Abstract
Background
Evidence showed possible benefits of a less gestational weight gain (GWG) than the US Institute of Medicine (IOM) recommendation in gestational diabetes mellitus (GDM) pregnancy. Here, we aimed to explore an appropriate GWG range in GDM women according to adverse pregnancy outcomes.
Methods
We enrolled all the singleton GDM pregnant women (n = 14,213) from January 2015 to December 2018 in Xi'an, Northwest China. According to the pre-pregnancy body mass index (BMI), they were classified into the Underweight (< 18.5 kg/m2), Normal weight (18.5–24.9 kg/m2), Overweight (25.0–29.9 kg/m2) and Obesity (≥ 30.0 kg/m2) group, respectively. Logistic regression analysis was used to calculate the odds ratio (OR) and 95% confidence intervals (95% CI). The appropriate ranges of GWG were determined based on a significant protective association (OR < 1).
Results
Totally, 12,712 participants were finally recruited. There were 1180 (9.3%), 9134 (71.9%), 2097 (16.5%), and 301 (2.4%) patients in the Underweight, Normal weight, Overweight, and Obesity groups, respectively. Adverse outcomes increased with the elevation of pre-pregnancy BMI. Among them, the risk of cesarean section was the highest, followed by large for gestational age (LGA), small for gestational age (SGA), preeclampsia, and gestational hypertension. Through the analysis of the risk of adverse outcomes in continuous GWG categories in each group, an ideal GWG range obtained in this study was as follows: 10–15.9 kg, 8–11.9 kg, 6–7.9 kg, and -5–3.9 kg for the Underweight, Normal weight, Overweight and Obesity group, respectively. Furthermore, the ranges in this study were more protective for adverse outcomes than those from IOM.
Conclusions
Based on the adverse pregnancy outcomes of over 12 thousand participants, our findings showed a more stringent GWG range for GDM women than the IOM criteria recommendation.
Similar content being viewed by others
Background
Gestational diabetes mellitus (GDM) is diagnosed during the 2nd or 3rd trimester of pregnancy that is not either preexisting type 1 or type 2 diabetes [1]. A meta-analysis involving 79,064 Chinese participants showed that the total incidence of GDM in mainland China was 14.8% (95% confidence interval of 12.8–16.7%) [2]. GDM has been reported to be associated with a lot of adverse pregnancy outcomes, such as large for gestational age (LGA), macrosomia, shoulder dystocia, cesarean section, pregnancy-induced hypertension, and pre-eclampsia [3, 4]. Long-termly, GDM may increase the risk for glucose metabolism disorders and dyslipidemia after pregnancy in mothers, and childhood obesity, insulin resistance, and atherosclerotic lipid properties in offspring [5, 6].
Excessive gestational weight gain (GWG) usually leads to both short-term and long-term adverse pregnancy outcomes directly itself and indirectly as a mediator of GDM [7]. Studies have shown that women with excessive GWG are more likely to have abdominal obesity and increased metabolic diseases [8, 9]. With the implementation of China's comprehensive two-child policy, the weight retention caused by excessive GWG will be another important fuse for a new round of metabolic diseases in pregnancy [10]. In addition to affecting the mothers, GWG is also an independent predictor of obesity and total body fat distribution in infancy offspring and affects offspring’s cardiovascular metabolism in their adulthood [11, 12]. Besides, insufficient GWG also doesn’t benefit the offspring by increasing the risk of premature delivery, small gestational age (SGA), and low birth weight [13, 14].
Therefore, an appropriate GWG suggestion is urgently needed for women with GDM. Unfortunately, there are currently no specific guidelines in any country, including China. In China, the clinical guidelines revised by the US Institute of Medicine (IOM) in 2009 [15], recommended GWG for all pregnant women according to pre-pregnancy body mass index (BMI) is still being used. Not to mention in women with GDM, such a recommendation is even inappropriate for pregnant women with normal glucose metabolism [16]. A previous study has shown that in GDM pregnancy, GWG less than the recommended weight will be beneficial [17]. However, the ideal range of GWG has not been determined. Therefore, using the data from the Xi’an longitudinal mother–child cohort (XAMC) study, we explored the appropriate ranges of GWG for GDM women with different pre-pregnancy BMI categories.
Methods
Population and data sources
The present analysis is based on data from the XAMC study, which was established in January 2013 and the enrollment will be expected to end in January 2023. The research protocol and basic information were previously published [18]. Based on the dynamically conducted XAMC study, we enrolled all the singleton GDM pregnant women (14,213) from January 2015 to December 2018 from Northwest Women’s and Children’s Hospital, Xi’an, Northwestern of China. The inclusion criteria were as follows: women who were diagnosed with GDM by receiving a 75 g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation according to criteria of the International Association of Diabetes in Pregnancy Study Groups (IADPSG) (75 g OGTT fasting blood glucose ≥ 5.1 mmol/L, or 1 h blood glucose ≥ 10.0 mmol/L, or 2 h blood glucose ≥ 8.5 mmol/L) [19]; full-term singleton pregnancy (gestational age ≥ 37 weeks); completed data including height, weight, GWG, maternal and fetal outcomes and so on. The exclusion criteria were non-gestational diabetes or diabetes diagnosed before pregnancy; multiple births; premature delivery; abortion or induced labor; incomplete or incorrect data. The protocol was approved by the ethical committee of Xi’an Jiaotong University (XJTU 2016-053) and the Northwest Women and Children’s Hospital (NWCH 2012-013). All women provided gave written informed consent. The principles of the Helsinki Declaration were followed throughout the study.
Maternal pre-pregnancy BMI and GWG
When firstly diagnosed being pregnant (usually before 6 gestational weeks), women’s maternity booklet was created at hospital and information of height and weight were measured by a professional medical staff and recorded as pre-pregnancy height and weight. Maternal pre-pregnancy BMI was calculated by dividing the pre-pregnancy weight by the square of the height. The gestational weight gain (GWG) was calculated by subtracting pre-pregnancy weight from the weight measured before delivery. The Underweight, Normal weight, Overweight and Obesity group were defined with pre-pregnancy BMI < 18.5 kg/m2, 18.5–24.9 kg/m2, 25.0–29.9 kg/m2 and ≥ 30.0 kg/m2, respectively.
Pregnant outcomes
Adverse outcomes of this study were defined as the presence of at least one of the following outcomes: gestational hypertension, preeclampsia, cesarean delivery, and SGA or LGA. Gestational hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg occurring for the first time after 20 weeks of pregnancy. Pre-eclampsia was defined as gestational hypertension plus proteinuria. LGA was defined as an infant whose birth weight was above the 90th percentile of the average birth weight of the same gestational age, whereas SGA was below the 10th percentile according to the INTERGROWTH-21st Project [20].
Data quality control
In this study, two trained data collectors performed parallel data entry using the Epidata 3.1 software. When completed, a consistency check was performed. Other personnel with professional knowledge checked the outliers, which were defined when beyond 3 times the standard deviation of the average. Data judged as outliers were double-checked and set as missing values if it were not a data entry error. The missing value analysis is in Additional file 1: Table S1 and S2.
Statistical analysis
Quantitative data were tested by analysis of variance or rank-sum test, and qualitative data were tested by Chi-square. The absolute risk was calculated as the percentage of women with adverse outcomes in each combination of the BMI and GWG categories. The Logistic regression analysis was used to calculate the odds ratio (OR) and 95% confidence intervals (95% CI) of adverse outcomes for each GWG range in a specific pre-pregnancy BMI group with the adjustment of age, parity, gestational week, and previous cesarean section history. The reference for each GWG category was the GWG beyond the range. The risk of adverse outcomes in continuous GWG categories in each group was analyzed. The appropriate GWG ranges were determined based on whether the GWG range was a protective factor for adverse outcomes (OR value < 1). According to the obtained GWG ranges or the IOM recommended ones (12.5–18.0 kg, 11.5–16.0 kg, 7.0–11.5 kg, and 5.0–9.0 kg for the Underweight [BMI < 18.5 kg/m2], the Normal weight [BMI 18.5–24.9 kg/m2], the Overweight [BMI 25.0–29.9 kg/m2] and the Obesity [BMI ≥ 30.0 kg/m2], respectively), the sensitivity, specificity, positive predictive value and negative predictive value were calculated. Furthermore, the Net Reclassification Index (NRI) was calculated to assess the prediction ability of GWG obtained in this study and the one from IOM. P < 0.05 was considered statistically significant.
Results
Basic characteristics of participants
As shown in Additional file 1: Figure S1, a total of 12,712 participants were finally enrolled in this study, of which 9.3% (1180) were in the Underweight group; 71.9% (9134) in the Normal weight group; 16.5% (2097) in the Overweight group and 2.4% (301) in the Obesity group (Table 1). Compared with the Normal weight group, the Underweight group was younger and the Overweight group was older. The proportion of participants with a family history of diabetes and hypertension in each group increased from the Underweight group to the Obesity group (8.1%, 9.6%, 11.5%, and 16.9%, respectively for diabetes while 10.4%, 14.7%, 17.6%, and 22.9%, respectively for hypertension). More participants had a history of previous cesarean section or adverse pregnancy in the Normal weight group and Overweight group than that in the Underweight group whilst the situation was the same as multiparous. The majority of basic characteristics were comparable between the Obese group and the Overweight group.
The GWG and the indicators of glucose metabolism
The median total GWG decreased with pre-pregnancy BMI, which was 15.0 (12.0–18.0) kg, 14.0 (11.0–17.0) kg, 12.0 (9.0–15.0) kg, and 10.0 (7.0–14.0) kg for the Underweight, Normal weight, Overweight and Obesity group, respectively. The OGTT data showed that the blood glucose level also increased with pre-pregnancy BMI. In detail, the fasting blood glucose was 5.11, 5.19, 5.29, and 5.29 mmol/L, the 1 h blood glucose was 9.05, 9.24, 9.64, and 9.79 mmol/L, while the 2 h blood glucose was 7.89, 7.96, 8.07 and 7.78 mmol/L in the Underweight, Normal weight, Overweight and Obesity group, respectively. There was no significant difference in OGTT level between the Overweight group and the Obesity group. Meanwhile, the late pregnancy HbA1c levels increased with pre-pregnancy BMI as well. The birth weight of the fetus in the Normal weight group (3415 [3150–3690] g) was higher than the Underweight group (3250 [3012–3500] g) but smaller than the Overweight group (3500 [3210–3800] g) and Obesity group (3560 [3210–3900] g). No significant difference in birth weight between the Overweight group and the Obesity group was reported (Table 1).
Incidence of adverse pregnancy outcomes
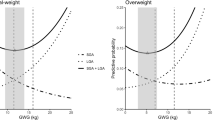
Overall, adverse outcomes increased with pre-pregnancy BMI, which were 39.6%, 56.5%, 72.5%, and 80.1% in the Underweight, Normal weight, Overweight, and Obesity groups, respectively. The incidence of gestational hypertension and preeclampsia increased sequentially both from 0.8% in the Underweight group to 10.3% and 12.0% in the Obesity group, respectively. The cesarean section rate of the Normal weight group (45.2%) was higher than that of the Underweight group (31.3%) but lower than that of the Overweight group (59.9%) and Obesity group (67.8%). In addition, the Obesity group had the highest risk of LGA (36.5%), and the Underweight group was the lowest (10.4%). No significant difference was found in the incidence of cesarean section and LGA between the Overweight and the Obesity group. Although the incidence of SGA among the four groups was comparable, it decreased gradually with pre-pregnancy BMI (Table 1). Generally, the absolute risk of adverse outcomes increased with the increase of GWG in all groups (Fig. 1). Among all the adverse outcomes mentioned in this study, the risk of cesarean section was the highest, followed by LGA, SGA, preeclampsia, and gestational hypertension. Interestingly, in the Underweight and the Normal weight group, the risk of SGA was higher than that of LGA only when the GWG was lower. This phenomenon occurred when the GWG was less than 12 kg in the Underweight group and 4 kg in the Normal weight group. However, the risk of LGA was always higher than that of SGA in the other two groups (Additional file 1: Table S3).
Absolute Risk for Adverse Maternal or Infant Outcomes. Absolute risk = (No. of participants with the adverse outcomes/No. of participants in GWG category within pre-pregnancy BMI group) × 100. The total GWG was divided by the group distance of 2 kg, except for the first GWG range and the last GWG range of each pre-pregnancy BMI group
The appropriate GWG range and its verification
As shown in Additional file 1: Table S4, the risk of adverse outcomes increased by 1.153 (95% CI 1.139–1.167) and 1.021 (95% CI 1.014–1.029) for each increase of 1 unit of pre-pregnancy BMI and GWG, respectively. We next analyzed the risk of adverse outcomes in continuous GWG categories in each group (Fig. 2). The GWG category with a protective association was defined as the appropriate GWG range. As shown in Additional file 1: Table S5–S8, the appropriate GWG range was 10–15.9 kg, 8–11.9 kg, 6–7.9 kg, and -5–3.9 kg for the Underweight, Normal weight, Overweight, and the Obesity group, respectively.
Associations of GWG Categories with Adverse Outcomes. The filled circles and error bars indicate OR and 95% CI, respectively. The red area represents the optimal GWG range according to this study, the area with black slant lines represents the GWG ranges recommended by IOM. The overlapped area is shown in red with black slant lines. The total GWG was divided by the group distance of 2 kg, except for the first GWG ring and the last GWG range of each pre-pregnancy BMI group. The GWG category with a protective association was defined as the optimal GWG range. Combining the results of the four groups of models, the results of Model 3 are used for the Underweight group, the results of Model 1 are used for the Normal weight group and Overweight group, and the results of Model 2 are used for the Obesity group
As shown in Table 2, the sensitivity of the optimal GWG range obtained in this study was all higher than that recommended by IOM, while the specificity was the opposite. Except for SGA, positive and negative predictive values were higher than those of IOM. To compare the accuracy of the prediction capabilities of the two GWG recommended ranges, the Net Reclassification Index (NRI) was calculated. The NRI value was 0.065 > 0, indicating that the predictive ability of GWG recommended ranges in this study was better compared with the ones from IOM (Additional file 1: Table S9).
Discussion
An appropriate GWG is crucial to reducing the risk of adverse pregnancy outcomes for GDM women and their offspring. Here, using the data from the XAMC cohort, we explored GWG ranges for GDM women with different pre-pregnancy BMI, which are stricter than the IOM recommendation. In detail, the ideal GWG range was 10–15.9 kg, 8–11.9 kg, 6–7.9 kg, and -5–3.9 kg for the Underweight, Normal weight, Overweight, and Obesity group, respectively.
In this study, we found that not only the blood glucose and HbA1c, but also the incidence of adverse outcomes, gestational hypertension, pre-eclampsia, cesarean section, and LGA raised with the increase in pre-pregnancy BMI. Nevertheless, pre-pregnancy BMI cannot be changed for pregnant women who have already been diagnosed with GDM. Furthermore, even in women of normal weight before pregnancy, excessive GWG will also have a moderate long-term impact on the cardiometabolic risk factors of adult offspring [12]. On the contrary, GWG is modifiable, and it still had a positive impact on the fetus even with the intervention conducted in the third trimester of pregnancy. Moreover, an appropriate GWG can attenuate the influence of pre-pregnancy BMI on childhood obesity [21]. Currently, the optimal GWG recommended by IOM is the most authoritative and widely used for all pregnant women. Accordingly, GDM women who had insufficient GWG were found to be at the lowest risk of adverse outcomes compared to those who had appropriate or excessive GWG [22]. A stricter GWG recommendation is needed for women with GDM [17, 23]. Consistently, our findings showed that, for GDM patients, the GWG ranges of each pre-pregnancy BMI category were stricter and lower than those recommended by IOM. Notably, among all participants included in the study, nearly one in five were overweight or obese before conception. In our study, the appropriate GWG range of the Obesity group was less than 4 kg and the lower limit even is -5 kg, indicating that weight loss during pregnancy in obese women with GDM may reduce adverse pregnancy outcomes.
Among all adverse pregnancy outcomes, cesarean section was the one with the highest incidence. A prospective birth cohort study in Southwest China showed that controlling GWG can reduce the incidence of cesarean section, and the optimal level of GWG in reducing the rate of cesarean section is more stringent than the IOM recommendation [24]. Importantly, we also observed a similar phenomenon in GDM women. When the GWG in each group was within the recommended ranges, the risk of cesarean section was significantly reduced, and more importantly, the risk was much lower in the ranges obtained in our study than in those from IOM. The situation of all other adverse outcomes was similar except for SGA. It has been found that a lower GWG will increase the risk of SGA [25]. Notably, since the GWG ranges obtained in our study were stricter, it significantly increased the risk of SGA. Considering LGA was the second major adverse outcomes with a remarkable high incidence than SGA in this study and the risk of LGA can be reduced within the recommended range of this study in all groups, the recommended range of GWG in our study should be more favorable for an appropriate birth weight if corresponding measures are taken to avoid the occurrence of SGA.
Clinically, when a pregnant woman comes to see a doctor for the first time, the doctor should recommend an appropriate GWG based on her pre-pregnancy BMI to reduce their risk of getting GDM and adverse pregnancy outcomes. If she is at a high risk of developing GDM, the doctor can directly advise her of an appropriate GWG during the entire pregnancy based on our recommendation. By the 24–28th week of pregnancy, if she is diagnosed with GDM, the doctor can conduct diet, exercise, and even insulin treatments, to control both blood sugar and weight gain. Although the ideal ranges we recommended are for the entire pregnancy, the doctor can also subtract the weight gain value before being diagnosed with GDM from them to roughly suggest the weight gain space for the patient in the later pregnancy.
GWG is necessary to ensure a healthy fetus, but excessive GWG, especially in women with GDM, has been associated with a high risk of adverse pregnancy outcomes. Women with different pre-pregnancy BMI have different GWG expectations. Existing guidelines for GWG from IOM have several key limitations [26], and it is not suitable for women with GDM. This study pooled GDM individual participant data from the XAMC study to explore optimal GWG ranges for women with different pre-pregnancy BMI. The ability of the optimal GWG range in this study to correctly predict adverse pregnancy outcomes in pregnant women with GDM is higher than that of IOM, and the GWG recommended range of IOM has a higher ability to correctly determine the absence of adverse pregnancy outcomes in pregnant women with GDM. As we described in the Clinical Implication, our findings will provide a chance for women with GDM to reduce the risk of getting adverse outcomes because of being suggested an appropriate GWG. However, whether women with GDM and those who are at high risk of being diagnosed with GDM can achieve the target GWG and whether this recommended GWG range can be promoted clinically remain to be further verified. This requires more effective and convenient interventions for GDM women to be developed. Notably, the ranges in our study were only based on the very short-term adverse pregnancy outcome. To make them more precise and have a greater guiding role in the clinical work, the long-term adverse outcomes are under observation with the follow-up of the XAMC study.
Our research had some limitations. Firstly, the timing and pattern of GWG will affect pregnancy outcomes [27, 28]. Consistent with the IOM guidelines, this study used total GWG to identify optimal GWG ranges instead of GWG per week because it does not have a linear pattern. Since this was a retrospective study, the dynamic changes of GWG were not obtained so it was impossible to evaluate how GWG patterns affected pregnancy outcomes in our study. Secondly, we only analyzed five important adverse outcomes (gestational hypertension, pre-eclampsia, cesarean section, LGA, and SGA) instead of all possible ones. This may lead to a possibility that the missing of some information may inevitably make this range suboptimal. Thirdly, information on the diet and physical activity of pregnant women was not available which may bring some bias. Fourth, although there is a big sample, the women are all from one single place in China and only a few adverse outcomes were evaluated, so the results cannot affirm that the proposed GWG rages can be used for all women with GDM around the world. A study of 1,309,136 pregnant women showed that the BMI of women from the United States and Europe was higher than that of Asia, and the GWG expectation was different [29]. Deputy et al. showed that in addition to the association between pre-pregnancy BMI and GWG, race-ethnicity is also related to GWG. Compared with white women of normal weight, the probability of insufficient GWG mainly occurs in blacks, Hispanics, and Asians with normal weight [30]. Besides, the loss of weight during pregnancy is a very controversial concern in the current research. Despite these limitations, our research also has several important strengths. Firstly, to the best of our knowledge, this is the first time appropriate GWG ranges are explored for women with GDM with different pre-pregnancy BMI categories. Secondly, the data obtained from such a large population made the results more convincing although they were only from a tertiary hospital in western China. Thirdly, many efforts were made to find optimal GWG in normal weight pregnant women and only a single outcome, such as birth weight or cesarean section was used [24, 31, 32]. Here, we analyzed adverse outcomes including five common ones to explore and assess the optimal range of GWG. Finally, a planned long-term follow-up based on the XAMC cohort is ongoing, which will verify the clinical value of this range and provide more valuable information for the exploration of a more suitable GWG for pregnant women with GDM.
Conclusions
In conclusion, based on the adverse pregnancy outcomes of over 12 thousand participants in northwest of China, our findings showed a more stringent GWG range for GDM women than the IOM criteria recommendation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GDM:
-
Gestational diabetes mellitus
- GWG:
-
Gestational weight gain
- IOM:
-
Institute of medicine
- CI:
-
Confidence intervals
- OR:
-
Odds ratio
- LGA:
-
Large for gestational age
- SGA:
-
Small for gestational age
- BMI:
-
Body mass index
- XAMC:
-
Xi’an longitudinal mother–child cohort
- OGTT:
-
Oral glucose tolerance test
- IADPSG:
-
International Association of Diabetes in Pregnancy Study Groups
- XJTU:
-
Xi’an Jiaotong University
- NWCH:
-
Northwest Women and Children’s Hospital
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- NRI:
-
Net Reclassification Index
References
American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–27. https://doi.org/10.2337/dc18-S002.
Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig. 2019;10:154–62. https://doi.org/10.1111/jdi.12854.
Aburezq M, AlAlban F, Alabdulrazzaq M, Badr H. Risk factors associated with gestational diabetes mellitus: the role of pregnancy-induced hypertension and physical inactivity. Pregnancy Hypertens. 2020;22:64–70. https://doi.org/10.1016/j.preghy.2020.07.010.
Benhalima K, De Landtsheer A, Van Crombrugge P, Moyson C, Verhaeghe J, Verlaenen H, Vercammen C, Maes T, Dufraimont E, De Block C, et al. Predictors of neonatal adiposity and associations by fetal sex in women with gestational diabetes mellitus and normal glucose-tolerant women. Acta Diabetol. 2021;58:341–54. https://doi.org/10.1007/s00592-020-01619-0.
Kaseva N, Vaarasmaki M, Sundvall J, Matinolli HM, Sipola M, Tikanmaki M, Heinonen K, Lano A, Wehkalampi K, Wolke D, et al. Gestational diabetes but not prepregnancy overweight predicts for cardiometabolic markers in offspring twenty years later. J Clin Endocrinol Metab. 2019;104:2785–95. https://doi.org/10.1210/jc.2018-02743.
Yefet E, Schwartz N, Sliman B, Ishay A, Nachum Z. Good glycemic control of gestational diabetes mellitus is associated with the attenuation of future maternal cardiovascular risk: a retrospective cohort study. Cardiovasc Diabetol. 2019;18:75. https://doi.org/10.1186/s12933-019-0881-6.
Jin C, Lin L, Han N, Zhao Z, Liu Z, Luo S, Xu X, Liu J, Wang H. Excessive gestational weight gain and the risk of gestational diabetes: comparison of intergrowth-21st standards, IOM recommendations and a local reference. Diabetes Res Clin Pract. 2019;158:107912. https://doi.org/10.1016/j.diabres.2019.107912.
Widen EM, Whyatt RM, Hoepner LA, Ramirez-Carvey J, Oberfield SE, Hassoun A, Perera FP, Gallagher D, Rundle AG. Excessive gestational weight gain is associated with long-term body fat and weight retention at 7 y postpartum in African American and Dominican mothers with underweight, normal, and overweight prepregnancy BMI. Am J Clin Nutr. 2015;102:1460–7. https://doi.org/10.3945/ajcn.115.116939.
McClure CK, Catov JM, Ness R, Bodnar LM. Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. Am J Clin Nutr. 2013;98:1218–25. https://doi.org/10.3945/ajcn.112.055772.
Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph17249517.
Gaillard R, Welten M, Oddy WH, Beilin LJ, Mori TA, Jaddoe VW, Huang RC. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: a prospective cohort study. BJOG. 2016;123:207–16. https://doi.org/10.1111/1471-0528.13700.
Hrolfsdottir L, Rytter D, Olsen SF, Bech BH, Maslova E, Henriksen TB, Halldorsson TI. Gestational weight gain in normal weight women and offspring cardio-metabolic risk factors at 20 years of age. Int J Obes (Lond). 2015;39:671–6. https://doi.org/10.1038/ijo.2014.179.
Hannaford KE, Tuuli MG, Odibo L, Macones GA, Odibo AO. Gestational weight gain: association with adverse pregnancy outcomes. Am J Perinatol. 2017;34:147–54. https://doi.org/10.1055/s-0036-1584583.
Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, Li N, Hu G, Corrado F, Rode L, et al. Association of gestational weight gain with maternal and infant outcomes a systematic review and meta-analysis. Jama. 2017;317:2207–25.
Rasmussen KM, Yaktine AL, Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines, editors. Weight gain during pregnancy: Reexamining the guidelines. Washington (DC), US: National Academies Press; 2009. pp. 1–13. https://doi.org/10.17226/12584
Ukah UV, Bayrampour H, Sabr Y, Razaz N, Chan WS, Lim KI, Lisonkova S. Association between gestational weight gain and severe adverse birth outcomes in Washington State, US: a population-based retrospective cohort study, 2004–2013. PLoS Med. 2019;16:e1003009. https://doi.org/10.1371/journal.pmed.1003009.
Viecceli C, Remonti LR, Hirakata VN, Mastella LS, Gnielka V, Oppermann ML, Silveiro SP, Reichelt AJ. Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta-analysis. Obes Rev. 2017;18:567–80. https://doi.org/10.1111/obr.12521.
Ji J, He Z, Qu P, Gao J, Zhang W, Wu P, Wei J, Zhang T, Ma ZF, Luo X, et al. The Xi’an longitudinal mother-child cohort study: design, study population and methods. Eur J Epidemiol. 2021;36:223–32. https://doi.org/10.1007/s10654-020-00704-6.
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, International Association of D, Pregnancy Study Groups Consensus P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. https://doi.org/10.2337/dc09-1848.
Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68. https://doi.org/10.1016/s0140-6736(14)60932-6.
Liang H, Yin C, Dong X, Acharya G, Li X. Clusters of week-specific maternal gestational weight gain pattern and their association with birthweight: an observational cohort study. Acta Obstet Gynecol Scand. 2017;96:1251–60. https://doi.org/10.1111/aogs.13204.
Xie X, Liu J, Pujol I, Lopez A, Martinez MJ, Garcia-Patterson A, Adelantado JM, Ginovart G, Corcoy R. Inadequate weight gain according to the institute of medicine 2009 guidelines in women with gestational diabetes: frequency, clinical predictors, and the association with pregnancy outcomes. J Clin Med. 2020. https://doi.org/10.3390/jcm9103343.
Xu Q, Ge Z, Hu J, Shen S, Bi Y, Zhu D. The association of gestational weight gain and adverse pregnancy outcomes in women with gestational diabetes mellitus. Endocr Pract. 2019;25:1137–50. https://doi.org/10.4158/ep-2019-0011.
Huang L, Zhang J, Sun H, Dong H, Li R, Cai C, Gao Y, Wu C, Lan X, Zeng G. Association of gestational weight gain with cesarean section: a prospective birth cohort study in Southwest China. BMC Pregnancy Childbirth. 2021;21:57. https://doi.org/10.1186/s12884-020-03527-1.
Zhang CX, Lai JQ, Liu KY, Yang NH, Zeng G, Mao LM, Li ZN, Teng Y, Xia W, Dai N, et al. Optimal gestational weight gain in Chinese pregnant women by Chinese-specific BMI categories: a multicentre prospective cohort study. Public Health Nutr. 2021. https://doi.org/10.1017/s1368980021001622.
Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MA, Chatzi L, Chrousos GP, LifeCycle Project-Maternal O, Childhood Outcomes Study G, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321:1702–15. https://doi.org/10.1001/jama.2019.3820.
Darling AM, Werler MM, Cantonwine DE, Fawzi WW, McElrath TF. Timing and amount of gestational weight gain in association with adverse birth outcomes. Epidemiology. 2019;30:695–705. https://doi.org/10.1097/ede.0000000000001055.
Wu Y, Wan S, Gu S, Mou Z, Dong L, Luo Z, Zhang J, Hua X. Gestational weight gain and adverse pregnancy outcomes: a prospective cohort study. BMJ Open. 2020;10:e038187. https://doi.org/10.1136/bmjopen-2020-038187.
Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, Black MH, Li N, Hu G, Corrado F, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. Bmc Med. 2018;16:153. https://doi.org/10.1186/s12916-018-1128-1.
Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773–81. https://doi.org/10.1097/aog.0000000000000739.
Devlieger R, Ameye L, Nuyts T, Goemaes R, Bogaerts A. Reappraisal of gestational weight gain recommendations in obese pregnant women: a population-based study of 337,590 births. Obes Facts. 2020;13:333–48. https://doi.org/10.1159/000508975.
Zheng W, Huang W, Zhang L, Tian Z, Yan Q, Wang T, Li G, Zhang W. Suggested gestational weight gain for chinese women and comparison with institute of medicine criteria: a large population-based study. Obes Facts. 2021. https://doi.org/10.1159/000509134.
Acknowledgements
Thanks to the staff of Northwest Women's and Children's Hospital for their support of this study.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81874263) and the Key Special Project of the Development Center for Medical Science & Technology of the National Health Commission of the People’s Republic of China (W2015CAE060).
Author information
Authors and Affiliations
Contributions
Conceptualization, XL, JG, JJ and YM; data curation, JG, ZH, WZ, PW, XG, DC, ZX and CL; funding acquisition, XL and YM; investigation, ZH, WZ, DC and ZX; project administration, XL, ZH, JJ, PW and XG; resources, JJ and YM; software, CL; supervision, YM; visualization, CL; writing—original draft, XL and JG; writing—review & editing, ZH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the ethical committee of Xi’an Jiaotong University (XJTU 2016-053) and the Northwest Women and Children's Hospital (NWCH 2012-013). All women provided gave written informed consent. The principles of the Helsinki Declaration were followed throughout the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flow chart of the participants. Table S1. Description of Missing value. Table S2. Chi-square test for missing values. Table S3. Description of outcomes by GWG category. Table S4. Univariate logistic regression analysis of continuous GWG and pre-pregnancy BMI with adverse outcomes. Table S5. Associations of GWG categories with adverse outcomes in the Underweight group*. Table S6. Associations of GWG categories with adverse outcomes in Normal weight group*. Table S7. Associations of GWG categories with adverse outcomes in Overweight group*. Table S8. Associations of GWG categories with adverse outcomes in Obesity group*. Table S9. The results of adverse outcomes predicted by the logistic regression models in Table 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, X., Gao, J., He, Z. et al. What is an appropriate gestational weight gain for women with gestational diabetes mellitus: based on the adverse pregnancy outcomes of over 12 thousand participants?. Diabetol Metab Syndr 14, 166 (2022). https://doi.org/10.1186/s13098-022-00940-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00940-8