Abstract
Background
The aim of this systematic review and meta-analysis was to determine the effect of olive leaf extract (OLE) supplementation on cardiovascular-related variables, including lipid, glycemic, inflammatory, liver and renal-related factors, as well as blood pressure.
Methods
PubMed, ISI Web of Science, Scopus, and Cochrane library were searched, up to October 2021, for relevant controlled trials. Mean differences and standard deviations were pooled for all outcomes, using a random-effects model. The methodological quality, as well as quality of evidence were assessed using standard tools.
Results
Twelve studies (n = 819 participants) were included in our analyses. Overall analyses showed that OLE supplementation significantly decreased triglyceride (TG) levels (WMD = − 9.51 mg/dl, 95% CI − 17.83, − 1.18; P = 0.025; I2 = 68.7%; P-heterogeneity = 0.004), and systolic blood pressure (SBP) (WMD = − 3.86 mmHg, 95% CI − 6.44, − 1.28 mmHg; P = 0.003; I2 = 19.9%; P-heterogeneity = 0.28). Subgroup analyses also revealed a significant improvement in SBP (− 4.81 mmHg) and diastolic blood pressure (− 2.45 mmHg), TG (− 14.42 mg/dl), total cholesterol (TC) (− 9.14 mg/dl), and low-density lipoprotein-C (LDL-C) (− 4.6 mg/dl) measurements, in patients with hypertension. Significant reductions were also observed in TC (− 6.69 mg/dl), TG (− 9.21 mg/dl), and SBP (− 7.05 mmHg) in normal-weight individuals. However, no meaningful changes were seen in glucose hemostasis, liver and kidney, or inflammatory markers.
Conclusion
The present study revealed that supplementation with OLE yielded beneficial effects for blood pressure and lipid profile in adults, especially in patients with hypertension. As the quality of evidence for glucose hemostasis variables, liver, kidney, and inflammatory markers, were low-to-very low, higher quality RCTs may impact the overarching results.
This study was registered at PROSPERO with the code CRD42022302395.
Similar content being viewed by others
Background
Cardiovascular disease (CVD) is one of the most prominent noncommunicable diseases (NCDs), accounting for the most NCD deaths in the world [1]. Modifiable unhealthy behaviors, such as sedentary lifestyle, smoking, and unhealthy food habits, are regarded as important contributors to the widespread prevalence of CVDs [2, 3], which occur concurrently in overweight/obesity, hypertension, dyslipidemia, hyperglycemia, and inflammation [3, 4].
Oxidative stress and chronic inflammation are among the biggest contributing factors in CVD pathogenesis and progression, and they have recently been introduced as the key targets for the prevention and treatment of CVDs [5]. Moreover, anomalies in glucose metabolism, such as elevated fasting blood glucose (FBG) and insulin resistance, as well as dyslipidemia and elevated blood pressure (BP), are demonstrably associated with a higher risk of CVD [2].
Meanwhile, interventional and epidemiological evidence supports the beneficial effects of antioxidants and antioxidant-rich diets on CVD risk factors [6,7,8,9]. Among different antioxidant-rich foods, olive oil, a typical component of the Mediterranean diet, is known as one of the most important health-protective agents, mainly due to its high content of polyphenols [10]. In addition to olive oil, leaves of the olive tree (known as Olea europaea L.) have been widely used in traditional remedies in Mediterranean countries [11]. The olive leaf extract (OLE) contains a high amount of phenolic antioxidant, named oleuropein, which is markedly higher than those found in olive fruit or olive oil [12, 13]. In recent years, the role of OLE in improvement of CVD-related variables has gained attention in clinical trial investigations in the general population, which mostly include its lipid-lowering [14, 15], anti-obesity [16], blood pressure-lowering [17], and anti-diabetic effects [18]. However, some other studies failed to show any significant improvements in body mass index (BMI) [19, 20], glucose hemostasis [17, 19], plasma lipids, [21], or cytokines [20]. Moreover, a previous meta-analysis of five human investigations reported that OLE supplementation did not have any significant effects on diastolic blood pressure (DBP), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), and a slight improvement in systolic blood pressure (SBP) among patients with hypertension [22]. However, this study omitted two eligible studies [23, 24], and also did not examine the effect of OLE in the general population [22].
The exact mechanism of action of the health-related beneficial effects of OLE is not well understood; although some putative explanations have been proposed. Animal studies have showed that OLE exerts antidiabetic effects through increased peripheral glucose uptake, postprandial insulin secretion, and stimulation of glucagon-like peptide-1 (GLP-1) secretion [25, 26]. Furthermore, it has been shown that lipid peroxidation is inhibited through induced catalase activity following OLE supplementation in rats [27]. In addition, except for the oleuropein content of OLE, other constituents, such as hydroxytyrosol, are shown to have positive effects on glucose metabolism [28, 29].
Thus, we sought to investigate whether OLE could improve the major cardiovascular-related variables, including lipid profile, glucose hemostasis, blood pressure, as well as liver/kidney and inflammatory markers in the general adult population, by conducting a systematic review and meta-analysis of randomized clinical trials (RCTs).
Methods
This systematic review and meta-analysis was prepared in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [30] and was registered at PROSPERO with the code CRD42022302395.
Data sources and search strategy
According to the PICOS tool for performing search strategies in systematic reviews and meta-analysis, endorsed by Cochrane Collaborations [31], the related components consisted of “adult populations” with any health conditions, OLE supplementation as the “intervention”, a concurrent placebo group as the comparator, and randomized controlled trial investigations as the “study design”. Accordingly, the related Medical Subject Headings (MeSH) and non-MeSH terms were used to search PubMed, ISI Web of Science, Scopus, and the Cochrane library, from inception to October, 2021. No restrictions were considered regarding the language, year of publication, the type of populations or the outcomes of measure. The reference lists of the included studies and related reviews were also checked to identify other potential missing studies. More details of the search strategy are provided in Additional file 1.
Study selection
Two reviewers (MM and SS) independently reviewed the titles and abstracts of all records. Randomized-controlled trials were considered to be eligible if they: (1) had either a parallel or crossover design with at least two weeks of OLE supplementation; (2) included adult male or female participants aged 18 years and older, with any health condition; and (3) reported mean and standard deviation (SD) values of change (or provided sufficient data to calculate these variables) for at least one of the cardiovascular-related markers, including glucose indices, lipid profile, liver enzymes, kidney function, circulating inflammatory markers, and blood pressure, as the primary or secondary outcomes.
Studies were excluded if they: (1) used OLE or its active component (olea europaea) in combination with other interventions; (2) were conducted on children, adolescents, pregnant or lactating women; and/or (3) had an intervention duration of less than two weeks.
Data extraction
Two authors (SS and MM) extracted the following information for each study: the last name of the first author, year of publication, study location, study design, doses (mg/d) and duration (week) of intervention, any other intervention given to groups, sample size in each group, sex (male or female), age (y), health status of participants, and means before and after the intervention or mean changes and the corresponding SDs during the follow-up period.
Regarding the articles that reported the outcomes of interest in the same set of population, we included the study that had the greatest sample size or the longest follow-up duration. Extracted data for each outcome were finally converted to a specified unit. Any disagreements were discussed with the corresponding author (SS).
Study quality
Two reviewers (SS and MM) independently assessed the methodological quality of the eligible RCTs using the revised Cochrane risk-of-bias tool for randomized trials (RoB2), which consists of five main domains, including bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in the outcome measures, and bias related to the selection of reported results. Final judgments and overall risk of bias were defined as “Low” or “High” risk of bias or expressed as “Some Concerns” [32].
Certainty of evidence
The overall quality of evidence was evaluated, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool, independently by two reviewers (SS and MM). RCTs begin with a high quality of evidence, but the final quality may be downgraded by detecting the existence of study limitations, inconsistency, indirectness of evidence, and/or publication bias.
Statistical analysis
All of the outcomes for this meta-analysis were reported as continuous data, for which we calculated the weighted mean difference (WMD), with their associated 95% CIs, as the absolute mean difference in change of the outcome of interest between OLE supplementation and the placebo arm. According to the Cochrane recommendations [33], for studies in which the mean and SD changes from baseline were not reported, mean changes were computed as the post-intervention mean minus the pre-intervention mean, and the SD values were yielded via computing the correlation coefficient from the study that reported baseline SD measures in each arm. The correlation coefficients were 0.61 for FBS [19, 23, 34, 35], 0.73 for TC [23, 36, 37], 0.60 for TG [19, 23, 36, 37], 0.71 for LDL-C, 0.79 for HDL-C [19, 23, 34, 37], 0.62 for interlukine-6 (IL-6), 0.60 for interlukine-8 (IL-8), 0.66 for tumor necrosis factor-α (TNF-α) [23, 35], 0.49 for SBP, and 0.52 for DBP [23, 36, 37], and 0.5 for other outcomes. The effect sizes were pooled using the inverse variance random-effects method, which took into account the heterogeneity among studies [38]. Statistical heterogeneity was examined using the Q (P-value < 0.1) and I2 statistics. The I2 represented moderate heterogeneity if ranging 30% to 50%, serious heterogeneity if ranging 50–75%, and very serious heterogeneity if ranging 75–100%[39]. The potential sources of heterogeneity between studies were explored by conducting a series of predefined subgroup analyses based on sex, study design, study duration, and health status of participants [defined as the presence of overweight/obesity, hypertension, and/or hyperlipidemia]. Subgroup analyses were conducted if four studies or more were included for each outcome.
The sensitivity analysis of every outcome was conducted by excluding one study or a group of studies at the same time to discern whether the selected study influenced the overall results. Publication bias was evaluated through Begg’s funnel plots [40] and Egger’s regression symmetry test [41], if more than 10 studies were included. All statistical analyses were performed using STATA software (version 16.0, Stata Corporation, College Station, TX, USA), and a P < 0.05 was, a priori, considered statistically significant.
Results
Literature flow
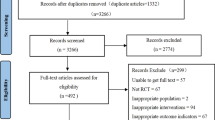
The primary search identified 723 articles, and after removing duplicates (n = 314), 344 records were excluded through screening of the titles and abstracts. We were unable to obtain the full-text of one article, despite contacting the corresponding author, and so, finally, 64 studies were reviewed in full-text. Twenty additional articles were excluded due to the irrelevant endpoints and design (Additional file 2. References 1–20). A further 32 articles were excluded for the following reasons: two studies were conducted on children (Additional file 2: References 21–22) and one among athletes (Additional file 2: Reference 23); ten articles applied multi-supplementation in the intervention group (Additional file 2: References 24–33); three studies used olives or olive extracts (Additional file 2: References 34–36), and four studies used olive pollen as the supplement (Additional file 2: References 37–40); one study was in-vitro research (Additional file 2: Reference 41), and two studies were food-industry investigations (Additional file 2: References 42–43). Seven studies did not consider any control group (Additional file 2: References 44–50); one study had insufficient data (Additional file 2: Reference 51), and another study had duplicate data from a previous publication (Additional file 2: Reference 52).
Finally, twelve eligible studies were included in the systematic review and meta-analysis [19,20,21, 23, 24, 34,35,36,37, 42,43,44]. The study selection process is presented in Fig. 1.
Characteristics of the included studies
The characteristics of the included studies are outlined in Table 1. All of the included studies were in English, except for three, which were in Japanese [34] and Farsi [24, 37]. Studies were conducted in Iran [24, 35, 37], the Netherlands [42, 43], Australia and New Zealand [20, 23], Japan [19, 34], Switzerland [21], Indonesia [36], and Israel [44]. Except for two studies that used the liquids [23] or beverages [34] of OLE, the rest of the studies used either tablets or capsules of OLE. All but two studies applied a parallel design [20, 23]. The duration and dose of OLE supplementation varied from 6 to 48 weeks, and 500 mg to 5 g per day, respectively. Two studies included males [20, 23], one study included females [42], and the rest of the studies enrolled both males and females. Studies were conducted among patients with type-2 diabetes mellitus [19, 44], hypertension [21, 23, 24, 35,36,37], dyslipidemia [34, 43], obesity [20], and osteopenia [42]. Eight studies considered placebo controls in their investigations [20, 21, 23, 24, 35, 37, 43, 44], and one study used a low concentration of green tea as the comparator [34]. One study examined the effect of OLE supplementation in combination with calcium, in which the calcium supplements were given to both the intervention and the control groups [42]. One investigation used captopril in the control group, which was excluded in the analysis of blood pressure [36]. None of the included studies assessed the pure bioactive compounds of OLE, and none reported any related adverse effects.
Risk of bias and quality of evidence
According to the overall quality assessment based on the Cochrane Collaboration Risk of Bias tool (Additional file 3), six studies were classified as “low” risk of bias (i.e., low risk of bias for all domains) [20, 23, 36, 37, 42, 43], three studies were classified as “some concerns” [24, 35, 44], and the three remaining studies were classified as “high” risk of bias [19, 21, 34]. To explain the details of the observed biases, three studies did not clearly explain the randomization and allocation concealment processes [19, 21, 34]. The blinding process was not considered in one study [21] and was not clearly explained in two investigations [19, 34]. One study had a “high” risk of bias [21] and three studies had “some concerns” risk of bias [19, 34, 35] due to the measurements of the outcomes where outcome assessors were not blinded to the study. One study did not clearly report the number of participants with missing outcome data [21] and three studies [21, 24, 44] were also shown to have insufficient data regarding the pre-specified analysis plan (bias due to the selection of the reported results).
According to the evaluation of the quality of evidence based on the GRADE system, the quality of evidence was found to be very low for the effect of OLE supplementation on HbA1c a, high sensitive C-reactive protein (hs-CRP), TNF-α, IL-6, and IL-8. A low quality of evidence was also observed for insulin, AST, ALT, ALP, and creatinine, as well as LDL-ox. A moderate quality of evidence was observed for that of OLE supplementation on FBS, LDL-C, HDL-C, TC, TG, and blood pressure (SBP and DBP) levels (Additional file 3).
Meta-analysis
Lipid profile
TC
Meta-analysis of seven studies [20, 21, 23, 36, 37, 42, 43], including 520 participants, showed that OLE supplementation had no significant effect on the levels of TC, and the heterogeneity between studies was found to be moderate (WMD = − 3.95 mg/dl, 95% CI − 9.97, 2.07; P = 0.2; I2 = 65.6%; P-heterogeneity = 0.008) (Fig. 2a). Subgroup analyses showed a significant reducing effect of OLE supplementation on TC levels in normal-weight participants (4 studies; WMD = − 6.69 mg/dl, 95% CI − 11.90, − 1.49; P = 0.01; I2 = 0.0%; P-heterogeneity = 0.45), and in patients with hypertension (4 studies; WMD = − 9.14 mg/dl, 95% CI − 13.80, − 4.47; P < 0.001; I2 = 7.5%; P-heterogeneity = 0.36) (Additional file 4).
LDL-C
Nine RCTs [19,20,21, 23, 34, 36, 37, 42, 43], including 616 participants, evaluated the effect of OLE on LDL-C levels. Results indicated that OLE supplementation yielded in an insignificant reduction in LDL-C measures and the between-study heterogeneity was reported to be medium (WMD = − 1.30 mg/dl, 95% CI − 5.25, 2.65; P = 0.52; I2 = 53.6%; P-heterogeneity = 0.03) (Fig. 2b). Subgroup analyses showed that LDL-C concentration decreased significantly in patients with hypertension (4 studies; WMD = − 4.60 mg/dl, 95% CI − 8.26, − 0.94; P = 0.014; I2 = 11.7%; P-heterogeneity = 0.33). Other potential sources of heterogeneity are reported in Additional file 4.
HDL-C
Among the included studies, nine investigations [19,20,21, 23, 34, 36, 37, 42, 43] with 616 participants assessed the effect of OLE on HDL-C concentrations and reported no significant related changes (WMD = 0.38 mg/dl, 95% CI − 1.08, 1.83; P = 0.61; I2 = 54.2%; P-heterogeneity = 0.03) (Fig. 2c). According to the subgroup analyses, the levels of HDL-C decreased significantly in male participants following OLE supplementation (2 studies; WMD = − 1.24 mg/dl, 95% CI − 2.42, − 0.07; P = 0.04; I2 = 0.0%; P-heterogeneity = 0.79). Additional file 4 shows further potential sources of heterogeneity.
TG
According to the pooled analysis of seven studies (539 participants) [20, 23, 34, 36, 37, 42, 43], OLE supplementation resulted in a significant decrease in TG levels with a moderate between-study heterogeneity (WMD = -9.51 mg/dl, 95% CI − 17.83, − 1.18; P = 0.025; I2 = 68.7%; P-heterogeneity = 0.004) (Fig. 2d). Subgroup analyses showed that OLE supplementation significantly decreased TG levels in participants with a normal body weight (4 studies, WMD = − 9.21 mg/dl, 95% CI − 18.14, − 0.29; P = 0.04; I2 = 0.0%; P-heterogeneity = 0.73), and participants with hypertension (3 studies, WMD = − 14.32 mg/dl, 95% CI − 19.36, − 9.28; P < 0.001; I2 = 20.2%; P-heterogeneity = 0.28) (Additional file 4).
Glucose homeostasis
In total, six RCTs [19, 23, 34,35,36, 43], with a total of 442 participants, examined the effect of OLE supplementation on FBS levels, and according to the pooled analysis, there was an insignificant reduction in blood FBS concentration (WMD = − 1.29 mg/dl, 95% CI − 2.70, 0.13; P = 0.07; I2 = 0.0%; P-heterogeneity = 0.43) (Fig. 3a). Based on the subgroup analyses, FBS levels decreased significantly after OLE supplementation in participants without dyslipidemia (4 studies; WMD = -1.73 mg/dl, 95% CI − 3.33, − 0.13; P = 0.03; I2 = 0.0%; P-heterogeneity = 0.47) (Additional file 4).
Moreover, pooling effect sizes from three RCTs (175 participants) [23, 35, 43, 44] showed no significant effect of OLE supplementation on HbA1c (Fig. 3b) (WMD = − 0.03%, 95% CI − 0.22, 0.16; P = 0.77; I2 = 78.2%; P-heterogeneity = 0.01), HOMA-IR (Fig. 3c) (3 studies, n = 221; WMD = − 0.14, 95% CI − 0.51, 0.0; P = 0.47; I2 = 53.4%; P-heterogeneity = 0.12), and blood insulin levels (Fig. 3d) (4 studies, n = 320 participants; WMD = − 0.93 μU/L, 95% CI − 2.59, 0.73; P = 0.27; I2 = 54.4%; P-heterogeneity = 0.09).
Blood pressure
SBP
Six studies [20, 21, 23, 24, 37, 43] examined the effect of OLE supplementation on the SBP measure (n = 372 participants). One study was excluded from the final analyses, since the control group received an anti-hypertension treatment [36]. The pooled effect sizes showed a significant reduction in SBP after OLE supplementation (WMD = − 3.86 mmHg, 95% CI − 6.44, − 1.28; P = 0.003; I2 = 19.9%; P-heterogeneity = 0.28) (Fig. 4a). Subgroup analyses also revealed that SBP was reduced significantly following OLE supplementation in participants with a normal lipid profile (4 studies; WMD = − 4.47 mmHg, 95% CI − 7.39, − 1.56; P = 0.003; I2 = 22.2%; P-heterogeneity = 0.27), individuals with normal body weight (3 studies; WMD = − 7.05 mmHg, 95% CI − 10.94, − 3.16; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.64), and patients with hypertension (4 studies; WMD = − 4.81 mmHg, 95% CI − 7.27, − 2.35; P < 0.001; I2 = 0.3%; P-heterogeneity = 0.39) (Additional file 4).
DBP
Pooled analyses of six studies [20, 21, 23, 24, 37, 43] (n = 372 participants) showed that OLE supplementation did not have any significant effect on DBP (WMD = − 1.18 mmHg, 95% CI − 3.09, 0.72; P = 0.22; I2 = 47.5%; P-heterogeneity = 0.09) (Fig. 4b). However, based on the subgroup analyses, a significant decrease in DBP was observed in participants with hypertension (4 studies; WMD = − 2.45 mmHg, 95% CI − 4.13, − 0.76; P = 0.004; I2 = 0.0%; P-heterogeneity = 0.84). Other possible sources of between-study heterogeneity are shown in Additional file 4.
Liver and kidney variables
Liver enzymes
According to the pooled analyses, no significant changes with no evidence of heterogeneity were observed in ALP (Additional file 5) [3 studies [35, 42, 43], n = 184; WMD = -0.41 μU/L, 95% CI − 6.06, 5.23; P = 0.89; I2 = 0.0%; P-heterogeneity = 0.47], AST (Additional file 5) [3 studies [35, 36, 43], n = 315; WMD = 0.22 μU/L, 95% CI − 1.63, 2.06; P = 0.47; I2 = 0.0%; P-heterogeneity = 0.68], and ALT levels (Additional file 5) [3 studies [35, 36, 43], n = 315; WMD = 0.33 μU/L, 95% CI − 0.96, 1.62; P = 0.62; I2 = 0.0%; P-heterogeneity = 0.763], after OLE supplementation. We were unable to perform subgroup analyses due to the limited number of studies.
Creatinine
Pooling data from three eligible studies [20, 23, 42] showed no significant related change in creatinine levels and no evidence of heterogeneity between studies (n = 285; WMD = − 0.03 mg/dl, 95% CI − 0.09, 0.03; P = 0.35; I2 = 0.0%; P-heterogeneity = 0.45) (Additional file 5).
Inflammatory markers
Compared to the control group, OLE supplementation had no significant effect on any of the inflammatory markers, including hs-CRP (Additional file 5) (3 studies, n = 238; WMD = 0.24 mg/dl, 95% CI − 0.20, 0.68; P = 0.28; I2 = 63.9%; P-heterogeneity = 0.06), IL-6 (Additional file 5) (4 studies, n = 220; WMD = 0.02 pg/ml, 95% CI − 0.28, 0.33; P = 0.88; I2 = 58.1%; P-heterogeneity = 0.07), IL-8 (Additional file 5) (3 studies, n = 188; WMD = -0.36 pg/ml, 95% CI − 0.99, 0.27; P = 0.26; I2 = 82.7%; P-heterogeneity = 0.003), TNF-α (Additional file 5) (3 studies, n = 186; WMD = − 0.31 pg/ml, 95% CI − 0.93, 0.32; P = 0.34; I2 = 75.1%; P-heterogeneity = 0.02), and LDL-ox (Additional file 5) (3 studies, n = 267; WMD = − 2.01 pg/ml, 95% CI − 5.78, 1.77; P = 0.30; I2 = 0.0%; P-heterogeneity = 0.47). There was some evidence of heterogeneity; however, the sources of heterogeneity remained unknown due to the low number of studies.
Sensitivity analysis and publication bias
A leave-one-out sensitivity analysis was performed to identify the influential studies. However, effect estimates remained stable for all outcomes. We also conducted the sensitivity analysis excluding studies with high risk of bias (studies with less quality), and the results remained unchanged (Additional file 6).
Publication bias was not assessed, since the number of included studies in each outcome was less than 10.
Discussion
This systematic review and meta-analysis of twelve RCTs indicated that OLE supplementation significantly decreased TG and SBP levels. The main results of subgroup analyses revealed that OLE may improve lipid profile and blood pressure more effectively in participants with hypertension and normal body weight.
A comprehensive pooling data demonstrated that BP-lowering treatments are associated with a lower risk for death and CVD events, especially when baseline SBP is 140 mmHg or higher [45]. Another large-scale meta-analysis found that a reduction of 5-mmHg in SBP was associated with a decreased risk of major CVD events by 10%, irrespective of previous diagnoses of CVD [46]. In accordance with a previous meta-analysis [22], we observed a 4.81 mmHg reduction in SBP following OLE supplementation in patients with hypertension, suggesting that OLE supplementation may be useful as an adjunct therapy in these patients. Based on the subgroup analyses, the conclusion that patients with hypertension would benefit more from OLE may also suggest that there would be a common mechanism through which OLE exert its beneficial effects on blood pressure as well as the other common risk factors for CVD.
The present study indicated that OLE supplementation only has short-term positive effects on blood pressure and lipid profiles, which may be attributed to the active constituents in OLE [47]. In particular, oleuropein and oleacein, are the major components of OLE, and are reported to possess acute anti-hypertensive activity through inhibition of angiotensin-converting enzyme (ACE) [48]. Furthermore, previous research showed that oleuropein has a short-term vasodilatory effect [49], as well as a direct calcium antagonistic action [50]. On the other hand, oleuropein has been identified as a ligand of the peroxisome proliferator-activated receptor-alpha (PPAR-α) [51]. Studies have found that PPAR-α agonists can effectively modulate lipid profile, especially TG, and these agonists are currently being used as important targets for the treatment of insulin resistance and dyslipidemia [52]. Furthermore, PPAR-α activation favorably downregulates the expression of proinflammatory genes and affects serum lipid levels. This may also justify our results regarding an insignificant reduction in TG levels when the analysis was restricted to obese participants; indeed, as previously stated, the beneficial effects of OLE on lipid profile have been observed in the short term, and because the adipose tissue increases inflammation, longer durations and higher doses of OLE supplementation may be needed to detect a significant difference. On the other hand, obese participants predominantly need lifestyle modifications and specific medications, rather than taking adjunct therapies such as OLE supplements [21]. Moreover, it has been shown that oleuropein decreases the activity of hydroxymethylglutaryl-CoA reductase, leading to a reduction in cholesterol synthesis in hepatocytes of rat [15]. However, surprisingly, we found that HDL-C levels decreased in men after OLE supplementation. It is of note that only two studies were included in this subgroup, which enrolled obese participants, and thus, a potential adverse effect in obese men should be monitored.
Our analyses failed to detect any significant changes in glucose hemostasis variables, as well as the inflammatory and liver/kidney markers. The most likely reason might be related to the limited number of included studies. Moreover, the baseline measures of these variables were within normal ranges across the included studies, except for two investigations which were conducted among patients with diabetes and pre-diabetes [19, 44]. Besides, the variable baseline characteristics of participants could be a further contributory factor to the null results, including physical activity, alcohol consumption, smoking, dietary fat intake and etc., which were not reported in all of the included studies [53]. The quality of evidence was shown to be low and very low for these outcomes, and more high-quality studies may change the results in the future.
In the present study, we used a standard methodology to perform a systematic review and meta-analysis to answer the question of whether OLE supplementation has an effect on cardiometabolic factors. To do this, we designed a comprehensive search strategy without considering the outcomes of measures to ensure that we found all the relevant studies. Although a previous meta-analysis tried to answer this question [22], serious limitations were present, including missing two eligible RCTs [23, 24] and splitting analyses based on the dose of OLE supplementation. In our study, various subgroups and sensitivity analyses were performed to determine the sources of heterogeneity, and we examined the methodological quality and the quality of evidence using standard tools.
Notwithstanding our methodological rigor, the majority of the assessed outcomes had low quality of evidence and nearly half of the included studies had either a moderate or high risk of bias. This might also be the main reason for the observed heterogeneous findings in the subgroup analyses. Moreover, evidence showed that the GRADE evaluation relies on metrics for judging heterogeneity and incoherence and may lack quantitative evaluation criteria [54]. In addition, we were unable to perform subgroup analyses for some of our interested outcomes including liver, kidney, and inflammatory markers, as well as insulin levels, due to the limited number of studies. This limitation also prevented us from examining the publication bias. Moreover, some of the included studies did not report the pure concentration of oleuropein, a bioactive component of OLE, which further limited us to perform subgroup analyses based on the concentration of oleuropein and determine which precise dose had the most favorable effects on the common risk factors associated with CVD. Additionally, although we considered some confounding factors such as duration of the intervention, baseline BMI, and the health status of participants in the subgroup analyses, many confounding factors, including dietary intakes, physical activity, alcohol consumption, and smoking were not taken into account in the final analyses.
Conclusion
The present systematic review and meta-analysis revealed that supplementation with OLE had a significant beneficial effect on TG and SBP in adults. Furthermore, we found that supplementation with OLE had more profitable effects on the improvement of TG, SBP, DBP, TC, and LDL-C measures among participants with hypertension and individuals with normal body weight. However, no meaningful changes were found in glucose hemostasis, liver and kidney variables, or inflammatory markers. Stronger RCT investigations, assessing different doses and durations of OLE, are required to better elucidate the effects of OLE supplementation.
Availability of data and materials
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
WHO. World Health Organization (WHO): noncommunicable diseases 2021. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=The%20main%20types%20of%20NCD,disease%20and%20asthma)%20and%20diabetes. Accessed Apr 2021.
Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–14.
Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020;8: 574111.
Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm Des. 2019;25:4063–84.
Donia T, Khamis A. Management of oxidative stress and inflammation in cardiovascular diseases: mechanisms and challenges. Environ Sci Pollut Res. 2021;28:34121–53.
Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, et al. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr. 2017;57:3218–32.
Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50.
Grosso G, Micek A, Godos J, Pajak A, Sciacca S, Galvano F, et al. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: systematic review and dose-response meta-analysis. Am J Epidemiol. 2017;185:1304–16.
Ma ZF, Zhang H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: a mini-review. Antioxidants. 2017. https://doi.org/10.3390/antiox6030071.
Gaforio JJ, Visioli F, Alarcón-de-la-Lastra C, Castañer O, Delgado-Rodríguez M, Fitó M, et al. Virgin olive oil and health: summary of the III international conference on virgin olive oil and health consensus report, JAEN (Spain) 2018. Nutrients. 2019. https://doi.org/10.3390/nu11092039.
El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67:632–8.
Silva S, Gomes L, Leitão F, Coelho AV, Boas LV. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci Technol Int. 2006;12:385–95.
Gariboldi P, Jommi G, Verotta L. Secoiridoids from Olea europaea. Phytochemistry. 1986;25:865–9.
Hadrich F, Mahmoudi A, Bouallagui Z, Feki I, Isoda H, Feve B, et al. Evaluation of hypocholesterolemic effect of oleuropein in cholesterol-fed rats. Chem Biol Interact. 2016;252:54–60.
Priore P, Siculella L, Gnoni GV. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J Nutr Biochem. 2014;25:683–91.
Lepore SM, Morittu VM, Celano M, Trimboli F, Oliverio M, Procopio A, et al. Oral administration of oleuropein and its semisynthetic peracetylated derivative prevents hepatic steatosis, hyperinsulinemia, and weight gain in mice fed with high fat cafeteria diet. Int J Endocrinol. 2015;2015: 431453.
Lockyer S, Corona G, Yaqoob P, Spencer JP, Rowland I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: a randomised, double-blind, placebo-controlled, cross-over trial. Br J Nutr. 2015;114:75–83.
Murotomi K, Umeno A, Yasunaga M, Shichiri M, Ishida N, Koike T, et al. Oleuropein-rich diet attenuates hyperglycemia and impaired glucose tolerance in type 2 diabetes model mouse. J Agric Food Chem. 2015;63:6715–22.
Araki R, Fujie K, Yuine N, Watabe Y, Nakata Y, Suzuki H, et al. Olive leaf tea is beneficial for lipid metabolism in adults with prediabetes: an exploratory randomized controlled trial. Nutr Res. 2019;67:60–6.
de Bock M, Derraik JG, Brennan CM, Biggs JB, Morgan PE, Hodgkinson SC, et al. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: a randomized, placebo-controlled, crossover trial. PloS ONE. 2013;8:e57622.
Perrinjaquet-Moccetti T, Busjahn A, Schmidlin C, Schmidt A, Bradl B, Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother Res. 2008;22:1239–42.
Ismail MA, Norhayati MN, Mohamad N. Olive leaf extract effect on cardiometabolic profile among adults with prehypertension and hypertension: a systematic review and meta-analysis. PeerJ. 2021;9: e11173.
Lockyer S, Rowland I, Spencer JPE, Yaqoob P, Stonehouse W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomised controlled trial. Eur J Nutr. 2017;56:1421–32.
Saberi M, Kazemisaleh D, Bolurian V. Effect of olive leaf on mild to moderate hypertension resistant to normal treatments. J Med Plants. 2008;7:52–9.
Al-Azzawie HF, Alhamdani MS. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006;78:1371–7.
Rafferty EP, Wylie AR, Elliott CT, Chevallier OP, Grieve DJ, Green BD. In vitro and in vivo effects of natural putative secretagogues of glucagon-like peptide-1 (GLP-1). Sci Pharm. 2011;79:615–21.
Jemai H, Fki I, Bouaziz M, Bouallagui Z, El Feki A, Isoda H, et al. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J Agric Food Chem. 2008;56:2630–6.
Pirozzi C, Lama A, Simeoli R, Paciello O, Pagano TB, Mollica MP, et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J Nutr Biochem. 2016;30:108–15.
Tabernero M, Sarriá B, Largo C, Martínez-López S, Madrona A, Espartero JL, et al. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014;5:1556–63.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from https://training.cochrane.org/handbook.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928.
Araki R, Fujie K, Nakata Y, Suzuki H, Matsui K, Uematsu K, et al. An exploratory study of the effects of continuous intake of olive leaf tea on physique and glucose and lipid metabolism. Nippon Eiyo Shokuryo Gakkaishi. 2018;71:121–31.
Javadi H, Yaghoobzadeh H, Esfahani Z, Reza Memarzadeh M, Mehdi MS. Effects of olive leaf extract on metabolic response, liver and kidney functions and inflammatory biomarkers in hypertensive patients. Pak J Biol Sci. 2019;22:342–8.
Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, Perrinjaquet-Moccetti T, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251–8.
Yaghoobzadeh H, Mehravar S, Javadi H, Memarzadeh MR, Mirhashemi SM. Determining cardiometabolic and antioxidant effects of olive leaf extract in patients with essential hypertension. J Qazvin Univ Med Sci. 2020. https://doi.org/10.32598/JQUMS.23.5.372.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629.
Filip R, Possemiers S, Heyerick A, Pinheiro I, Raszewski G, Davicco MJ, et al. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. Evid Based Complement and Altern Med. 2015;19:77–86.
Stevens Y, Winkens B, Jonkers D, Masclee A. The effect of olive leaf extract on cardiovascular health markers: a randomized placebo-controlled clinical trial. Eur J Nutr. 2021;60:2111–20.
Wainstein J, Ganz T, Boaz M, Bar Dayan Y, Dolev E, Kerem Z, Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food. 2012. https://doi.org/10.1089/jmf.2011.0243.
Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28–36.
Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Ramakrishnan R, Pinho-Gomes AC, Woodward M, Adler A, Agodoa L, Algra A. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–36.
Nediani C, Ruzzolini J, Romani A, Calorini L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants. 2019. https://doi.org/10.3390/antiox8120578.
Scheffler A, Rauwald HW, Kampa B, Mann U, Mohr FW, Dhein S. Olea europaea leaf extract exerts L-type Ca(2+) channel antagonistic effects. J Ethnopharmacol. 2008;120:233–40.
Zarzuelo A, Duarte J, Jiménez J, González M, Utrilla MP. Vasodilator effect of olive leaf. Planta Med. 1991;57:417–9.
Hansen K, Adsersen A, Christensen SB, Jensen SR, Nyman U, Smitt UW. Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicine. 1996;2:319–25.
Malliou F, Andreadou I, Gonzalez FJ, Lazou A, Xepapadaki E, Vallianou I, et al. The olive constituent oleuropein, as a PPARα agonist, markedly reduces serum triglycerides. J Nutr Biochem. 2018;59:17–28.
Pirat C, Farce A, Lebègue N, Renault N, Furman C, Millet R, et al. Targeting peroxisome proliferator-activated receptors (PPARs): development of modulators. J Med Chem. 2012;55:4027–61.
Kim HJ, Park HA, Cho YG, Kang JH, Kim KW, Kang JH, et al. Gender difference in the level of HDL cholesterol in Korean adults. Korean J Fam Med. 2011;32:173–81.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17: e1003082.
Funding
This work was supported by the Shahid Sadoughi University of Medical Sciences. The funding body has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
ER, SA, MM: conducted the literature search and performed data extraction and quality assessment; ER, SA: wrote the paper; CC, SS: provided critical review; SS: conceived the study and performed statistical analysis and had primary responsibility for final content; All authors provided feedback for the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Search strategy and table of excluded studies.
Additional file 2:
References of excluded studies.
Additional file 3:
Study quality and risk of bias assessment using Cochrane collaboration tool.
Additional file 4:
Meta-analysis showing the effect of OLE supplementation on total cholesterol based on several subgroups.
Additional file 5:
Forest plot of randomized controlled trials illustrating weighted mean differences in liver enzymes and creatinine.
Additional file 6:
Meta-analysis showing the effect of OLE supplementation on cardiovascular risk factors including studies with good quality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Razmpoosh, E., Abdollahi, S., Mousavirad, M. et al. The effects of olive leaf extract on cardiovascular risk factors in the general adult population: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr 14, 151 (2022). https://doi.org/10.1186/s13098-022-00920-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00920-y