Abstract
Background
Previous studies suggest intestinal dysbiosis is associated with metabolic diseases. However, the causal relationship between them is not fully elucidated. Gut microbiota evaluation of patients with congenital generalized lipodystrophy (CGL), a disease characterized by the absence of subcutaneous adipose tissue, insulin resistance, and diabetes since the first years of life, could provide insights into these relationships.
Methods
A cross-sectional study was conducted with patients with CGL (n = 17) and healthy individuals (n = 17). The gut microbiome study was performed by sequencing the 16S rRNA gene through High-Throughput Sequencing (BiomeHub Biotechnologies, Brazil).
Results
The median age was 20.0 years old, and 64.7% were female. There was no difference between groups in pubertal stage, BMI, ethnicity, origin (rural or urban), delivery, breastfeeding, caloric intake, macronutrient, or fiber consumption. Lipodystrophic patients presented a lower alpha diversity (Richness index: 54.0 versus 67.5; p = 0.008). No differences were observed in the diversity parameters when analyzing the presence of diabetes, its complications, or the CGL subtype.
Conclusion
In this study, we demonstrate for the first time a reduced gut microbiota diversity in individuals with CGL. Dysbiosis was present despite dietary treatment and was also observed in young patients. Our findings allow us to speculate that the loss of intestinal microbiota diversity may be due to metabolic abnormalities present since the first years of life in CGL. Longitudinal studies are needed to confirm these findings, clarifying the possible causal link between dysbiosis and insulin resistance in humans.
Similar content being viewed by others
Background
In recent years, studying the human microbiota and its relationship with health and disease processes has aroused the scientific community’s interest [1,2,3]. Numerous studies have suggested that intestinal dysbiosis is associated with metabolic diseases, including insulin resistance, diabetes, and obesity [4,5,6]. Dysbiosis is characterized by loss of microbiota diversity and alteration of its composition, promoting changes in the use and metabolism of diet components by bacteria, with impairment of the mechanisms of protection against invading pathogens [7, 8]. Besides, dysbiosis is associated with endotoxemia and chronic subclinical inflammation, among other mechanisms, which may culminate in predisposition to the development of insulin resistance and obesity [9, 10]. However, the causal relationship between intestinal dysbiosis and metabolic disorders is not fully elucidated despite this evidence.
Although some experimental studies with animal models have demonstrated the existence of biological plausibility for a cause-effect relationship [5, 11], it is not known whether the changes in the intestinal microbiota described in observational studies carried out in humans are due to the metabolic changes themselves or if the changes in the intestinal microbiota precede the appearance of such metabolic diseases. Several factors can influence the human microbiota, including age, genetic predisposition, geographic aspects, birth conditions, dietary habits, physical activity, and drug use [1]. These variables can act as co-founders in the studies available, and these relationships are not yet well established.
Studies on biological models of diseases that are associated with metabolic disorders of monogenic diseases could contribute to a better understanding of the effects of such changes on the intestinal microbiota. In this context, the study of patients with congenital generalized lipodystrophy (CGL), a hereditary disease characterized by the absence of subcutaneous adipose tissue, hypoleptinemia, severe insulin resistance, and diabetes with micro and macrovascular complications [12,13,14,15], could provide insights into these relationships. Thus, this study aims to describe the intestinal microbiota of patients with CGL, associating it with the metabolic disorders classically found in this condition.
Methods
Study design and patient enrollment
A cross-sectional study was carried out by the Brazilian Group for the Study of Inherited and Acquired Lipodystrophies (BRAZLIPO), Clinical Research Unit, Walter Cantídio University Hospital, Federal University of Ceará (UFC/EBSERH), from October 2019 to March 2020. Patients from the same state in Brazil, Ceará (CE), diagnosed with CGL followed by Federal University of Ceará (n = 17), aged between 1 and 40 years old, were included in this investigation. For the diagnosis of lipodystrophy, clinical and molecular criteria were used. The presence of generalized lipodystrophy since birth or early childhood stages was the main criteria for the clinical diagnosis of lipodystrophy. Other characteristics evaluated were acromegalic aspect, apparent muscle hypertrophy, prominent superficial veins (phlebomegaly), hepatomegaly, hypertriglyceridemia, and insulin resistance [16]. For molecular diagnosis, pathogenic variants in AGPAT2 or BSCL2 genes were considered for classification in the subtypes of CGL type 1 and CGL type 2, respectively [16]. The description of the main clinical and molecular features of the patients with CGL is provided in Table 1.
For the comparative group, 17 individuals matched for age and sex, non-diabetic, residing in the same state in Brazil, and without pathogenic variants in the AGPAT2 or BSCL2 genes were evaluated. Participants who used antibiotics two months before the start of this study with gastrointestinal diseases, liver failure, undergoing bariatric surgery, or using drugs with effects on the immune system were not included in any of the groups. For composition of the comparative group, we asked the volunteers with lipodystrophy and/or their guardians to indicate people from their community. After this indication, the researchers evaluated whether they met the study's inclusion criteria.
Study protocol
Clinical evaluation
Participants were submitted to medical and nutritional interviews and physical examinations. The variables evaluated in our analysis were sex, age, birth conditions, breastfeeding duration, personal and familial history, diagnosis of diabetes, hypertension, dyslipidemia, cardiovascular disease, macrovascular and microvascular complications, use of drugs, dietary habits, anthropometric measures, and blood pressure measurement.
Anthropometric measurements and arterial blood pressure were obtained following the previous recommendation [17]. Tanner’s pubertal classification was used to determine the pubertal stage [18]. Diabetes Mellitus (DM) was diagnosed based on the American Diabetes Association criteria [19]. Dyslipidemia was defined according to the recommendations of the National Cholesterol Education Program Adult Treatment Panel [20] regarding age and sex.
Nutritional evaluation
Food intake was assessed using 24-h food recalls collected by a single trained interviewer at two different times with a 15-day interval, according to the Automated Multiple Pass Method (AMPM) [21]. The first interview was in person, and the following interviews were by telephone. The results of the recall analysis were grouped based on the average and were evaluated using the following variables: total caloric value, percentage distribution of macronutrients, including carbohydrates, proteins, saturated fats, polyunsaturated, total cholesterol, and fibers.
Laboratory analysis
All blood and urine samples were collected after 10-h fasting. The blood samples were centrifuged at 3000 rpm for 10 min. Subsequently, the serum samples were stored at − 80 °C for further analysis. A biochemical evaluation was performed by determining glycemia, total cholesterol, HDL cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, and urine albumin-creatinine ratio using an enzymatic colorimetric method, according to the manufacturer’s instruction (HITACHI®–Roche). Glycated hemoglobin (HbA1c) was dosed by high-performance liquid chromatography (PREMIER®–Trinity Biotech). Insulin was determined by electrochemiluminescence (HITACHI®–Roche). Leptin was dosed using an enzyme immunoassay (AIKA®–Diasorin; REF: CAN-L-4260; analytical sensitivity: 0.5 ng/mL; variation coefficient intra-assay: 3.7–5.0%).
Molecular analysis of lipodystrophy
Genomic DNA was extracted from peripheral blood samples using a standard protocol. The entire coding region and the exon–intron boundaries of the AGPAT2 and BSCL2 genes were amplified by polymerase chain reaction using intronic oligonucleotide primer pairs (Additional file 1) using a 9700 thermal cycler (Thermofisher). The amplified products were purified using the QIAquick PCR Purification Kit (QIAGEN), followed by a sequencing reaction with the ABI PrismTM BigDye Terminator Kit (Applied Biosystems, Foster City, California, USA). The products of this reaction were subjected to electrophoresis in an ABI Prism 3100 Genetic Analyzer automatic DNA sequencer (Applied Biosystems, Foster City, California, USA). The obtained sequences were aligned with the AGPAT2 and BSCL2 reference sequences NG_008090.1 and NG_008461.1, respectively, using the UGENE tool to identify the mutational profile of the participants and their families. The sequence variants found were described according to the variant nomenclature proposed by the Human Genome Variation Society using the transcript reference sequences NM_006412.4 and NM_001122955.3 for the AGPAT2 and BSCL2, respectively.
Gut microbiome study
The gut microbiome study was carried out by GeneOne, DASA laboratory (https://geneone.com.br/). Participants were instructed to collect stool samples at their own homes. The samples were collected on two occasions, with an interval of 30 days. The first collection was made during medical care and laboratory tests. Stool samples were placed in a bottle with an appropriate preservative solution [22]. DNA was extracted with QIAGEN DNeasy PowerSoil Kit. Then, amplification of the V3-V4 region from the 16S rRNA gene, with primers 341F (CCTACGGGRSGCAGCAG) [23] and 806R (GGACTACHVGGGTWTCTAAT), was performed [24]. Preparation of the libraries from the PCR product was done with a proprietary protocol (BiomeHub Biotechnologies, Brazil). The libraries were sequenced using the MiSeq Sequencing System (Illumina Inc., USA) and the V2 kit, with 300 cycles and single-end sequencing. The sequences were analyzed using a proprietary pipeline previously described (BiomeHub Biotechnologies, Brazil) [25].
All DNA sequences were evaluated by quality control metrics, using the sum of the probabilities of error of their bases as a base, allowing at most 1% of accumulated error. Subsequently, the DNA sequences corresponding to the Illumina technology adapters were removed. Reads are then analyzed with the Deblur package v.1.1.0 [26] to remove possible erroneous reads, and identical sequences are grouped into oligotypes (clusters with 100% identity). Sequencing clustering with 100% identity provides a higher resolution for the amplicon sequencing variants. Next, VSEARCH 2.13.6 [27] was used to remove chimeric amplicons. We implemented an additional filter to remove amplicon sequence variants (ASVs) below the frequency cutoff of 0.2% in the final sample counts. The remaining ASVs in the samples are used for taxonomic assignment with the BLAST tool [28] against a reference genome database (encoderef16s_rev6, BiomeHub, SC, Brazil). This database is constructed with complete and draft bacterial genomes, focused on relevant bacteria for human microbiota, obtained from NCBI. It is composed of 11,750 sequences, including 1,843 different bacterial taxonomies.
Taxonomies are assigned to each ASVs using the lowest common ancestor (LCA) algorithm. If more than one reference can be assigned to the same ASV with equivalent similarity and coverage metrics (e.g., two distinct reference species mapped to ASV “A” with 100% identity and 100% coverage), the taxonomic assignment algorithm leads the taxonomy to the lowest level of possible unambiguous resolution (genus, family, order, class, phylum, or kingdom), according to similarity thresholds previously established [29].
Alpha diversity of the samples was measured by observed species, Shannon and Simpson index, and relative dominance [30]. The observed species index measures the number of species per sample, defined as “richness.” The relationship between phylum Bacteroidetes and Firmicutes, and the presence of bacteria with pro- and anti-inflammatory profiles were also analyzed.
For the characterization of dysbiosis, a decrease in alpha diversity was considered. However, the presence of bacteria with an anti-inflammatory profile was also considered (Akkermansia muciniphila, Bifidobacterium spp., Eubacterium rectale, Feacalibacterium prausnitzii, Lactobacillus spp., Prevotella copri, Roseburia spp., Veilonella spp., Odoribacter splanchnicus, Coprococcus, Bacteroides cellulosilyticus, Blautia spp.), as well as the presence of bacteria with pro-inflammatory activity (Escherichia coli, Klebsiella pneumoniae, Parasutterella spp., Fusobacteriaceae spp., Enterobacter hormaechei, Enterobacter asburiae, Bacteroides caccae, Sutterella wadsworthensis, Bilophila wadsworthia, Ruminococcus gnavus, Fusobacteria spp., Arcobacter butzleri, Bacteroides ovatus, Acinetobacter lwoffii, Clostridium perfringens, Clostridioides difficile, Proteobacteria, Bacteroides vulgatus, Haemophilus parainfluenzae, Enterobacter cloacae, Acinetobacter spp.).
Statistical analysis
Data were analyzed using the JAMOVI version 1.6.9 for macOS (Sydney, Australia). Continuous variables were described using the median (25th; 75th), and categorical variables using relative and absolute frequency. The Shapiro–Wilk test evaluated normality. The student’s t-test was used for continuous variables with a normal distribution. Mann–Whitney test was used for continuous variables with a non-parametric distribution. Association between categorical variables was performed using the Chi-square and Fisher’s exact test. Spearman's rank correlation coefficient (r) was calculated for correlation analysis. The level of statistical significance adopted for all tests was 5% (p < 0.05).
Results
The median age of patients was 20 years old (9.0; 31.0), 64.7% (n = 11) were female and 35.3% (n = 6) male. There was no difference in age, pubertal stage, ethnicity, or origin (rural or urban) between patients with CGL and healthy individuals. We did not observe differences between the groups regarding birth conditions (parturition type and time, hospitalization, or use of antibiotics in the first 30 days of life) and previous breastfeeding history. Regarding nutritional assessment, the groups did not differ in body mass index (BMI), caloric intake, and macronutrient and fiber intake adequacy (Table 2). Among patients with lipodystrophy, 12 (70%) were diagnosed with diabetes, and 11 (64.7%) were treated with metformin.
The patients with lipodystrophy presented less diversity, measured by the richness index (54.0 versus 67.5; p = 0.008) (Table 3). Among them, four patients (23.5%) had characteristics compatible with intestinal dysbiosis versus only one subject (5.9%) in the group of healthy individuals (p = 0.335).
A subanalysis of adult patient data showed that the richness index (54 vs. 70; p = 0.024) was lower in patients with CGL compared to healthy individuals.
There was no difference in the anti- and pro-inflammatory bacteria profiles between groups. We did not observe differences between samples collected at baseline and after 30 days in CGL and healthy groups (Additional file 2). The abundance composition of each bacterium data is presented as Additional file 3.
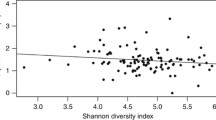
Patients with CGL had higher values of glycated hemoglobin, insulin, triglycerides, and urine albumin-creatinine ratio and lower values of leptin and HDL-c (Additional file 4). There was a positive correlation between leptin levels and Shannon index (r = 0,678; p < 0.001) (Fig. 1). No differences in diversity parameters were observed when analyzing the gender, age, CGL subtype, the presence of diabetes, and the use of metformin or insulin (Additional file 5).
Correlation matrix between leptin levels and gut microbiota diversity parameters in patients with congenital generalized lipodystrophy and healthy individuals (n = 34). There was a positive correlation between leptin levels and Shannon index (r = 0.678; p < 0.001). Spearman's rank correlation coefficient (r) was calculated for correlation analysis. Leptin was dosed using an enzyme immunoassay (AIKA®–Diasorin; REF: CAN-L-4260) and expressed in ng/mL
Discussion
In this study, we evaluated the gut microbiota of patients with CGL and demonstrated a reduction in bacterial diversity in individuals with severe hypoleptinemia and insulin resistance since childhood. Our data is the first to assess the presence of dysbiosis in CGL patients.
Metabolic alterations in CGL are genetically determined and result from mutations in specific genes related to adipogenesis [14]. Our patients present the two most common CGL subtypes, type 1 and type 2 CGL. Type 1 CGL is associated with AGPAT2 mutations. AGPAT2 gene is involved in the biosynthesis of glycerophospholipids and triglycerides. Type 2 CGL is caused by BSCL2 mutations and is considered a more severe form. BSCL2 gene is involved in the maturation of preadipocytes and adipocytes [12, 13].
The development of insulin resistance and diabetes in these young patients is independent of traditional factors related to the development of metabolic diseases, including diet, physical activity, and obesity. Patients with CGL have an absence of subcutaneous adipose tissue, which is associated with insulin resistance and diabetes mellitus in childhood or adolescence due to severe hypoleptinemia [12,13,14,15].
Leptin is a hormone predominantly produced in the adipose tissue and plays a central role in regulating energy metabolism and food intake [31]. In addition to its synthesis by adipocytes, leptin can be produced in smaller amounts by enteroendocrine cells (EEC) of the gastrointestinal (GI) tract [32, 33]. Leptin synthesis by adipose tissue can be regulated by short-chain fatty acids (SCFA), produced through the metabolization of complex carbohydrates by intestinal bacteria. Furthermore, experimental studies have shown that SCFA benefits insulin signaling, improving its peripheral tissue sensitivity [34]. Besides, gastrointestinal microbiota also influences leptin production by EECs [35,36,37].
It has been demonstrated that patients with CGL present hyperphagia due to reduced leptin [16]. Intestinal microbiota also influences the control of hunger/satiety by producing neurotransmitters such as serotonin, dopamine, and γ-aminobutyric acid (GABA) that can act locally in the enteric nervous system or transmit signals to the central nervous system through vagal afferent neurons. In addition, SCFA, mainly butyrate, acetate, and propionate, can bind to G protein-coupled receptors (GPCR), specifically to GPR41 and GPR43, in EECs, stimulating the release of anorexigenic hormones, such as glucagon-like peptide (GLP-1) and peptide YY (PYY) [34, 38, 39]. The imbalance of the intestinal microbiota can predispose to low-grade chronic inflammation, causing vagal remodeling and changes in the control of hunger/satiety, increasing the food intake [40, 41]. It is interesting to note, however, that most patients with CGL in this study had a normal BMI, consumed a diet with adequate macronutrients and fiber, and still presented microbiota alterations like those observed in previous studies with obese patients [42,43,44]. Thus, our findings raise the question that the lower diversity of the intestinal microbiota observed in patients with CGL may result from the metabolic alterations themselves.
Leptin can modulate many essential functions in the GI tract, including motility, absorption, growth, and immunity [45]. Leptin receptors are abundant in the GI tract and are located in the afferent and efferent vagus nerve endings [46]. Leptin regulates gastric motility, delaying gastric emptying, and presents a complex effect on the motility of the small bowel [47, 48]. Besides, leptin modulates the absorption of macronutrients in the GI tract [49], stimulates gut mucosal cell proliferation, and inhibits apoptosis [45].
In addition, mice leptin-deficient ob/ob or leptin receptor (LepRb)-null db/db mice present hyperphagia, obesity, and alterations in the gut microbiota [50, 51]. However, it remains unclear whether compositional changes in the gut microbiota are due to hyperphagia or physiologic changes associated with obesity or from other leptin actions independent of food intake and adiposity. Furthermore, Duggal et al. identified a mutation in the leptin receptor is associated with Entamoeba histolytica infection in children, suggesting a role for leptin signaling in the gut epithelium in the host's defense against intestinal pathogens [52].
Gut antimicrobial peptides (AMPs) secreted by Paneth cells represent the central mechanism by which the host influences the gut microbiome [53]. AMPs not only defend against enteric pathogens but also have the capacity to alter the composition of commensal microbes [54]. Rajala et al. suggested that leptin action might modulate bacterial populations within the gut by controlling the expression of AMPs. Their data demonstrated a decreased mRNA expression of gut AMPs in leptin receptor (LepR)-deficient db/db mice, suggesting a potential role for LepRb signaling for AMP modulation, independent of food intake, in the host regulation of gut microbiota composition [55].
Our study demonstrated a positive correlation between leptin levels and the Shannon index, a well-known diversity index used in microecological studies. This alpha diversity index is a quantitative indicator of the number of bacteria present in a stool sample, whose value increases when the number of species and the evenness increases—the higher the Shannon index value, the higher the community diversity [56]. Our data, together with the evidence presented in the previous studies by Duggal et al. [52] and Rajala et al. [55], make us speculate that hypoleptinemia could promote changes in the intestinal microbiome in patients with CGL.
Moreover, diabetes and hyperglycemia are also associated with modifications in the gut microbiota [57]. Diabetes can cause gastrointestinal disturbances, mainly associated with microangiopathic complications, including neuropathy. Autonomic neuropathy is related to changes in intestinal motility, leading to reduced intestinal transit, bacterial overgrowth, and microbiota imbalance. Diabetic angiopathy secondary to chronic hyperglycemia may also be associated with intestinal ischemia and the development of diabetic gastroenteropathy [58]. Besides, in animal models, hyperglycemia, through its action on type-2 glucose transporters present in intestinal epithelial cells, can change the integrity of the intestinal barrier by modifying the composition of mucus and the function of tight junctions. This effect promotes increased mucosal permeability, leading to the "leaky gut" associated with bacterial translocation [59]. Although this phenomenon has been associated with dysbiosis, it also may be due to hyperglycemia per se. Thus, in diabetic patients, the profile of bacteria in the intestine may act synergistically with hyperglycemia in developing endotoxemia and systemic inflammation, worsening metabolic disorders.
Many CGL patients in our study were using metformin. It is described that metformin affects intestinal microbiota composition and increases some bacterial species, such as Lactobacillus spp and Akkermnsia mucipniphila [60,61,62,63]. Akkemansia muciniphila is a bacterium in the intestinal mucus with a critical barrier function and one of the most relevant producers of SCFAs [64]. Although the use of metformin has been associated with changes in the intestinal bacterial microbiota, we did not observe these findings in our patients, and a reduction of microbiota diversity in CGL patients was observed despite metformin use.
It is also important to consider that the lipodystrophy subtype could influence the microbiome. Patients with CGL have reduced adiponectin levels, especially in type 1 CGL [16]. Adiponectin is an adipokine with an anti-inflammatory function associated with metabolic disturbance [65]. In an experimental study with suckling rats, Grases-Pintó et al. recently demonstrated that adiponectin supplementation might influence microbiota composition [66]. In our study, we found intestinal dysbiosis just in patients with type 1 CGL. However, we did not demonstrate differences in diversity parameters between groups with type 1 and type 2 CGL. As we did not evaluate the adiponectin levels, we could not establish any association between this adipokine and the microbiota diversity parameters in our analysis. The lack of adiponectin measurement is a limitation of our study and should be further explored in future studies.
Also, it is interesting to discuss that impairment of the seipin protein in patients with type 2 CGL could lead to neuronal dysfunction, especially motor neuron disease [67]. Although these diseases can lead to different neurological manifestations, we did not find studies showing changes in intestinal motility related to seipinopathies. Besides, our patients had no clinical signs of motor neuron disease or other neurological signs of seipinopathies.
We did not find any difference in the proportion between Bacteroidetes and Firmicutes phyla in patients with CGL and healthy controls. Although previous experimental studies have shown an increase in the proportion of Firmicutes, which could predispose to the development of obesity and metabolic disease [68, 69], recent studies have questioned these findings [70,71,72]. The relative abundance of the Bacteroidetes and Firmicutes phyla is highly variable between subjects from the same population. Many factors could influence the composition of the gastrointestinal microbiota, making it difficult to associate the ratio between Bacteroidetes and Firmicutes phyla with determining health status. Currently, although the gut microbiota could contribute to the development of obesity, the evidence suggesting an association between obesity and alterations of the Firmicutes/Bacteroidetes ratio is still questionable [73].
Answering all these questions is not straightforward. Most studies on the assessment of the gut microbiota in humans are subject to numerous biases that act as confounding factors. Here, we tried to control for possible confounders of relevance, such as age, gender, geographic location, ethnicity, birth conditions, breastfeeding, physical activity, and diet. We believe the rigorous selection of the comparative group was a strong point of this research. The main limitation of our study is the sample size; however, considering the rarity of CGL and the number of patients evaluated, our data are relevant to the literature.
CGL is a rare disease, which makes it exceedingly difficult to have a large enough sample size within a particular age group. However, this is critical because the development of the microbiome changes rapidly early in life. Thus, the data were analyzed according to the age group to understand the connections between microbiome and metabolism better. We observed a reduction of alpha diversity even in the sub-analyses of adult patients, a subgroup with a more stable microbiome [1].
We use the term "dysbiosis" to characterize patients with reduced alpha diversity and altered profile of the pro- and anti-inflammatory bacteria [1]. However, we understand that there is too much variability in the definition of dysbiosis. We also emphasize that the main parameters to assess the healthy gut microbiota in this study were the alpha diversity indexes, which give less subjectivity to the analysis, reinforcing the results found.
Our data allow us to hypothesize some inferences about causality between gut microbiota and metabolic disease once we evaluate patients considered as biological models to study the absence of adipose tissue and leptin deficiency. It seems to us that there is a dual pathway in the modulation between the microbiota and metabolic disease. In our patients, a role of hypoleptinemia in the loss of gut microbiota diversity is possible once we observed dysbiosis in patients who still had no hyperglycemia or diabetes. Due to the small sample size and rarity of CGL, we believe the hypotheses generated here could be tested further in animal models or via comparisons to people with type 2 diabetes.
Still, it is interesting to make a parallel with obese patients in this context. Could the hyperleptinemia observed in obese patients modify the microbiota? Is there resistance to leptin in the receptors of the vagus nerve endings in the GI tract? Would this imply changes in intestinal motility and microbiota in obese patients? All these questions are relevant to understanding the relationship between obesity and microbiota. Studying the impact of leptin replacement in the treatment of CGL could establish better inferences about this relationship.
Lastly, it is necessary to consider the potential therapeutic effect of microbiota manipulation on metabolic disease management. Several drugs with prebiotic and probiotic action have been studied with variable effects on metabolic outcomes [74,75,76,77]. Besides, advances in engineered bacteria using synthetic biological methods reflect a new possibility for microecological therapy. Experimental studies with animal models of diabetes and obesity have been conducted with incipient but promising results [57]. Understanding the role of microbiota and its metabolites, such as SCFAs, in the leptin synthesis by EECs could result in developing strategies to minimize the repercussions of hypoleptinemia in patients with CGL.
Conclusion
In summary, this is the first study to demonstrate a reduction of gut microbiota diversity in individuals with CGL. Reduced gut microbiota diversity was present despite dietary treatment and was also observed in young patients. Our findings allow us to speculate that the loss of intestinal microbiota diversity may be due to metabolic abnormalities present since the first years of life in CGL. Longitudinal studies are needed to confirm these findings, clarifying the possible causal link between dysbiosis and insulin resistance in humans.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due individual privacy of the participants could be compromised but are available from the corresponding author on reasonable request.
Abbreviations
- AMP:
-
Antimicrobial peptides
- AMPM:
-
Automated multiple pass method
- ASV:
-
Amplicon sequence variants
- BMI:
-
Body mass index
- BRAZLIPO:
-
Brazilian Group for the Study of Inherited and Acquired Lipodystrophies
- CGL:
-
Congenital generalized lipodystrophy
- DM:
-
Diabetes mellitus
- EEC:
-
Enteroendocrine cells
- GABA:
-
γ-Aminobutyric acid
- GI:
-
Gastrointestinal
- GLP-1:
-
Glucagon-like peptide 1
- GPCR:
-
G protein-coupled receptors
- HbA1c:
-
Glycated hemoglobin
- LCA:
-
Lowest common ancestor
- PYY:
-
Peptide YY
- SCFA:
-
Short-chain fatty acids
References
Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–70.
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–10.
Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17(2):322–32.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–5.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, Trancassini M, Mancini C, Cicerone C, Corazziari E, Pantanella F, Schippa S. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39(1):1–12.
Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16(7):1024–33.
Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–15.
Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7(10): e47713.
Luoto R, Kalliomäki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes. 2010;34(10):1531–7.
Garg A. Lipodystrophies. Am J Med. 2000;108(2):143–52.
Patni N, Garg A. Congenital generalized lipodystrophies—new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11(9):522–34.
Akinci B, Oral EA, Neidert A, Rus D, Cheng WY, Thompson-Leduc P, Cheung HC, Bradt P, de Freitas MCF, Montenegro RM, Fernandes VO, Cochran E, Brown RJ. Comorbidities and survival in patients with lipodystrophy: an international chart review study. J Clin Endocrinol Metab. 2019;104(11):5120–35.
Ponte CMM, Fernandes VO, Gurgel MHC, Vasconcelos ITGF, Karbage LBAS, Liberato CBR, Negrato CA, Gomes MB, Montenegro APDR, Montenegro Júnior RM. Early commitment of cardiovascular autonomic modulation in Brazilian patients with congenital generalized lipodystrophy. BMC Cardiovasc Disord. 2018;18(1):6.
Akinci B, et al. Natural history of congenital generalized lipodystrophy: a nationwide study from Turkey. J Clin Endocrinol Metab. 2016;101(7):2759–67.
National High Blood Pressure Education P. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Bethesda: National Heart, Lung, and Blood Institute (US); 2004.
Tanner JM. Growth at adolescence. 2nd ed. Oxford: Blackwell; 1962.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S13–28.
National Cholesterol Education Program. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–421.
Moshfegh AJ, et al. The US department of agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–32.
Christoff AP, Cruz GNF, Sereia AFR, Yamanaka LE, Silveira PP, Oliveira LFV. End-to-end assessment of fecal bacteriome analysis: from sample processing to DNA sequencing and bioinformatics results. BioRxiv. 2019. https://doi.org/10.1101/646349.
Wang Y, Qian P-Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE. 2009;4(10): e7401.
Caporaso JG, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4.
Cruz GNF, Christoff AP, de Oliveira LFV. Equivolumetric protocol generates library sizes proportional to total microbial load in 16S amplicon sequencing. Front Microbiol. 2021;26(12): 638231.
Amir A, McDonald D, Navas-Molina JA, Kopylova E, Morton JT, Xu ZZ, et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems. 2017;2:e191-16.
Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4: e2584.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Yarza P, Yilmaz P, Pruesse E, Glöckner F, Ludwig W, Schleifer K-H, et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 2014;12:nrmicro3330.
Magurran AE, McGill BJ, editors. Biological diversity: frontiers in measurement and assessment, vol. 12. Oxford: Oxford Univ Press; 2011.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. https://doi.org/10.1038/372425a0 (Erratum in: Nature 1995 Mar 30;374(6521):479).
Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–3.
Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–83.
Cani PD, Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab. 2016;5(9):743–52.
Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci CMLS. 2016;73:737–55.
Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol. 2013;13:954–8.
Park HJ, Lee SE, Kim HB, Isaacson RE, Seo KW, Song KH. Association of obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J Vet Intern Med. 2015;29:43–50.
De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut and Liver. 2012;6(1):10–20.
Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12(49):1–9.
Sen T, Cawthon CR, Ihde BT, et al. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol Behav. 2017;173:305–17.
Klingbeil E, De La Serre CB. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):1254–60.
Le Chatelier E, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6.
Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2008;457:480–4.
Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio. 2016;7:e01018-16.
Yarandi SS, Hebbar G, Sauer CG, Cole CR, Ziegler TR. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27(3):269–75.
Zhang J, Scarpace PJ. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol. 2009;158(2):475–82.
Martínez V, Barrachina MD, Wang L, Taché Y. Intracerebroventricular leptin inhibits gastric emptying of a solid nutrient meal in rats. NeuroReport. 1999;10(15):3217–21.
Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124(1):98–103.
Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 2005;54(2):348–54.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–5.
Geurts L, Lazarevic V, Derrien M, et al. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:149.
Duggal P, Guo X, Haque R, et al. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121:1191–8.
Porter E, Bevins C, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–70.
Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83.
Rajala MW, Patterson CM, Opp JS, Foltin SK, Young VB, Myers MG Jr. Leptin acts independently of food intake to modulate gut microbial composition in male mice. Endocrinology. 2014;155(3):748–57.
Kim BR, Shin J, Guevarra R, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27(12):2089–93.
Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, Meng L, Xin Y, Jiang X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117: 154712.
Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26(5):611–24.
Thaiss CA, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–83.
Forslund K, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–6.
Wu H, et al. Metformin alters the gut microbiome of individuals with treatment-naïve type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–8.
Zhang Q, Hu N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;16(13):5003–14.
Rodriguez J, Hiel S, Delzenne NM. Metformin: old friend, new ways of action-implication of the gut microbiome? Curr Opin Clin Nutr Metab Care. 2018;21(4):294–301.
Dao MC, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–36.
Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148(3):293–300.
Grases-Pintó B, Abril-Gil M, Castell M, Rodríguez-Lagunas MJ, Burleigh S, Fåk Hållenius F, Prykhodko O, Pérez-Cano FJ, Franch À. Influence of leptin and adiponectin supplementation on intraepithelial lymphocyte and microbiota composition in suckling rats. Front Immunol. 2019;9(10):2369.
Ito D. BSCL2-related neurologic disorders/seipinopathy. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews. Seattle: University of Washington, Seattle; 1993–2022.
De Bandt JP, Waligora-Dupriet AJ, Butel MJ. Intestinal microbiota in inflammation and insulin resistance relevance to humans. Curr Opin Clin Nutr Metab Care. 2011. https://doi.org/10.1097/MCO.0b013e328347924a.
Zou Y, Ju X, Chen W, Yuan J, Wang Z, Aluko RE, He R. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020. https://doi.org/10.1039/c9fo01524h.
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–4.
Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–5.
Patil DP, Dhotre DP, Chavan SG, Sultan A, Jain DS, Lanjekar VB, Gangawani J, Shah PS, Todkar JS, Shah S, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–57.
Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474.
Hsieh M-C, et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8(1):16791.
Pedret A, et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes. 2019;43(9):1863–8.
Salminen MK, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis. 2002;35(10):1155–60.
Rozman V, et al. Characterization of antimicrobial resistance in lactobacilli and bifidobacteria used as probiotics or starter cultures based on integration of phenotypic and in silico data. Int J Food Microbiol. 2020;314: 108388.
Acknowledgements
R.M.M.J. would like to thank for the financial support by INCT for Diabetes and Obesity, BiomeHub, and DASA. The authors would like to thank Antônio Viana for assistance with the statistical analyses (Clinical Research Unit, University Hospital, Federal University of Ceará).
Funding
This work was supported by INCT (National Institute of Science and Technology) for Diabetes and Obesity - CNPq Grant number 465693/2014-8 and FAPESP Grant number 2014/50907-5. This work was partially supported by BiomeHub and DASA, Brazil.
Author information
Authors and Affiliations
Contributions
CMMP and ACOS wrote the main manuscript. RMMM, CMMP, MHCGC, VOF and JEL participated in the study design. CMMP, MHCGC, VOF and GECP participated in data collection. CBD, ADH, JEL performed the genetic analysis. ACOS and LFVO performed the microbiome analysis. All authors to have approved the submitted version (and any substantially modified version that involves the author's contribution to the study), and to have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Walter Cantídio University Hospital, Fortaleza, CE (protocol number: 3.342.082). Participants and guardians were included after signing the Free and Informed Consent Form (ICF).
Competing interests
M.H.C.G.C., A.C.O.S., A.D.H., S.P.B., T.S.C., G.A.C., and J.E.L. are GeneOne-DASA employees, and L.F.V.O. is BiomeHub employee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Oligonucleotide primer pairs used for the amplification of the AGPAT2 and BSCL2 coding regions.
Additional file 2.
Microbiome analysis in each collection time (t0 and t1) by group.
Additional file 3.
Abundance composition of each bacterium data.
Additional file 4.
Biochemical and hormonal analysis.
Additional file 5.
Diversity parameters analyzed by the age, gender, the presence of diabetes, CGL subtype, and the use of metformin or insulin.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Montenegro Junior, R.M., Ponte, C.M.M., Castelo, M.H.C.G. et al. Reduced gut microbiota diversity in patients with congenital generalized lipodystrophy. Diabetol Metab Syndr 14, 136 (2022). https://doi.org/10.1186/s13098-022-00908-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00908-8