Abstract
Background
A main factor contributing to insufficient glycemic control, during basal/bolus insulin therapy, is poor self-management bolus. Insulin bolus administration frequency is strongly associated with glycated hemoglobin (A1c) in Type 1 Diabetes (T1D). In the present study, we analyzed the performance of two-bolus calculator’s software that could be accessible to T1D patients from a Public Health Service to improve glycemic time in range (TIR) and A1c.
Methods
This prospective, controlled, randomized, parallel intervention clinical trial was carried out with 111 T1D participants on basal/bolus therapy [multiple daily insulin injections (MDI) or subcutaneous infusion pump (CSII)] with basal A1c ≥ 8.5% for 24 weeks. Patients were divided into 3 groups: 2 interventions: COMBO® (bolus calculator) and GLIC (mobile application) and 1 control (CSII group). Anthropometrics and metabolic variables were assessed on basal, 3 and 6 months of follow-up.
Results
TIR was increased in 9.42% in COMBO group (29 ± 12% to 38.9 ± 12.7%; p < 0.001) in 8.39% in the GLIC® group (28 ± 15% to 36.6 ± 15.1%; p < 0.001) while remained stable in CSII group (40 ± 11% to 39.3 ± 10.3%). A1c decrease in 1.08% (p < 0.001), 0.64% (p < 0.001) and 0.38% (p = 0.01) at 6 months in relation to basal in the COMBO, GLIC and CSII respectively. Daily basal insulin dose was reduced by 8.8% (p = 0.01) in the COMBO group.
Conclusion
The COMBO and a mobile applicative (GLIC) bolus calculator had a similar and a good performance to optimize the intensive insulin treatment of T1D in the public health system with increase in the TIR and reduction in A1C without increase hypoglycemia prevalence.
Similar content being viewed by others
Background
The Brazilian Type 1 Study Group (BrazDiab1SG), a multicenter study carried out in 20 public care centers reported that only 10% of Type 1 Diabetes (T1D) participants achieved glycated hemoglobin (A1c) within the target (< 7%), with the national A1c average in these patients being 9.1% [1]. Several factors related to poor glycemic control of these participants are educational level, economic status, age, duration of T1D, adherence to the dietary plan, number and frequency of hypoglycemia, difficult to access a specialized care, the inputs for daily minimum blood glucose measures, among other [2, 3]. Beside this, it had been shown that regardless of treatment insulin schedule 31% of Brazilian T1D people were also overweight, what might potentially increase of insulin resistance [4].
Optimizing postprandial glucose control is known to aid in the reduction of A1c, hypoglycemia risk, glycemic variability, and weight gain [5, 6]. However, A1c reductions are also markedly dependent to patient lifestyle, treatment adherence and self-attitude. Specifically, in T1D, where the endogenous insulin secretion is absent or very low, the good glycemic control depends on rapid insulin bolus. In this way, today, several tactics have been proposed to optimize these insulin boluses [7,8,9,10].
Omission of self-monitoring blood glucose levels and “forgetting” meals boluses correction, even by patients using continuous subcutaneous insulin infusion (CSII), are directly related to an increase in A1c [11]. Moreover, performing a complex insulin bolus calculation in according to blood glucose (BG) level, meal ingestion, past and present physical activities and target limits goals need to be considerate and could be collaborated to fail in this task. Also, fear of hypoglycemia is one of the main factors that hinders the issue of insulin bolus, partially because manual calculation increases its risk [12, 13].
By comparing manual and automated methods to calculate insulin boluses to cover all these factors in people with T1D, a study revealed an approximately tenfold higher error rate for manual than automated insulin boluses. Therefore, most individuals felt more confident (83%) and preferred (87%) to use the automated method for calculating insulin boluses [14].
Insulin bolus calculators, come together to CSIIs systems, however, it uses still restricted to a 7% of Brazilian T1D population, due to their high cost [2, 15].
Multiple daily insulin (MDI) injection therapy is the main form of intensive insulin therapy for people with Brazilin T1D, either with long insulin analogs associated with fast analogs, or more frequently yet with human NPH insulin associated with human short-acting insulin or fast analog. Beside they have insulin free of charge from by Brazilian Public Health System, many have low adherence to treatment, about self-monitoring of blood glucose (SMBG) and meal insulin bolus administration. Justifications to this behavior are many, as lack of inputs provided by public agencies, lack of time, irregular mealtimes, forgetting insulin bolus doses, or pain at the site of both the SMBG and the subcutaneous insulin infusion. A great percentage of MDI-treated T1D individuals use empirical insulin dose or worse, missed boluses. These became more complex and frustrating when we relate it to Brazilian socioeconomic conditions.
Hence, it is pertinent to explore the insulin bolus calculators, possible to use in a T1D population with poorly glycemic control assisted in a public health service to optimize MDI therapy.
Methods
Design
A prospective, controlled, randomized, parallel intervention trial for 24 weeks in a Brazilian Tertiary Public Health Center to compare three insulin bolus calculators to increase time in range (TIR) and decrease A1c in T1D adults with poor glycemic control.
All patients included in the study signed an informed consent form.
The inclusion criteria were age between 18 and 45 years old, T1D definition by The American Diabetes Association (ADA) criteria [16], on MDI (insulin basal/bolus) therapy with fast-acting insulin analogs or CSII without continuous glucose-sensor and A1c > 8.5%. The exclusion criteria were intestinal, liver, kidney diseases, psychopathy and hemoglobinopathies.
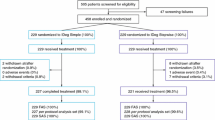
Initially 203 T1D adults were selected from the electronic medical record to participate in the study including CSII, GLIC® APP (Brazilian insulin bolus calculator application) and COMBO® (Smart Control Accu—Check COMBO® insulin bolus calculator).
The intervention groups studied were composed of 168 T1D patients, using MDI, randomized into COMBO (n = 82) and GLIC(n = 86) groups, there was a drop out of 49 people in the COMBO group and 43 people in the GLIC group.
CSII group (n = 35): was the control group representing patients previously using CSII in the routine care of Diabetes Center (Fig. 1).
All participants underwent a period of diabetes education review and orientation (signs and symptoms of hyperglycemia, hypoglycemia and ketosis, guidance for sick days, exercise guidance, training on carbohydrate counting).
Total daily insulin dose (TD) was individualized through the calculation by patient’s weight multiplied by 0.7 to 1.0 U/Kg. Insulin Carbohydrate ratio and Sensitivity factor was calculated by the IR/CHO 450/TD, and FS = 1800/TD rule, and proper handling of technology implemented [17] was carried out by one of the investigators (VM).
Insulin bolus calculators
GLIC® APP: suggested insulin dose is based on an algorithm that considers the input of multiple variables, such as: sensitivity factors, carbohydrate-insulin ratio and fat-insulin, blood glucose value and targets, residual or active insulin, food intake (carbohydrates, fat, and other macronutrients).
COMBO®: bolus calculation considers some parameters: post-prandial glucose increasing, insulin duration, waiting time for decrease glucose, health events, and exercises, beyond the medical prescription (sensitivity factors, carbohydrate-insulin ratio, and glycemic target).
Demographic, glycemic parameters and glycated hemoglobin evaluations
Anthropometric and clinical assessment were weight (kg), height(cm), waist circumference (WC) (cm), body mass index (BMI) (kg/m2) and blood pressure (mmHg).
Capillary blood glucose (mg/dL): the COMBO group used Perform® reagent strips while the GLIC group used Gluco Leader® Glucometer with Enhance II® reagent strips, and data were extracted via the Glico Sys® platform.
Time in Range (TIR) was percentage of time of capillary glycemic values between 70 and 180 mg/dL, extracted from glucometers upload.
Average blood glucose (AG): Blood glucose (mg/dL) was extracted from glucometer data uploaded the Accu-Chek Smart Pix®—Roche® platform.
Glycemic targets for adjusting insulin doses in basal-bolus therapies were in according to ADA orientations, such as fasting and pre-prandial glycemia between 70 to 130 mg/dL and 2 h after meals < 180 mg/dL, respectively [18]. Patients were asked to attend diabetes clinic at Visit one (basal), Visit 2 (3 months) and Visit 3 (6 months) of follow-up. When necessary, phone calls completed the presential appointment. Fasting and post prandial glycemia was reassessed at each visit (Basal, 3 and 6 months).
Hypoglycemia: Hypoglycemia was considered capillary glycemia below 70 mg/dL.
Glycated Hemoglobin: high pressure chromatography, certified by the National Glycohemoglobin Standardization Program (NGSP) Reference Value: 3.4 to 5.6% [19].
Diet, physical activity, and compliance evaluations
Diet adherence was defined as following at least 80% of the reported diet.
Physical activity: Participants were asked how many times physical activity was planned for each week and was classified as: Yes: physical activity performed 35 min three times a week, and No: less than three times a week or no activity reported.
Compliance with the intervention device was confirmed by the upload of insertion data of four capillary blood glucose per day and grams of carbohydrates into the GLIC app https://gliconline.net/entrar and the Combo Accu-Chek Smart Pix®—Roche®20.
Were considered adherence use of the intervention device during all the study time considering on uploaded data during appointment the detection of at least 3 capillary glucose measured by day.
Statistical analysis
For descriptive analysis, continuous variables are expressed as summary measures (mean, median, standard deviation, and quartiles) while categorical variables are expressed as percentages.
To compare the groups in relation to continuous variables with repeated measures, nonparametric two-way ANOVA was used. For binary categorical variables, a mixed logistic model was used, with group as a fixed effect and individuals as a random effect.
To verify the difference between continuous measures within the same group, nonparametric one-way ANOVA was used, as well as the Mann–Whitney paired test for two-by-two comparisons.
For variables with single measures, the student’s t-test was used to compare groups in relation to continuous variables for variables that follow a normal distribution (Anderson–Darling test). The nonparametric Mann–Whitney and Brunner-Munzel tests were respectively used for homogeneous and heterogeneous variables (Bartlett test). For categorical variables, Fisher's exact test was used.
Linear regression was carried out withA1c variation as an outcome and Adherence and Group as explanatory variables. The significance level adopted in the tests was 0.05. Two-tailed hypotheses were considered. R software version 3.6.0 was used to perform all analyses.
Results
Enrollment and participants characteristics
The three groups studied had similar age range, diabetes duration and ketoacidosis frequency at diagnosis. Women frequency was greater in CSII group than in the intervention groups. These data are shown on Table 1.
Intra-groups Comparations
Clinical parameters and daily insulin dose
These measures shown differences only in COMBO group. In this group some clinical and daily insulin (IU/kg/day) parameters significantly different after 6 months of study. Basal insulin (IU/kg/day) was reduced by 8.8% (p = 0.01), weight increased 2% (p = 0.007), waist circumference (WC) increased 5.4% (p < 0.001) and systolic blood pressure (SBP) reduced in 8.1% (Table 2).
Glycemic parameters
After 6 months of follow-up blood glucose mean reduced 5.8% in GLIC (216.4 ± 38.8 mg/dL (Mean ± SD) to 203.85 ± 44.99 mg/dL; p = 0.039), 7% in COMBO (224.5 ± 49.6 mg/dL to 209.7 ± 40.92 mg/dL (p = 0.012) and remained stable in CSII group (197.6 ± 28.1 mg/dL to 197.3 ± 30.4 mg/dL.
Blood glucose standard deviation (GSD) was reduced only in GLIC® group (100.3 ± 23.2 mg/dL to 90.6 ± 24.1 mg/dL) at 6 months; p = 0.003) (Table 2).
After 6 months of intervention TIR was increased in 9.42% in COMBO group (29 ± 12% to 38.9 ± 12.7%; p < 0.001) in 8.39% in the GLIC® group (28 ± 15% to 36.6 ± 15.1%; p < 0.001) while remained stable in CSII group (40 ± 11% to 39.3 ± 10.3%) (Fig. 2).
A1c had a mean reduction of -1.08% (9.9% ± 1.3% to 8.8 ± 1.3%; p < 0.001) in COMBO® Group, -0.64% in GLIC® group (9.6 ± 1.5% to 9.0 ± 1.4%; p < 0.001), and -0.38% (9.0 ± 0.5% to 8.6 ± 0.8%; p = 0.01) in CSII group (Fig. 3).
Inter group comparisons
Clinical parameters and daily insulin dose
WC was significantly different in GLIC® and COMBO® groups. In the baseline (82.81 ± 9.5 vs 82.88 ± 11.13 cm; p = 0.010) and after 6 months (83.89 ± 9.31 vs 87.34 ± 11.67; p = 0.010) respectively. While SBP was reduced only in the COMBO® group, (123.59 ± 12.46 mmHg vs 113.56 ± 12.93 mmHg; p = 0.030) (Table 3).
Total basal insulin administration in COMBO® was higher than CSII Group at time zero (28.0 ± 8.5 vs 24.27 ± 8.41 IU/day; p = 0.022) (Table 3).
The total dose of bolus at 6 months follow up at GLIC® was significantly lower than CSII group (27.53 ± 11.47 vs 28.3 ± 9.63 IU/Kg/day; p = 0.029) (Table 3).
Glycemic parameters
Blood glucose mean shown a tendence (p = 0.06) to significance difference only between COMBO vs CSII (209.75 ± 40.92 vs 197.38 ± 30.43 mg/dL) groups after 6 months intervention (Table 3).
TIR was significant different between COMBO group and CSII group 38.3% 12.7% ± vs 39.3% ± 10.5%; p < 0.001 and GLIC group vs CSII group (33.3% ± 15.1% vs 39.3% ± 10.5%; p = 0.002) at 6 months of follow-up (Table 3).
A1c was significantly different between COMBO vs CSII groups (8.8% ± 1.3% vs 8.6% ± 0.8%; p = 0.05) and COMBO vs GLIC groups (8.8% ± 1.3% vs 9.0% ± 1.4%; p = 0.05) at 6 months of follow-up (Fig. 3).
There was no significant difference in hypoglycemia frequency among all groups at any time of the study: basal, 3 and 6 months (Table 2).
Discussion
The use of two different technologies (GLIC®) and Smart control glucometer (COMBO®) as a bolus calculator (> 3 capillary blood glucose measures a day) significantly increased TIR and reduced A1c without increasing hypoglycemia prevalence in a group of adults T1D with poor glycemic control from a Public Diabetes Care Center.
After 6 months of intervention during follow-up TIR increased in 9.42% in COMBO® group and 8.39% increase in the GLIC® group. At the end of the study (6 months of follow-up) TIR of GLIC® (36.3%), COMBO® (38.3%) and CSII (40%) groups were similar. Beside of these TIRs still far from the minimum goal of TIR propose to a good glycemic control (> 70%) it was like TIR found in the DCCT participants were also using SMBG (41 ± 16%). The increase in TIR got in the COMBO® and GLIC® groups (9.4 and 8.4% respectively) was near to 10% that was shown to be sufficient to reduce the risk to diabetic retinopathy and microalbuminuria [21]. Recently these data was confirmed by the International Consensus for Continuous Monitoring where was demonstrated that 5% increase in TIR contributes to reduce chronic diabetic complications, so an increase of almost 9% in TIR in our intervention groups has potentially a clinical impact to reduce diabetes complications [22]. We also found a decrease in mean blood glucose in the GLIC® and COMBO® groups as in blood glucose SD in the GLIC® group, two important components of glycemic variability.
Glycemic variability (GV), referring to oscillations in blood glucose levels, is usually defined by the measurement of fluctuations of glucose or other related parameters of glucose homeostasis over a given interval of time (i.e., within a day, between days or long term). GV is considered independent risk factors for diabetes-related complications related to possible vascular damage due to excessive glucose fluctuations and an increased risk of hypoglycaemia, and can be better evaluated using Mean Glycemia, standard deviation and its coefficient of variation (CV = AG/SD × 100). [23]
Both Daily or Between Days short-term or long-term glycemic variability (A1c) should be considered independent risk factors for diabetes-related complications like an increased risk of a major cardiovascular diseases, diabetic retinopathy, sensory neuropathy, diabetic nephropathy, predisposes symptoms of depression and association with decline in cognitive function. It has also been associated with an increased risk of mortality in diabetic population. [24]
The reduction of A1c was more in COMBO® group (1.08%) than in GLIC® group (0.64%). This could be a result of less adherence to the GLIC® applied, probably due to various steps to reach final insulin bolus calculation. Nevertheless, a meta-analysis that evaluated the effectiveness of cell phone application in diabetes management and storage and collection of data for healthcare team analysis revealed a 0.44% decrease in A1c [25]. This systematic review and meta-analysis of efficacy of mobile APPs to support care of people with diabetes, confirmed that A1c could be improved when it strengthens the perception of self-care and allow remote access to health care professional, but an important characteristic measured in these studies was participants education degree and the ability in adopting new technologies [25]. In addition, a recent Brazilian study carried out with a group of adults with T1D or latent autoimmune diabetes of adult (LADA), undergoing treatment with MDI plus carbohydrate counts or MDI plus fixed doses of insulin compared to CSII also observed that using SMBG device connected to a cell phone application contributed to better glycemic control and increased adherence to tasks of self-care [26].
Specifically in relation to A1c reduction (1.08%) in COMBO® group those might reflect better insulin treatment adherence with this device. This result on A1c was better than it was found by the ABACUS study (reduction of 0.7% in A1c) when evaluating T1D that used the Accu-Check Aviva Expert BG meter® bolus calculator that resembles the COMBO® device use in our study [12].
Comparation between CSII and GLIC® groups shown that there was insulin dose bolus reduction in the GLIC® group (3.3%) while there was an increase of 7.1% in the CSII group. This reflects better adherence to bolus in the CSII group due to easiness of self-explanatory management of the device determining the exact basal and bolus dose in the profile while GLIC® device depends on data entry, which is often inputted irregularly. However, there was an increase in TIR and one reduction in A1c, as there was an increase in adherence to meal insulin bolus due to ease of carbohydrate counting by APP.
The reduction in basal/bolus insulin dose ratio in COMBO® group can reflect an optimization of insulin therapy in this group resulting greater A1c reduction. Optimized metabolic control was also demonstrated by WC increased, SBP reduction without raising in hypoglycemia prevalence.
Although cost-effective analyses were not done in this study, it is important to consider that the Smart Control Accu-Check COMBO® Bolus Calculator costs (~ US$ 243.00) and 150 reagent strips/month (~ US$ 50.00) but it was free of charge from SUS) to use the GLIC® are cheaper than CSII costs (~ US$ 2,727.00 per unit plus US$ 181.00 a month by supplies). [27, 28]
In our study, all groups used SMBG but is important to comment that better results would have been possible if continuous glucose monitoring system (CGMS) were used, as demonstrated by COMISAIR study were CGMS impacted glycemic outcomes more than insulin delivery method, CSII or MDI [29].
Study limitations
Herein, we performed a prospective study using low-income T1D people from the Public Health System. Of note, there was a high frequency of participants drop out due to socioeconomic conditions, such as difficulty in maintaining internet or cell phone data. Another limitation was we did not evaluate our participant’s education level because results favoring intervention groups might have been better if they had more scholar education degree.
The GLIC® bolus calculators were performed after the insertion of blood glucose measured by a glucometer provided by the SUS but sometimes, they were measured but not inserted in the APP. In addition, both COMBO device bolus calculator and the GLIC® used SMBG instead of CGM, which made it difficult to assess hypoglycemia. However, it reflects real-life challenge faced by most of T1D Brazilian population who do not have access to CGM [30, 31].
People with T1D still struggle to attain ideal glycemic control and avoid long-term complications despite these advancements in diabetes technology and diabetes education. The major cause of this can be attributed to the huge number of variables that influence the glucose control and must be considered when determining how much insulin is required for a meal. Some factors were not studied in this paper as low education level, smoking, living alone, exercising infrequently, nutrition factors which include low intake of fruit and vegetables, use of caffeine, alcohol consumption, biological or pathologic factors as menstruation, and celiac disease [32].
Finally, it is worth considered that this study was done during Covid-19 pandemic which made difficult to patients attend all presential appointment.
Conclusion
This study demonstrated that administration of intensive insulin treatment to poor glycemic control (A1c > 8.5%) T1D adults from public health system, can be optimized (reduction in A1c and an increase in TIR without an increase in hypoglycemia), using bolus calculators by intelligent glucometer (COMBO®) or mobile APP (GLIC®) became its like CSII users.
Thus, the facilitators in basal/bolus insulin therapy associated to seek guidance from professionals trained in diabetes education should be used to overcome personal and general difficulties found in a health system of developing countries to achieve better glycemic control and potentially collaborate to reduce the precocious diabetes chronic complications frequently found in these patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gomes MB, Coral M, Dib SA, et al. Prevalence of adults with type 1 diabetes who meet the goals of care in daily clinical practice: a nationwide multicenter study in Brazil. Diabetes Res Clin Pract. 2012;97:63–70.
Gomes MB, Negrato CA. Adherence to insulin therapeutic regimens in patients with type 1 diabetes. A nationwide survey in Brazil. Diabetes Res Clin Pract. 2016;120:47–55.
Gomes MB, Rodacki M, Negrato CA, et al. The impact of ethnicity, educational and economic status on the prescription of insulin therapeutic regimens and on glycemic control in patients with type 1 diabetes. A nationwide study in Brazil. Diabetes Res Clin Pract. 2017;134:44–52.
Negrato CA, Lauris JRP, Gomes MB, et al. Increasing incidence of type 1 diabetes between 1986 and 2015 in Bauru Brazil. Diabetes Res Clin Pract. 2017;127:198–204. https://doi.org/10.1016/j.diabres.2017.03.014.
Gross TM, Kayne D, Juth S, et al. A bolus calculator is aneffective means of controlling postprandial glycemia inpatients oninsulin pump therapy. Diabetes Technol Ther. 2003;5:365–9.
Zisser H, Wagner R, Jendrike N, et al. Clinical performance of three bolus calculators in subjects with Type 1 Diabetes Mellitus: a head-to-head-to-head comparison. Diabetes Technol Ther. 2010;12:955–61.
Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12:42–6.
Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health. 2015;25:43–52.
Monnier L, Colette C, Dunseath GJ, et al. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263–9.
Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA (1c). Diabetes Care. 2003;26:881–5.
Eiland L, McLarneyM DA, et al. App-based insulin calculators: current and future state. Curr Diab Rep. 2018;18:123–36.
Ziegler R, Cavan DA, Barnard K, et al. Use of an insulin bolus advisor improves glycemic control in Multiple Daily Insulin Injection (MDI) therapy patients with suboptimal glycemic control First results from the ABACUS trial. Diabetes Care. 2013;36:3613–9.
Colin IM, Paris I. Glucose meters with built- in automated bolus calculator: gadget or real value for insulin—treated diabetics patients? Diabetes Therapy. 2013;4:1–11.
Sussman A, Taylor EJ, Patel MBS, et al. Performance of a glucose meter with a built—in automated bolus calculator versus manual bolus calculation in insulin using subjects. J Diabetes Sci Technol. 2012;6:339–44.
Gomes MB, Negrato CA, Cobas R, Tannus LRM, Brazilian Type 1 Diabetes Study Group (BrazDiab1SG). Determinants of intensive insulin therapeutic regimens in patients with type 1 diabetes: data from a nationwide multicenter survey in Brazil. Diabetol Metabolic Syndrome. 2014;6:67–76.
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes 2021. Diabetes Care. 2021;44(Suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: Dose Adjustment for Normal Eating (DAFNE) randomized controlled trial. BMJ. 2002;325:746–52.
Targets G. Standards of medical care in diabetes 2021—American Diabetes Association. Diabetes Care. 2021;44:S73–84.
Little RR. Glycated hemoglobin standardization—National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41:1191–8.
Datye KA, Boyle CT, Simmons J, et al. Timing of meal insulin and its relation to adherence to therapy in Type 1 Diabetes. J Diabetes Sci Technol. 2018;12:349–55.
Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400–5.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–603.
Ceriello A, Monnier L, Owen D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2018. https://doi.org/10.1016/S2213-8587(18)30136-0.
Zhou Z, Sun B, Huang S, et al. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc Diabetol. 2020;19(1):102. https://doi.org/10.1186/s12933-020-01085-6
Bonoto BC, Araújo VE, Godói IP, et al. Efficacy of mobile apps to support the care of patients with Diabetes Mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth. 2017;5:e4.1-16.
Oliveira FM, Calliari LEP, Feder CKR, et al. Efficacy of a glucose meter connected to a mobile app on glycemic control and adherence to self-care tasks in patients with T1DM and LADA: a parallel-group, open-label, clinical treatment trial. Arch Endocrinol Metab. 2021;65:185–97.
Gomes MB, Santos DC, Marcela Haas Pizarro MH, et al. Relationship between health care insurance status, social determinants and prevalence of diabetes-related microvascular complications in patients with type 1 diabetes: a nationwide survey in Brazil. Acta Diabetol. 2019;56:697–705.
Thabit H, Hovorka R. Continuous subcutaneous insulin infusion therapy and multiple daily insulin injections in type 1 Diabetes Mellitus: a comparative overview and future horizons. Expert Opin Drug Deliv. 2016;13:389–400.
Soupal J, Petruzelkov L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR Study. Diabetes Care. 2020;43:37–43.
Brito M, Deborah G, Santos C, et al. Relationship between health care insurance status, social determinants and prevalence of diabetes-related microvascular complications in patients with type 1 diabetes: a nationwide survey in Brazil. Acta Diabetol. 2019;56:697–705.
Cavalcante R, Matheus ASM, Würdig B, et al. The influence of demographic, social-educational determinants and diabetes management on agreement between glucometer and logbook and its impact on glycemic control in patients with type 1 diabetes: a follow-up study. Diabetol Metab Syndr. 2019;11:1–9.
Huo L. Deng W. Shaw EJ. Factors associated with glycemic control in type 1 diabetes patients in China: A cross-sectional study. J Diabetes Investig. 2020;11(6):1575–1582. https://doi.org/10.1111/jdi.13282
Acknowledgements
Roche Diagnostics (Brazil) for donation of bolus calculator Smart Control Accu-Check COMBO®, Leandro Ferreira and Augusto Cesar Zamami, received consulting fees for their involvement in the statistical analysis, Editage (Liebert) received consulting fees for provided editing service. No other potential conflicts of interest relevant to this article were reported.
Funding
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), process number: 169556/2018–2.
Author information
Authors and Affiliations
Contributions
MVA, GMAL and DSA had the original idea for the study, wrote the study proposal and protocol and developed the manuscript. MVA applied and wrote the study proposal and protocol, and developed the manuscript, study measures and intervention, developed patient instructional materials, and provided training. DSA and GM. reviewed the manuscript. All authors read and approved the final manuscript and take full responsibility for the accuracy of its content. MVA is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the patients, physicians, and clinic staff for participating in this study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participants were included in the study based on the ethical principles for Medical Research Involving Human Subjects in accordance with the Declaration of Helsinki and were divided according to the type of intervention administered. The study was approved by the Teaching and Research Commission of the Federal University of São Paulo-Brazil and the Medical Ethics Committee (Protocol number: 0969/2018). All patients included in the study signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Montanari, V.A., Gabbay, M.A.L. & Dib, S.A. Comparison of three insulin bolus calculators to increase time in range of glycemia in a group of poorly controlled adults Type 1 diabetes in a Brazilian public health service. Diabetol Metab Syndr 14, 129 (2022). https://doi.org/10.1186/s13098-022-00903-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00903-z