterone mediated by G protein-coupled estrogen receptor activation. Front Pharmacol. 2015;6:34.
Li X, et al. 11β-Hydroxysteroid dehydrogenase type 1 in obese subjects with type 2 diabetes mellitus. Am J Med Sci. 2017;354(4):408–14.
Weiner J, et al. Molecular mechanisms of vaspin action–from adipose tissue to skin and bone, from blood vessels to the brain. Adv Exp Med Biol. 2018;1111:159–88.
Miao Z-W, et al. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin. 2020;41(12):1525–30.
Popescu M, et al. Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy. Drug Des Devel Ther. 2018;12:1985.
Roshanzamir F, et al. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2018;41(1):33–47.
Parida S, Siddharth S, Sharma D. Adiponectin, obesity, and cancer: clash of the bigwigs in health and disease. Int J Mol Sci. 2019;20(10):2519.
Mok JX, et al. A new prospective on the role of melatonin in diabetes and its complications. Horm Mol Biol Clin Investig. 2019. https://doi.org/10.1515/hmbci-2019-0036.
Bailey CJ. GIP analogues and the treatment of obesity-diabetes. Peptides. 2020;1(125):170202.
Hauser AS, et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(12):829–42.
Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Investig. 2006;116(3):598–606.
Tyagi S, et al. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236.
Choi S-S, Park J, Choi JH. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 2014;47(11):599.
Saini R, Badole SL. Bioactive Compounds Increase Incretins with Beneficial Effects on Diabetes. In: Glucose Intake and Utilization in Pre-Diabetes and Diabetes. Amsterdam: Elsevier; 2015. p. 349–53.
Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4(6):525–36.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Invest. 2010;1(1–2):8–23.
Shah U, Kowalski TJ. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam Horm. 2010;84:415–48.
Pola S, et al. Discovery of a potent G-protein-coupled receptor 119 agonist for the treatment of type 2 diabetes. Bioorganic Med Chem. 2021;35:116071.
Jang YK, et al. Design, synthesis, and biological evaluation of aryl N-methoxyamide derivatives as GPR119 agonists. Bioorg Med Chem Lett. 2017;27(16):3909–14.
Furman B, et al. Targeting β-cell cyclic 3′ 5′ adenosine monophosphate for the development of novel drugs for treating type 2 diabetes mellitus. a review. J Pharmacy Pharmacol. 2004;56(12):1477–92.
Albrechtsen NJW, et al. Targeting the intestinal L-cell for obesity and type 2 diabetes treatment. Expert Rev Endocrinol Metab. 2014;9(1):61–72.
Zhu C, et al. Discovery of phenyl acetamides as potent and selective GPR119 agonists. Bioorg Med Chem Lett. 2017;27(5):1124–8.
Neelamkavil SF, et al. Discovery of MK-8282 as a potent G-protein-coupled receptor 119 agonist for the treatment of type 2 diabetes. ACS Med Chem Lett. 2018;9(5):457–61.
Ekberg JH, et al. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology. 2016;157(12):4561–9.
Harada K, et al. Design and synthesis of novel and potent GPR119 agonists with a spirocyclic structure. Bioorg Med Chem Lett. 2018;28(7):1228–33.
Overton H, Fyfe M, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008;153(S1):S76–81.
Khan MZ, He L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology. 2017;113:639–51.
Roberts GP, et al. Comparison of human and murine enteroendocrine cells by transcriptomic and peptidomic profiling. Diabetes. 2019;68(5):1062–72.
Alquier T, et al. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes. 2009;58(11):2607–15.
Luo J, et al. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0046300.
Omar B, Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63(7):2196–202.
Offermanns S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu Rev Pharmacol Toxicol. 2014;54:407–34.
Watterson KR, et al. Treatment of type 2 diabetes by free fatty acid receptor agonists. Front Endocrinol. 2014;5:137.
Reutrakul S, et al. Lower nocturnal urinary 6-sulfatoxymelatonin is associated with more severe insulin resistance in patients with prediabetes. Neurobiol Sleep Circadian Rhythms. 2018;4:10–6.
Yeğin ZA, et al. The Impact of Melatonin on Glucose Homeostasis. Turkish J Endocrinol Metab. 2009;13:3.
Owino S, et al. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PloS ONE. 2016;11(1):e0148214.
Pandi-Perumal SR, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–53.
Mühlbauer E, et al. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606(1–3):61–71.
Cipolla-Neto J, et al. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–81.
Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81.
Wang Y, et al. 11β-Hydroxysteroid dehydrogenase type 1 shRNA ameliorates glucocorticoid-induced insulin resistance and lipolysis in mouse abdominal adipose tissue. Am J Physiol-Endocrinol Metab. 2015;308(1):E84–95.
Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83.
Lechleitner M, et al. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J Intern Med. 2000;248(1):67–76.
Ruan H, et al. Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-κB activation by TNF-α is obligatory. Diabetes. 2002;51(5):1319–36.
Esfahani M, et al. Adiponectin: an adipokine with protective features against metabolic syndrome. Iran J Basic Med Sci. 2015;18(5):430.
Fukuhara A, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–30.
Li Z-Y, et al. Adipocyte Metrnl antagonizes insulin resistance through PPARγ signaling. Diabetes. 2015;64(12):4011–22.
Ushach I, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol. 2015;156(2):119–27.
Ushach I, et al. Meteorin-like/Meteorin-β is a novel immunoregulatory cytokine associated with inflammation. J Immunol. 2018;201(12):3669–76.
Smitka K, Marešová D. Adipose tissue as an endocrine organ: an update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med Rep. 2015;116(2):87–111.
Heiker JT, et al. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell Mol Life Sci. 2013;70(14):2569–83.
Feng R, et al. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract. 2014;106(1):88–94.
Lappano R, et al. MIBE acts as antagonist ligand of both estrogen receptor α and GPER in breast cancer cells. Breast Cancer Res. 2012;14(1):1–13.
Sharma G, et al. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154(11):4136–45.
Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology. 2011;152(8):3030–9.
Wong MS, Hawthorne WJ, Manolios N. Gene therapy in diabetes. Self/nonself. 2010;1(3):165–75.
Jadhav MSD, et al. Gene therapy-challenges & success. Gene Therapy. 2020;4:3.
Wang M. Inhibitors of 11β-hydroxysteroid dehydrogenase type 1 in antidiabetic therapy. Diabetes-Perspectives Drug Therapy. 2011. https://doi.org/10.1007/978-3-642-17214-4_6.
Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93(3):1139–206.
Wyrwoll CS, Holmes MC, Seckl JR. 11β-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol. 2011;32(3):265–86.
Berthiaume M, et al. Depot-specific modulation of rat intraabdominal adipose tissue lipid metabolism by pharmacological inhibition of 11β-hydroxysteroid dehydrogenase type 1. Endocrinology. 2007;148(5):2391–7.
Morton NM, et al. Novel adipose tissue–mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1–deficient mice. Diabetes. 2004;53(4):931–8.
Zhang X, et al. Inhibition of forkhead box O1 protects pancreatic β-cells against dexamethasone-induced dysfunction. Endocrinology. 2009;150(9):4065–73.
Walker B. Cortisol—cause and cure for metabolic syndrome? Diabet Med. 2006;23(12):1281–8.
Hug C, Lodish HF. Diabetes, obesity, and Acrp30/adiponectin. Biotechniques. 2002;33(3):654–62.
Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6.
Berg AH, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–53.
Stefan N, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57(10):2762–7.
Pal D, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–85.
Roshanzamir F, et al. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2018;41(1):33–47.
Goustin A-S, Abou-Samra AB. The “thrifty” gene encoding Ahsg/Fetuin-A meets the insulin receptor: Insights into the mechanism of insulin resistance. Cell Signal. 2011;23(6):980–90.
Mathews ST, et al. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem Biophys Res Commun. 2006;350(2):437–43.
Oh K-J, et al. Metabolic adaptation in obesity and type II diabetes: myokines, adipokines and hepatokines. Int J Mol Sci. 2017;18(1):8.
Kim M-K, et al. Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J Mol Biol. 2006;362(1):66–77.
Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15(18):1851–62.
Fernández-Real JM, et al. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30(3):616–21.
Sandeep S, et al. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56(4):565–70.
Kralisch S, et al. Interleukin-6 is a negative regulator of visfatin gene expression in 3T3-L1 adipocytes. Am J Physiol-Endocrinol Metab. 2005;289(4):E586–90.
Fasshauer M, et al. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin Endocrinol. 2007;66(3):434–9.
Moschen AR, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748–58.
Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41(8):718–26.
Dahl TB, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115(8):972–80.
Samal B, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–7.
Ognjanovic S, et al. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26(2):107–18.
Yonezawa T, et al. Visfatin is present in bovine mammary epithelial cells, lactating mammary gland and milk, and its expression is regulated by cAMP pathway. FEBS Lett. 2006;580(28–29):6635–43.
Samara A, et al. Visfatin, low-grade inflammation and BMI. Clin Endocrinol (Oxf). 2008;69(4):568–74.
Li ZY, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther. 2014;20(4):344–54.
Rao RR, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–91.
Miao Z-W, et al. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin. 2020;41(12):1525–30.
Lee JH, et al. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res Clin Pract. 2018;135:7–10.
Dadmanesh M, et al. Lower serum levels of Meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PloS ONE. 2018;13(9):e0204180.
Zhang S-L, et al. Aggravated ulcerative colitis caused by intestinal Metrnl deficiency is associated with reduced autophagy in epithelial cells. Acta Pharmacol Sin. 2020;41(6):763–70.
Chung HS, et al. Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res Clin Pract. 2018;136:100–7.
AlKhairi I, et al. Increased expression of meteorin-like hormone in type 2 diabetes and obesity and its association with irisin. Cells. 2019;8(10):1283.
Wang K, et al. Serum levels of meteorin-like (Metrnl) are increased in patients with newly diagnosed type 2 diabetes mellitus and are associated with insulin resistance Medical science monitor: international. Med J Exp Clin Res. 2019;25:2337.
El-Ashmawy HM, et al. Association of low serum Meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res Clin Pract. 2019;150:57–63.
Wang C, et al. Serum metrnl level is correlated with insulin resistance, but not with β-Cell function in type 2 diabetics. Med Sci Monit Int Med J Exp Clin Res. 2019;25:8968.
Chavan SS, et al. Identification of pigment epithelium-derived factor as an adipocyte-derived inflammatory factor. Mol Med. 2012;18(8):1161–8.
Kim JK. Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity in vivo. In: Type 2 diabetes. Amsterdam: Springer; 2009. p. 221–38.
Galhardo J, Hunt L, Shield J. Serum levels of pigment epithelium-derived factor (PEDF) are positively associated with acanthosis nigricans in obese adolescents. Diabet Med. 2012;29(7):e117–20.
Hida K, et al. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res. 2000;41(10):1615–22.
Dimova R, Tankova T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. BioMed Res Int. 2015;2015:1.
Carrión M, et al. The adipokine network in rheumatic joint diseases. Int J Mol Sci. 2019;20(17):4091.
Youn B-S, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57(2):372–7.
Nakatsuka A, et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes. 2012;61(11):2823–32.
O’Dowd BF, et al. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47(2):310–3.
Takada Y, et al. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240(3):737–41.
Barton M, Prossnitz ER. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol Metab. 2015;26(4):185–92.
Otto C, et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149(10):4846–56.
Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76.
Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24(3):214–20.
Meyer MR, et al. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. 2011;203(1):259–69.
Xu J, et al. Reversal of diabetes in mice by intrahepatic injection of bone-derived GFP-murine mesenchymal stem cells infected with the recombinant retrovirus-carrying human insulin gene. World J Surg. 2007;31(9):1872–82.
Yechoor V, Chan L. Gene therapy progress and prospects: gene therapy for diabetes mellitus. Gene Ther. 2005;12(2):101–7.
Babiker A. and MAl dubayee, anti-diabetic medications: how to make a choice? Sudan J Paed. 2017;17(2):11.
Cobble M. Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4(1):1–10.
Acknowledgements
We would like to acknowledge all authors for idea, execution and implementation. We would also appreciate management for giving the opportunity to writing this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, SD, SC, DM and SG; literature review, SC, MS, N, RD, VG, DM, SG: formal analysis, SD, SC, SG, writing–original draft preparation, SD, SC; writing–review and editing, MS, DM, RD, SG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Yes on my behalf of all authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dhankhar, S., Chauhan, S., Mehta, D.K. et al. Novel targets for potential therapeutic use in Diabetes mellitus. Diabetol Metab Syndr 15, 17 (2023). https://doi.org/10.1186/s13098-023-00983-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-00983-5