Abstract
Background
Familial partial lipodystrophy (FPL) is a rare genetic disease characterized by body fat abnormalities that lead to insulin resistance (IR). Clinical conditions linked to milder IR, such as type 2 diabetes (T2D) and metabolic syndrome, are associated with abnormalities of the hypothalamic–pituitary–adrenal (HPA) axis, but little is known about its activity in FPL.
Methods
Patients meeting the clinical criteria for FPL were subjected to anthropometric, biochemical and hormone analyses. A genetic study to identify mutations in the genes encoding peroxisome proliferator-activated receptor gamma (PPARγ) was performed. Polycystic ovary syndrome and hepatic steatosis were investigated, and the patient body compositions were analyzed via dual X-ray energy absorptiometry (DXA). The HPA axis was assessed via basal [cortisol, adrenocorticotrophic hormone (ACTH), cortisol binding globulin, nocturnal salivary cortisol and urinary free cortisol (UFC)] as well as dynamic suppression tests (cortisol post 0.5 mg and post 1 mg dexamethasone).
Results
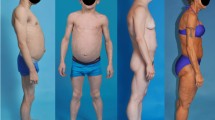
Six patients (five female and one male) aged 17 to 42 years were included. In DXA analyses, the fat mass ratio between the trunk and lower limbs (FMR) was > 1.2 in all phenotypes. One patient had a confirmed mutation in the PPARγ gene: a novel heterozygous substitution of p. Arg 212 Trp (c.634C>T) at exon 5. HPA sensitivity to glucocorticoid feedback was preserved in all six patients, and a trend towards lower basal serum cortisol, serum ACTH and UFC values was observed.
Conclusions
Our findings suggest that FPL is not associated with overt abnormalities in the HPA axis, despite a trend towards low-normal basal cortisol and ACTH values and lower UFC levels. These findings suggest that the extreme insulin resistance occurring in FPL may lead to a decrease in HPA axis activity without changing its sensitivity to glucocorticoid feedback, in contrast to the abnormalities in HPA axis function in T2D and common metabolic syndrome.
Similar content being viewed by others
Background
Familial partial lipodystrophy (FPL) is characterized by the selective loss of subcutaneous adipose tissue, such as in the limbs and gluteal area, and increased fat deposits are observed in other corporal segments, such as the face, trunk and abdomen [1,2,3,4,5]. Seven main FPL clinical variables have been described [1, 3,4,5], and, among the main genes studied, the genes encoding lamin A/C (LMNA) and peroxisome proliferator-activated receptor gamma (PPARγ) have been most thoroughly investigated [3,4,5,6,7]. The actual prevalence of FPL is not well established because it depends on sparse cases reported in the literature. In addition, FPL is a probable subdiagnosis of those patients who likely present with common metabolic syndrome [2, 3, 8, 9].
The loss of protective subcutaneous fat [3, 4] leads to severe insulin resistance (IR) in FPL and the early onset of related complications, such as type 2 diabetes (T2D) and other metabolic syndrome components [1,2,3,4,5,6,7,8,9,10,11]. The reduction of adipokines (adiponectin and leptin) may contribute to problems with insulin sensitivity [12].
Familial partial lipodystrophy carriers present phenotypic similarities with hypercortisolism and are frequently mistaken for patients with Cushing syndrome (CS) [1, 3, 6, 9]. Our hypothesis is that the dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis may be involved in this syndrome, as observed in common obese states [13, 14]; however, to our knowledge, this aspect has not been specifically elucidated in FPL.
Peripheral and/or central changes in cortisol action appear to be involved in the development of metabolic syndrome, which is associated with obesity. Subtle alterations in HPA axis activity, such as elevated circulating concentrations of ACTH in the morning, changes in pulsatility [15], higher responsiveness of the HPA axis to neuropeptides [16], increased cortisol production [17] and decreased cortisol suppression in response to low doses of dexamethasone [18, 19], are observed in metabolic syndrome. The increased peripheral production of cortisol in adipose tissue caused by an increase in the expression and activity of enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) in the visceral fat deposits of obese individuals suggests a possible role for hypercortisolic tissue in metabolic syndrome [13, 20,21,22].
Because of the practical difficulty of molecular diagnoses, the diagnosis of FPL is essentially clinical [2, 9, 23, 24]; thus, the clinical features of this disease must be addressed. Given that lipodystrophy represents a severe phenotypic spectrum of insulin resistance, in this study, we sought to provide insights on the contribution of insulin resistance to HPA axis abnormalities.
Methods
This paper presents a cross-sectional study that includes subjects in outpatient follow-up in the endocrinology department of two public hospitals in Goiânia, Brazil: Hospital Alberto Rassi–General Hospital of Goiânia (HGG) and Hospital das Clínicas of the Federal University of Goiás (HC-UFG). The protocol was approved by the ethics committees of these hospitals, and all patients provided written informed consent.
Clinical and anthropometric characteristics
This study included patients with clinical characteristics of lipodystrophy subtype FPL, which is defined by the American Association of Clinical Endocrinologists (AACE) as follows: gradual loss of subcutaneous adipose tissue in extremities and/or gluteal regions with fat accumulation in intra-abdominal areas or in the face and neck; presence of acanthosis nigricans, PCOS symptoms, hypertriglyceridemia or diabetes with severe insulin resistance, evidence of hepatic steatosis, preeminent muscularity and phlebomegaly in the extremities and/or a family history of a similar phenotype of lipodystrophy [2]. The exclusion criteria were history of autoimmune diseases, patients undergoing antiretroviral therapy and patients who recently used glucocorticoids.
Anthropometric measures were obtained by the same endocrinologist for all patients, and these measurements included body weight (kg); height (m); body mass index (BMI; weight in kilograms divided by the height squared in meters); waist circumference (WC; measured from the midpoint between the costal edge and the iliac crest in cm); and hip circumference (measured at the trochanter major in cm), which was used to determine the waist-to-hip ratio (WHR). Body adiposity index (BAI) was estimated as a percentage (%) according to the following formula: BAI = (Hip circumference/Height1,5) − 18 [25]. The adopted metabolic syndrome criteria were those recommended by the International Diabetes Federation [26].
Body fat evaluation
Body fat was analyzed via dual-energy X-ray absorptiometry (DXA) with the GE Lunar iDXA System (encore software version 2011, Madison, WI) to determine the following variables: total body fat (%), truncal fat (%), limb fat (%) and the android/gynoid fat ratio (A/G R). The fat mass ratio (FMR) was estimated as follows: FMR = [truncal fat (%)/[fat lower limbs (%)] [27].
Biochemical and basal hormonal evaluation
Blood samples were collected in the morning (08:00 h) after a 12 h overnight fast. The measured biochemical variables included the glucose level, lipid profile and liver enzyme levels (enzymatic assay); glycated hemoglobin HbA1c (high-performance liquid chromatography—HPLC); insulin (chemiluminescence assay); and leptin (radioimmunoassay). The hormonal variables measured by the chemiluminescence assay included prolactin, thyrotropin (TSH), free thyroxine (T4), total testosterone, sex hormone-binding globulin (SHBG), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels. In female patients, we also determined the dehydroepiandrosterone (DHEA), DHEA sulfate, androstenedione and 17-hydroxyprogesterone levels via radioimmunoassay.
Insulin resistance was estimated via a homeostatic model assessment (HOMA-IR) using the following formula: [insulin (mU/L) × glucose (mmol/L)/22.5].
Ultrasonography evaluation
Hepatic steatosis was investigated via upper abdominal ultrasonography (Philips HD.XE, transducer 3.5 MHz). A complementary evaluation for polycystic ovary syndrome (PCOS) was performed via pelvic ultrasound using the same equipment. We considered the PCOS criteria recommended by the International Consensus of Rotterdam [28].
Genetic studies
Venous blood samples (in EDTA collection tubes) were collected from all patients. The genetic studies were performed at the Laboratory of Molecular Pharmacology at the School of Health Sciences, University of Brasília, Brazil. Genomic DNA was extracted by a salting out method, and regions corresponding to exons of the PPARγ gene were amplified via PCR using specific primers. DNA sequencing was performed by the Sanger method.
Evaluation of the hypothalamic–pituitary–adrenal (HPA) axis
First, a basal evaluation of the HPA axis was performed via a chemiluminescence assay, and the measured parameters included the levels of serum cortisol (08:00 h), adrenocorticotrophic hormone (ACTH), 24-h urinary free cortisol (UFC) [three samples] and nocturnal salivary cortisol (at 23:00 h). The cortisol binding globulin (CBG) levels were measured via radioimmunoassay. In a second step, the study evaluated HPA axis sensitivity to dexamethasone (DEX). Plasma cortisol was measured after low doses of oral DEX (0.5 mg and 1 mg) overnight (at 23:00 h) with a 1-week interval. HPA axis suppression was considered normal at plasma cortisol levels < 1.8 µg/dL. Patients without suppression after 1 mg DEX were administered a 2 mg DEX suppression test [29].
Results
The study included six patients (P1–P6) who met the clinical criteria of FPL, with five females and one male (P2). The patients belonged to four unrelated families, with kinship between P1 and P2 (aunt and nephew) and between P5 and P6 (mother and daughter). The clinical, anthropometric, body composition and biochemical characteristics of the study participants are summarized in Tables 1 and 2.
Three patients had obesity defined by BMI value, and all but one had abdominal obesity. Clinical and biochemical evidence of severe insulin resistance was observed, which was disproportionate to the BMI. Moreover, a female patient (P5) was referred to the endocrinology department for an investigation of probable Cushing syndrome after a finding of precocious coronary disease (myocardial infarction at 36 years of age).
Hyperandrogenemia was detected in four females based on the elevated values of testosterone. All females met the criteria for PCOS. The male patient had normal basal levels of the gonadotropic axis.
The six patients had ultrasonographic evidence of hepatic steatosis. In the genetic study, patient P3 was found to carry the following mutation in the PPARγ gene at exon 5: a novel heterozygous substitution p.Arg212Trp (c.634C>T).
The basal and dynamic evaluations of the HPA axis are described in Table 3. We observed a tendency towards lower values of basal serum cortisol and UFC as well as circulating levels of basal ACTH, which was in the lower third of the normal range in five patients. Abnormalities in nocturnal salivary cortisol levels were not observed. All but one patient showed post-overnight 0.5 mg dexamethasone suppression of morning serum cortisol, and post-overnight 1 mg dexamethasone cortisol suppression was observed in all six participants.
Discussion
Patient clinical histories illustrate that these individuals usually seek medical attention years after the initial abnormalities of body fat distribution are detected [2, 9, 10]. Considering the challenge of the clinical diagnosis of lipodystrophy, two guidelines have already been developed in an attempt to organize clinical findings that could help with the earlier diagnosis of this disease [2, 23]. In this study, the reduction in subcutaneous adipose tissue was mild in the three youngest patients, which is consistent with a report showing that the adipose loss in FPL is gradual and progressive [2,3,4,5, 9]. The patient’s family history may represent a key parameter for the precocious identification of new and atypical cases, thus indicating the importance of screening the relatives of syndrome carriers [30].
According to the AACE consensus for lipodystrophy detection [2], PCOS represents a clinical criterion for insulin resistance, and its prevalence in FPL is approximately 25% [3]. Hyperinsulinemia results in hyperandrogenemia and anovulation [9, 31]. Therefore, the body fat distribution must be evaluated during the physical examination in females with PCOS clinical criteria.
The leptin serum concentrations among the six patients varied. Although low levels of leptin are expected during lipodystrophy, leptin assays and reference values have not been well defined for this clinical entity [2, 23]. The direct correlation between leptinemia and body fat mass appears to be well established [32]. Although BMI has already been positively correlated with leptin levels in lipodystrophy [33], this correlation is not consistently observed in patients with this disease [30, 34].
Therefore, decreases in the levels of leptin provide better therapeutic guidance than the diagnostic criteria of partial subtypes of lipodystrophy syndromes [9, 23]. Leptin replacement therapy has shown promising results in improving the metabolic outcomes of lipodystrophy syndromes, such those related to glucose metabolism, lipid profile and liver homeostasis [35].
The specific evaluation of the HPA axis in FPL represents a new aspect of the present study. This axis activity has previously been studied in antiretroviral-associated lipodystrophy, an acquired condition associated with insulin resistance related to anti-retroviral therapy. Axis hyperactivity and glucocorticoid hypersensitivity were reported in HIV-related lipodystrophy [36], despite preserved negative feedback sensitivity [37]. Our data suggest that the FPL condition may lead to a decrease in HPA axis activity, despite not changing its sensitivity to glucocorticoid feedback or cortisol circadian rhythmicity.
These findings contrast with the abnormalities in HPA axis function that were previously shown in conditions sharing phenotypic features with FPL, such as obesity, T2D and metabolic syndrome. The latter conditions have been described as pseudo-Cushing states [29] and are associated with a mild increase in UFC and an incomplete cortisol response after a 1 mg DEX test [38]. However, these signs were not observed in our study. Suppression after low oral DEX doses was also reported in other pseudo-Cushing conditions, such as obese prediabetic patients [39], although compared with healthy individuals, metabolic syndrome patients could demonstrate increased post-DEX cortisol levels, indicating a reduced ability to suppress the HPA axis [40].
The interactions between adipose tissue and the adrenal gland mainly involve obese states, and evidence suggests the possible hyperactivity of the HPA axis in obesity-related metabolic disorders [13,14,15,16,17,18,19, 41]. In this series of six lipodystrophy patients, however, we observed a trend of lower basal cortisol and ACTH values as well as decreased mean values for three of the analyzed UFC samples. Similar findings of low UFC have been described in female patients with abdominal obesity [42] and patients with antiretroviral-associated lipodystrophy [43]. Reduced basal cortisol concentrations were found in metabolic syndrome and attributed to an increased clearance of cortisol [14, 44].
In fact, the literature does not provide a consensus in support of a strong relationship between systemic cortisol and obesity or metabolic syndrome [45]. Most reports evaluating this linkage (adipose tissue and HPA axis) essentially consider visceral obesity. The primary disturbance in FPL is the loss of subcutaneous fat, and our findings suggest that the different types of adipocytes contribute to insulin resistance via distinct mechanisms. Although essentially speculative, these findings may also suggest that other features of the more common and milder forms of insulin resistance might indeed affect the HPA axis. Thus, it is not possible to rule out that other adipose tissue-derived factors may regulate HPA axis function. The possible stimulation of hyperinsulinemia in the HPA axis remains an undefined question [42].
Some drugs may have important implications for the interpretation of tests performed to evaluate HPA axis. However, the patients’ current medications in the present case series (described in Table 1) probably did not influence our findings. Two patients (P3 and P5) were using fenofibrate, a lipid lowering drug that can increases UFC levels assessed by HPLC, but does not affect assessment by chemiluminescence assay used in this study [29]. It is also important to note that CBG levels were normal in five of the studied patients; thus, CBG likely did not interfere with the plasma cortisol dosages and apparently did not represent an insulin resistance marker [46, 47].
Considering the phenotypic diversity among FPL patients [48], the body composition analysis by DXA constitutes an important tool for reinforcing clinical diagnoses of lipodystrophy [49]. All patients in our series had an FMR > 1.2, and the cut-off point suggested in a previous study involving FPL type 2 carriers had a sensitivity of 88.9% and a specificity of 93.8% for clinical diagnosis [50]. The index also demonstrated prognostic relevance, indicating that the severity of FPL is determined by the degree of loss of subcutaneous adipose tissue [2,3,4, 8, 51].
We recognize several limitations of this study. In addition to the small sample size, another limitation was the absence of a control group matched according to age and BMI. Additionally, five patients did not have a genotype diagnosis yet, representing a difficulty observed in clinical practice caused by the limited access to genetic testing. Even with advances in molecular biology, the genetic basis of the disease has not been identified for a considerable number of FPL patients [7]. We were not able to search for mutations in other candidate genes for lipodystrophy, such as LMNA, but this genetic testing represents a future project.
The HPA axis was assessed using basal measures and dynamic suppression tests to evaluate its sensitivity to dexamethasone. Since cortisol action is also regulated by at a peripheral level, we acknowledge that description of HPA axis activity would be more detailed by the evaluation of 11βHSD1 activity in adipose tissue. There is the evidence indicating increased activity of this enzyme in visceral fat in the setting of abdominal obesity and insulin resistance, thereby increasing cortisol generation from cortisone [13, 20,21,22, 44]. Therefore, it would be of interest to evaluate 11βHSD1 expression and activity in the visceral adipose tissue of patients with lipodystrophy syndromes.
Despite these limitations, this study highlighted important insights into a challenging disease for which early recognition is based on clinical criteria [2, 23] and broadened our knowledge of PPARγ mutations in lipodystrophy. The evaluation of HPA axis involvement in FPL provided insights into an uncertain and new aspect of the disease.
Conclusions
In conclusion, we found no overall dysregulation of the HPA axis sensitivity to glucocorticoid feedback in patients with a clinical diagnosis of FPL and no evidence of hyperactivity. However, this topic should be further clarified for this specific condition. Our findings point to the complex effects of adipose tissue abnormalities in HPA axis function, and it is not possible to rule out that other adipose tissue-derived factors may regulate HPA axis activity.
Abbreviations
- AACE:
-
American Association of Clinical Endocrinologists
- ACTH:
-
adrenocorticotrophic hormone
- BMI:
-
body mass index
- BAI:
-
body adiposity index
- CBG:
-
cortisol-binding globulin
- CS:
-
Cushing syndrome
- DEX:
-
dexamethasone
- DXA:
-
dual X-ray energy absorptiometry
- FMR:
-
fat mass ratio
- FPL:
-
familial partial lipodystrophy
- HBP:
-
high blood pressure
- HOMA-IR:
-
homeostatic model assessment of insulin resistance
- HPA:
-
hypothalamic–pituitary–adrenal axis
- HPLC:
-
high-performance liquid chromatography
- IR:
-
insulin resistance
- PCOS:
-
polycystic ovary syndrome
- PDC:
-
post-dexamethasone cortisol
- PPARγ:
-
peroxisome proliferator-activated receptor gamma
- TA:
-
adipose tissue
- T2D:
-
type 2 diabetes
- UFC:
-
urinary free cortisol
- WC:
-
waist circumference
- WHR:
-
waist-to-hip ratio
- 11βHSD1:
-
11β-hydroxysteroid dehydrogenase type 1
References
Hussain I, Garg A. Lipodystrophy syndromes. Endocrinol Metab Clin N Am. 2016;45:783–97.
Handelsman Y, Oral EA, Bloomgarden ZT, Brown RJ, Chan JL, Einhorn D, Garber AJ, Garg A, Garvey WT, Grunberger G, Henry RR, Lavin N, Tapiador CD, Weyer C, American Association of Clinical Endocrinologists. The clinical approach to the detection of lipodystrophy—an AACE Consensus Statement. Endocr Pract. 2013;19:107–16.
Garg A. Clinical review: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–25.
Capeau J, Magré J, Caron-Debarle M, Lagathu C, Antoine B, Béréziat V, Lascols O, Bastard JP, Vigouroux C. Human lipodystrophies: genetic and acquired diseases of adipose tissue. Endocr Dev. 2010;19:1–20.
Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–34.
Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59:16–23.
Hegele RA, Joy TR, Al-Attar SA, Rutt BK. Thematic review series: adipocyte Biology. Lipodystrophies: windows on adipose biology and metabolism. J Lipid Res. 2007;48:1433–44.
Cortés VA, Fernández-Galilea M. Lipodystrophies: adipose tissue disorders with severe metabolic implications. J Physiol Biochem. 2015;71:471–8.
Vantyghem MC, Balavoine AS, Douillard C, Defrance F, Dieudonne L, Mouton F, Lemaire C, Bertrand-Escouflaire N, Bourdelle-Hego MF, Devemy F, Evrard A, Gheerbrand D, Girardot C, Gumuche S, Hober C, Topolinski H, Lamblin B, Mycinski B, Ryndak A, Karrouz W, Duvivier E, Merlen E, Cortet C, Weill J, Lacroix D, Wémeau JL. How to diagnose a lipodystrophy syndrome. Ann Endocrinol. 2012;73:170–89.
Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7:137–50.
Vigouroux C, Caron-Debarle M, Le Dour C, Magré J, Capeau J. Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol. 2011;43:862–76.
Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395.
Baudrand R, Vaidya A. Cortisol dysregulation in obesity-related metabolic disorders. Curr Opin Endocrinol Diab Obes. 2015;22:143–9.
Matos AFG, Moreira RO, Guedes EP. Neuroendocrinology of the metabolic syndrome. Arq Bras Endocrinol Metab. 2003;47:410–20.
Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BA, Svensson J, Dallman M, McEwen B, Björntorp P. The activity of the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes Res. 2000;8:487–95. https://doi.org/10.1038/oby.2000.61.
Pasquali R, Vicennati V. Activity of the hypothalamic–pituitary–adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S47–9.
Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–7.
Jessop DS, Dallman MF, Fleming D, Lightman SL. Resistance to glucocorticoid feedback in obesity. J Clin Endocrinol Metab. 2001;86:4109–14.
Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, de Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R, Study Group on Obesity of the Italian Society of Endocrinology. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab. 2002;87:166–75.
Wake DJ, Walker BR. 11β-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol Cell Endocrinol. 2004;215:45–54.
Espíndola-Antunes D, Kater CE. Adipose tissue expression of 11beta-hydroxysteroid dehydrogenase type 1 in Cushing’s syndrome and in obesity. Arq Bras Endocrinol Metab. 2007;51:1397–403.
Dube S, Norby BJ, Pattan V, Carter RE, Basu A, Basu R. 11β-Hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: implications in obesity and diabetes. J Clin Endocrinol Metab. 2015;100:E70–6.
Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101:4500–11.
Huang-Doran I, Sleigh A, Rochford JJ, O’Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207:245–55.
Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, Xiang AH, Watanabe RM. A better index of body adiposity. Obesity. 2011;19:1083–9.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International association for the Study of obesity. Circulation. 2009;120:1640–5.
Freitas P, Santos AC, Carvalho D, Pereira J, Marques R, Martinez E, Sarmento A, Medina JL. Fat mass ratio: an objective tool to define lipodystrophy in HIV-infected patients under antiretroviral therapy. J Clin Densitom. 2010;13:197–203.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40.
Caldas D, Silva Júnior WS, Simonetti JP, Costa EV, Farias ML. Biochemical, hormonal and genetic evaluation of the families of two Brazilian patients with type 2 familial partial lipodystrophy. Arq Bras Endocrinol Metabol. 2013;57:583–93.
Pahuja I, De P, Sharma N, Kulshreshtha B. Polycystic ovarian syndrome in patients with lipodystrophy: report of 2 cases with review of literature. Indian J Endocrinol Metab. 2012;16:1022–5.
Chehab FF. Minireview: obesity and lipodystrophy—where do the circles intersect? Endocrinology. 2008;149:925–34.
Wong SP, Huda M, English P, Bargiotta A, Wilding JP, Johnson A, Corrall R, Pinkney JH. Adipokines and the insulin resistance syndrome in familial partial lipodystrophy caused by a mutation in lamin A/C. Diabetologia. 2005;48:2641–9.
Godoy-Matos AF, Moreira RO, Valerio CM, Mory PB, Moises RS. A new method for body fat evaluation, body adiposity index, is useful in women with familial partial lipodystrophy. Obesity. 2012;20:440–3.
Rodrigues AJ, Neeman T, Giles AG, Mastronardi CA, Paz Filho G. Leptin replacement therapy for the treatment of non-HAART associated lipodystrophy syndromes: a meta-analysis into the effects of leptin on metabolic and hepatic endpoints. Arq Bras Endocrinol Metabol. 2014;58(8):783–97.
Kino T, Chrousos GP. Human immunodeficiency virus type-1 accessory protein Vpr: a causative agent of the AIDS-related insulin resistance/lipodystrophy syndrome? Ann N Y Acad Sci. 2004;1024:153–67.
Yanovski JA, Miller KD, Kino T, Friedman TC, Chrousos GP, Tsigos C, Falloon J. Endocrine and metabolic evaluation of human immunodeficiency virus-infected patients with evidence of protease inhibitor-associated lipodystrophy. J Clin Endocrinol Metab. 1999;84(6):1925–31.
Pecori Giraldi F. PseudoCushing: why a clinical challenge? J Endocrinol Invest. 2015;38:1137–9.
Casulari LA, Dondi D, Celotti F, da Silva FV, Reis CE, da Costa TH. Effects of caloric restriction and low glycemic index diets associated with metformin on glucose metabolism and cortisol response in overweight/obese subjects: a case series study. Diabetol Metab Syndr. 2015;7:65.
Martins CS, Elias D, Colli LM, Couri CE, Souza MC, Moreira AC, Foss MC, Elias LL, de Castro M. HPA axis dysregulation, NR3C1 polymorphisms and glucocorticoid receptor isoforms imbalance in metabolic syndrome. Diabetes Metab Res Rev. 2017;33(3).
Kargi AY, Iacobellis G. Adipose tissue and adrenal glands: novel pathophysiological mechanisms and clinical applications. Int J Endocrinol. 2014;2014:614074.
Vicennati V, Pasquali R. Abnormalities of the hypothalamic–pituitary–adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration. J Clin Endocrinol Metab. 2000;85:4093–8.
Martin IP, Breen PA, Weigle DS. Absence of hypersensitivity to glucocorticoids in antiretroviral-associated lipodystrophy. Obes Res. 2003;11:21–4.
Wajchenberg BLO. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738.
Abraham SB, Rubino D, Sinaii N, Ramsey S, Nieman LK. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity. 2013;21:E105–17.
Lewis JG, Borowski KK, Shand BI, George PM, Scott RS. Plasma sex hormone-binding globulin, corticosteroid-binding globulin, cortisol, and free cortisol levels in outpatients attending a lipid disorders clinic: a cross-sectional study of 1137 subjects. Horm Metab Res. 2010;42:274–9.
Lewis JG, Shand BI, Elder PA, Scott RS. Plasma sex hormone-binding globulin rather than corticosteroid-binding globulin is a marker of insulin resistance in obese adult males. Diabetes Obes Metab. 2004;6:259–63.
Mory PB, Crispim F, Freire MB, Salles JE, Valério CM, Godoy-Matos AF, Dib SA, Moisés RS. Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations. Eur J Endocrinol. 2012;167:423–31.
Valerio CM, Godoy-Matos A, Moreira RO, Carraro L, Guedes EP, Moises RS, Mory PB, de Souza LL, Russo LA, Melazzi AC. Dual-energy X-ray absorptiometry study of body composition in patients with lipodystrophy. Diabetes Care. 2007;30:1857–9.
Valerio CM, Zajdenverg L, de Oliveira JE, Mory PB, Moyses RS, Godoy-Matos AF. Body composition study by dual-energy x-ray absorptiometry in familial partial lipodystrophy: finding new tools for an objective evaluation. Diabetol Metab Syndr. 2012;4:40.
Godoy-Matos AF, Valério CM, Bragança JB, Oliveira Rde A, Zagury RL, Lustosa Rde P, Camargo GC, Nascimento CA, Moreira RO. Evaluation of epicardial adipose tissue in familial partial lipodystrophy. Diabetol Metab Syndr. 2015;7:29.
Authors’ contributions
DEA conceived the original concept for the evaluation of the HPA axis in FPL, which was expanded by AAA and CPE to include the other studied aspects. CPE, DEA and AAA participated in the design and coordination of the study and drafted the manuscript. CPE was responsible for the patient clinical evaluation and medical visits. CLL and MSC performed the genetic studies. APMM and NR assisted in the analysis and discussion of the results. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Dr. Syrion de Oliveira for providing support for the biochemical and hormonal analyses and Dr. Leonardo Normanha for kindly allowing us to perform the DXA study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the ethics committees of the following institutions: Hospital Alberto Rassi (General Hospital of Goiânia), Hospital das Clínicas (Federal University of Goiás) and the School of Health Sciences, University of Brasilia.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Elias, C.P., Antunes, D.E., Coelho, M.S. et al. Evaluation of the hypothalamic–pituitary–adrenal axis in a case series of familial partial lipodystrophy. Diabetol Metab Syndr 11, 1 (2019). https://doi.org/10.1186/s13098-018-0396-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-018-0396-4