Abstract
Introduction
Diabetic foot ulcers (DFU) are one of the leading long-term complications experienced by patients with diabetes. Dipeptidyl Peptidase 4 inhibitors (DPP4is) are a class of antihyperglycemic medications prescribed to patients with diabetes to manage glycaemic control. DPP4is may also have a beneficial effect on DFU healing. This study aimed to determine vildagliptin's effect on inflammatory markers and wound healing.
Trial design
Prospective, randomized, double-blind, placebo-controlled, single-center study.
Methods
Equal number of participants were randomized into the treatment and placebo groups. The treatment was for 12 weeks, during which the participants had regular visits to the podiatrist, who monitored their DFU sizes using 3D camera, and blood samples were taken at baseline, six weeks, and 12 weeks during the study for measurement of inflammatory markers. In addition, demographic characteristics, co-morbidities, DFU risk factors, and DFU wound parameters were recorded.
Results
50 participants were recruited for the study, with 25 assigned to placebo and 25 to treatment group. Vildagliptin treatment resulted in a statistically significant reduction of HBA1c (p < 0.02) and hematocrit (p < 0.04), total cholesterol (p < 0.02), LDL cholesterol (p < 0.04), and total/HDL cholesterol ratio (P < 0.03) compared to the placebo group. Also, vildagliptin had a protective effect on DFU wound healing, evidenced by the odds ratio (OR) favoring the intervention of 11.2 (95% CI 1.1–113.5; p < 0.04) and the average treatment effect on the treated (ATET) for vildagliptin treatment group showed increased healing by 35% (95%CI; 10–60, p = 0.01) compared to placebo with the model adjusted for microvascular complications, smoking, amputation, dyslipidemia, peripheral vascular disease (PVD) and duration of diabetes.
Conclusions
Vildagliptin treatment was effective in healing DFU in addition to controlling the diabetes. Our findings support the use of DPP4is as a preferred option for treating ulcers in patients with diabetes. Further studies on a larger population are warranted to confirm our findings and understand how DPP4is could affect inflammation and DFU healing.
Similar content being viewed by others
Introduction
Diabetic patients are at exceptionally high risk for poor wound healing in general and foot ulcers in particular. The lifetime risk for developing chronic foot ulcers has been estimated to reach about 15–20% [1, 2]. Despite considerable advances in diabetic care, foot ulcers are responsible for a high number of lower limb amputations associated with decreased quality of life and increased mortality [2,3,4]. The significant risk factors for diabetic foot ulcers (DFU) are neuropathy and peripheral vascular disease. Diabetes induces complex vascular changes, promoting accelerated atherosclerosis and hypercoagulability, complicating foot ulcers, which can be assessed indirectly by many inflammatory markers [5].
Dipeptidyl peptidase-4 inhibitors (DPP4is) are a group of antihyperglycemic medications for managing type 2 diabetes mellitus. Several animal studies have suggested numerous beneficial anti-inflammatory effects of DPP4is beyond the effects on blood glucose alone [6,7,8]. Investigation into the anti-inflammatory property of DPP4i-vildagliptin in a human setting has shown benefits such as reducing oxidative stress and inflammation [9]. Treatment with a DPP4i may offer an attractive blood glucose reduction with the synergistic mechanism of action while exerting additional wound healing properties. Reduction of levels of inflammatory markers is of great clinical importance and has been shown to correlate with improvement of diabetic wound healing [10, 11].
In the present study, we focused on the potential anti-inflammatory properties of vildagliptin subjects with DFU. The critical representative serum markers for the study were chosen to represent alterations in inflammation markers, including interleukin 6 (IL-6), an inflammatory marker that markedly increased in individuals with DFU [10,11,12]. High circulating levels of high-sensitivity C-reactive protein (hs-CRP) and low levels of adiponectin, a hormone secreted from the adipose tissue with regulatory metabolic function, have also been reported to be associated with DFU. Reduction in hs-CRP levels represents a molecular hallmark of wound healing, while elevated adiponectin levels seem to protect against DFU progression [11]. Tumour necrosis factor-α (TNF-α) and transforming growth factor-beta 1 (TGF-β1) represent pro-inflammatory cytokines that are markedly increased and may serve as a good prognosis for diabetic foot syndrome [11, 13].
The primary objective of this study was to investigate whether Vildagliptin (100 mg/day) + standard of care (SOC) for DFU aids DFU healing compared to placebo + SOC over 12 weeks of treatment. In addition, we examined the effects of vildagliptin (100 mg/day) on circulating levels of interleukin-6 (IL-6), C-reactive protein (CPR), TNF-α, interleukin 1 beta (IL-1 beta), TGF-β, platelet reactivity, and adiponectin representing critical molecular markers of inflammation, thrombogenicity and wound healing, respectively. This study expanded on the known anti-glycemic effects of DPP4 inhibitors such as vildagliptin and informed on its beneficial effect on DFU wound healing, possibly by reducing inflammation, though the exact molecular mechanism remains to be identified.
Study design
This study was a 12-week single-center, two-campus, randomized, double-blind, placebo-controlled clinical trial to compare Vildagliptin (100 mg/day) + SOC (intervention arm) with placebo + SOC (control arm), a ratio of 1:1. Informed consent was obtained from all participants prior to commencement of the study.
Study participants were randomised by an independent pharmacist using www.randomizer.org [14]. The pharmacist provided the vildagliptin and placebo tablets in a sealed envelope to the study nurse. The study nurse then provided tablets based on the randomization allocation sequentially to the participants.
Inclusion criteria
Subjects ≥ 18 years of age diagnosed with type 2 diabetes on diet only or on any non-DPP4i medication were assessed. Patients with existing diabetes index foot ulcer grade A1 or higher, according to the University of Texas Wound Classification System of Diabetic Foot Ulcers, were included. A foot ulcer is defined as any full-thickness skin defect existing for a minimum of 14 days. In patients with multiple diabetic foot ulcers, the index foot ulcer is defined as the foot ulcer with the largest wound area at the time of inclusion. A suboptimal HBA1c ≥ 7.0% being an indication for use of vildagliptin was documented within 12 weeks prior to study inclusion or on the day of study inclusion.
Exclusion criteria
The primary exclusion criteria included type 1 diabetes and current index foot ulcer of any non-diabetic pathophysiology. Also, major surgery up to 6 months before the day of enrolment or any planned surgery prior to study completion, including any major surgical intervention for the diabetic foot ulcer, were considered exclusion criteria. Patients with hypersensitivity to vildagliptin, one of its excipients, or any other contraindication for vildagliptin use, including a pre-treatment ALT or AST > 3 × ULN (upper limit of normal), were excluded. Patients undergoing treatment with normothermic or hyperbaric oxygen therapy, enzymatic debridement, having Charcot's foot, renal impairment (defined as eGFR less than 60 ml/min/1.73 m2 for more than three months), and on GLP-1 analogs or DPP4is and on any other clinical trials were also excluded.
All participants were to remain on study medication and followed up for 12 weeks even if their index ulcer had completely healed prior to the end of the 12-week observation period.
This study was performed using the national and international guidelines: the ICH/GCP guidelines (Guidance on Good Clinical Practice [CPMP/GCP/135/95] and Guidance on Good Clinical Practice [CPMP/GCP/135/95] annotated with Therapeutic Goods Administration (TGA) comments [DSEB, July 2000]), the NHMRC National Statement on Ethical Conduct in Human Research (2007) and all other applicable Australian Commonwealth, State or Territory laws or guidelines of Regulatory Authorities as well as following the ethical principles that have their origin in the Declaration of Helsinki. The final study protocol and the final version of the written informed consent form, case report form (CRF), and patients' home blood glucose monitoring diaries were approved in writing by an independent ethics committee (IEC) HREC/13/QTHS/65 and the Trial Registration: ACTRN12613000418774. Written informed consent was obtained from all subjects prior to commencement of the study.
Laboratory procedures
Morning fasting venous blood samples were taken for determining levels of inflammatory markers and was centrifuged after 30 min of collection for 12 min at 3000 rpm. 0.1 ml of venous plasma was stored at − 80 °C for analysis as a batch to minimize variance. Serum levels of various candidates were measured using the ELISA kit as per manufacturer’s instructions. Details of ELISA kits were as follows: Interleukin 6, Interleukin 1β, Tumor Necrosis Factor-α, adiponectin, C-reactive protein and Transforming growth factor β (Abcam, Australia).
Sample size calculations and data analysis
The sample size calculation in this study was conducted to demonstrate superior efficacy of vildagliptin (100 mg/day) + SOC over placebo + SOC on wound healing from randomization to week 12. The appropriate sample size was attained by assuming an anticipated difference in wound healing of 20% for diabetic foot ulcer healing at week 12 between the intervention and control groups. A power set to 80% yielded a calculated sample size of 22 wounds per arm. Adding an estimated attrition rate of close to 15% = 3 ulcers per arm led to an overall planned sample size of a minimum of 25 wounds per arm and 50 wounds in total.
Data analysis
For patients with post-randomization data, the last study assessment was carried forward as their final assessment for analyses. These served as conservative estimates since the patients were expected to improve over time. Descriptive statistics were provided for all measured indices and clinical/demographic characteristics. All data were analyzed using SPSS Version 25 or Stata 16. Tests for normality were performed, and based on the outcome, parametric or nonparametric tests were used to determine the differences between the groups. The results were reported as mean ± standard deviation or median and interquartile range where appropriate. ANCOVA was used to determine the effect of treatment on variables at 12 weeks. Chi-squared analysis was performed for categorical variables. Binary logistic regression analysis was used to determine the factors for wound healing with confounding factors of microvascular complications, smoking, amputation, dyslipidemia, PVD, and year of diabetes included in the model. The average treatment effect (ATE) was calculated using a logit model with the inverse probability weights regression adjustment. P-value < 0.05 was to be considered significant.
Results
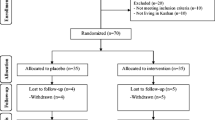
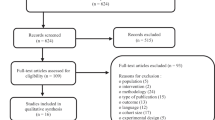
Eighty-eight patients were screened for eligibility, of which 38 did not meet the inclusion criteria. Fifty patients with proven DFU were recruited and randomized [14] in a 1:1 ratio to either vildagliptin and other antidiabetic therapies (n = 25) or placebo and other antidiabetic therapies (n = 25) for 12 weeks, as shown in Figs. 1 and 2. Seven participants withdrew during the study, 6 were lost to follow-up, and 4 had an amputation. An intention-to-treat analysis was undertaken for 50 ulcers n = 25 and n = 25 of the randomized placebo and vildagliptin groups, respectively (Fig. 2). With some patients having multiple ulcers, each group's total number of ulcers was 25. The demographic characteristics such as age, gender distribution, BMI, and duration of diabetes were all similar in the randomized groups (Table 1).
The clinical, biochemical parameters and inflammatory markers (baseline, week 6, and week 12) were analyzed at the end of the study. The two groups had no significant differences in most metabolic risk factors. Both the groups had a similar distribution of the other insulin and oral hypoglycaemic medications. The baseline characteristics of the biochemical parameters, inflammatory markers, and ulcer dimensions in the study population were similar in both control and vildagliptin-treated groups (Table 1).
Subjects on vildagliptin had lower levels of A1c; p < 0.02, cholesterol; p < 0.02, total/HDL cholesterol ratio; P < 0.03 and LDL cholesterol; p < 0.04 compared with controls. Other inflammatory markers, including IL-6, showed no significant differences (Table 2). C-reactive protein (CRP), WBC, neutrophils, ulcer length and width, and surface area were also reduced in the vildagliptin group compared to placebo (p < 0.05) (Table 2).
At the end of 12-week treatment period, of the total of 50 ulcers, 14 (28%) were completely healed. The factors for wound healing were determined using multivariate logistic regression analysis. The analysis revealed that vildagliptin has a favorable OR of 11.2 (95% CI 1.1–113.5, p < 0.04) with the model adjusted for microvascular complications, smoking, amputation, dyslipidemia, PVD, and duration of diabetes (Table 3). In addition, the average treatment effect on the treated (ATET) for the vildagliptin treatment group increased healing by 35% (95%CI; 10–60), p = 0.01 compared to placebo; adjusted for microvascular complications, smoking amputation, dyslipidemia, PVD, and year of diabetes.
Discussion
We have shown that vildagliptin treatment over 12 weeks can aid wound healing. This finding is in line with Marfella et al.’s previous report, that DDP4is may facilitate healing of chronic foot ulcers [15]. Our analysis showed the beneficial effect of vildagliptin in healing ulcers over 12 weeks with a favorable odds ratio of more than 11. Although DPP4is have shown promising wound healing properties [16], this study examined the effect of vildagliptin treatment over 12 weeks in patients with type 2 diabetes undergoing their SOC for DFU, representing a relatively short intervention period. Furthermore, vildagliptin treatment is efficacious in reducing HBA1c levels, whether treated as monotherapy [17] or combination therapies [18]. This study confirmed other metabolic and systemic effects of vildagliptin, such as improving lipids profile [19] and reducing systemic inflammation, thus aligned with previous findings [20,21,22]. Furthermore, our data substantiate previous findings since the study was a randomized clinical trial and double-blinded with one team involved in running the trial thus eliminating the possible inter-investigator variation.
Moreover, we attempted to quantify vildagliptin's potential effect on DFU healing and estimated a 35% increase in healing capacity compared to controls. This finding represents the clinically meaningful properties of vildagliptin in managing DFU [23]; however, the use of DPP4is for DFU treatment might not be without complications [24,25,26]. For instance, the U.S. Food and Drug Administration (FDA) warns that these drugs may cause joint pain that can be severe and disabling [27], suggesting that possible side effects should be borne in mind when using DPP-4is. However, we did not record any severe side effects associated with vildagliptin, possibly acknowledging only short-term use of this drug.
In conclusion, the vildagliptin treatment in DFU patients improves wound healing with an associated reduction in some inflammatory biomarkers. Our findings support that DPP4is inhibitors could be a preferred option for treating ulcers in patients with diabetes; however, long-term effects need to be determined precisely, including an extended follow-up to determine DFU reoccurrence following the vildagliptin treatment.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288–93.
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–28.
Malabu UH, Manickam V, Kan G, Doherty SL, Sangla KS. Calcific uremic arteriolopathy on multimodal combination therapy: still unmet goal. Int J Nephrol. 2012;2012: 390768.
Malabu U, Roberts L, Sangla K. Calciphylaxis in a morbidly obese woman with rheumatoid arthritis presenting with severe weight loss and vitamin D deficiency. Endocr Pract. 2011;17:e104–8.
Pendsey SP. Understanding diabetic foot. Int J Diabetes Dev Ctries. 2010;30:75–9.
Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;58:157.
Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–49.
Shimizu S, Hosooka T, Matsuda T, Asahara S-i, Koyanagi-Kimura M, et al. DPP4 inhibitor vildagliptin preserves-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J Mol Endocrinol. 2012;49:125.
Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes. Role of dipeptidyl peptidase-IV inhibition. Random Control Trial. 2012;35:2076–82.
Ahmad J, Zubair M, Malik A, Siddiqui MA, Wangnoo SK. Cathepsin-D, adiponectin, TNF-α, IL-6 and hsCRP plasma levels in subjects with diabetic foot and possible correlation with clinical variables: a multicentric study. Foot. 2012;22:194–9.
Zubair M, Malik A, Ahmad J. Plasma adiponectin, IL-6, hsCRP, and TNF-α levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian J Endocrinol Metab. 2012;16:769.
Tuttolomondo A, La Placa S, Di Raimondo D, Bellia C, Caruso A, et al. Adiponectin, resistin and IL-6 plasma levels in subjects with diabetic foot and possible correlations with clinical variables and cardiovascular co-morbidity. Cardiovasc Diabetol. 2010;9:50.
Biros E, Moran CS. Mini tryptophanyl-tRNA synthetase is required for a synthetic phenotype in vascular smooth muscle cells induced by IFN-γ-mediated β2-adrenoceptor signaling. Cytokine. 2020;127: 154940.
Marfella R, Sasso FC, Rizzo MR, Paolisso P, Barbieri M, et al. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diabetes Res. 2012;2012: 892706.
Long M, Cai L, Li W, Zhang L, Guo S, et al. DPP-4 inhibitors improve diabetic wound healing via direct and indirect promotion of epithelial-mesenchymal transition and reduction of scarring. Diabetes. 2017;67:518–31.
Cai L, Cai Y, Lu ZJ, Zhang Y, Liu P. The efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2012;37:386–98.
Mohan V, Zargar A, Chawla M, Joshi A, Ayyagari U, et al. Efficacy of a combination of metformin and vildagliptin in comparison to metformin alone in type 2 diabetes mellitus: a multicentre, retrospective, real-world evidence study. Diabetes Metab Syndr Obes. 2021;14:2925–33.
Shimodaira M, Niwa T, Nakajima K, Kobayashi M. Beneficial Effects of Vildagliptin on Metabolic Parameters in Patients with Type 2 Diabetes. Endocr Metab Immune Disord Drug Targets. 2015;15:223–8.
Younis A, Eskenazi D, Goldkorn R, Leor J, Naftali-Shani N, et al. The addition of vildagliptin to metformin prevents the elevation of interleukin 1ß in patients with type 2 diabetes and coronary artery disease: a prospective, randomized, open-label study. Cardiovasc Diabetol. 2017; 16:69
Wiciński M, Górski K, Wódkiewicz E, Walczak M, Nowaczewska M, Malinowski B. Vasculoprotective effects of vildagliptin. Focus on atherogenesis. Int J Mol Sci. 2020; 21:2275
Bi J, Cai W, Ma T, Deng A, Ma P, et al. Protective effect of vildagliptin on TNF-α-induced chondrocyte senescence. IUBMB Life. 2019;71:978–85.
Ishida Y, Murayama H, Shinfuku Y, Taniguchi T, Sasajima T, Oyama N. Cardiovascular safety and effectiveness of vildagliptin in patients with type 2 diabetes mellitus: a 3-year, large-scale post-marketing surveillance in Japan. Expert Opin Drug Saf. 2020;19:625–31.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88.
Tschöp MH, Stumvoll M, Ristow M. Opposing effects of antidiabetic interventions on malignant growth and metastasis. Cell Metab. 2016;23:959–60.
Sasaki T, Hiki Y, Nagumo S, Ikeda R, Kimura H, et al. Acute onset of rheumatoid arthritis associated with administration of a dipeptidyl peptidase-4 (DPP-4) inhibitor to patients with diabetes mellitus. Diabetol Int. 2010;1:90–2.
Acknowledgements
We thank Holger Jansen, Roy Rasalam, and Karen Hird for their support in the initial phase of the study.
Funding
We acknowledge the financial contribution of Novartis Australia in conducting this investigator-initiated trial.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology and funding acquisition; V.V, K.R, K.S and U.M. Investigation; V.V, K.R, J.G, J.B, K.S, S.J, O.H and U.M. Data curation V.V, S.J, O.H, J.G, J.B and U.M; Formal analysis; V.V, E.B; Roles/writing—original draft; V.V, E.B and U.M Writing—review and editing; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The final study protocol and the final version of the written informed consent form, case report form (CRF), and patients' home blood glucose monitoring diaries were approved in writing by an independent ethics committee (IEC) HREC/13/QTHS/65.
Competing interests
The authors do not have any financial associations that might conflict with the submitted article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vangaveti, V.N., Jhamb, S., Hayes, O. et al. Effects of vildagliptin on wound healing and markers of inflammation in patients with type 2 diabetic foot ulcer: a prospective, randomized, double-blind, placebo-controlled, single-center study. Diabetol Metab Syndr 14, 183 (2022). https://doi.org/10.1186/s13098-022-00938-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00938-2