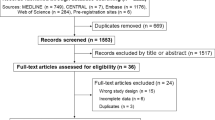
Abstract
Objective
To determine the effectiveness of a double-check protocol using Point-of-Care Ultrasound in the management of patients diagnosed with Acute Heart Failure in an Emergency Department.
Method
Prospective analytical cross-sectional observational study with patients diagnosed with Acute Heart Failure by the outgoing medical team, who undergo multi-organ ultrasound evaluation including cardiac, pulmonary, and inferior vena cava ultrasound.
Results
96 patients were included. An alternative diagnosis was found in 33% of them. Among the 77% where AHF diagnosis was confirmed, 73.4% had an underlying cause or condition not previously known (Left Ventricular Ejection Fraction less than 40% or moderate-severe valvulopathy). The introduction of the protocol had a clinically relevant impact on 47% of all included patients.
Conclusions
The implementation of a double-check protocol using POCUS, including cardiac, pulmonary, and inferior vena cava assessment in patients diagnosed with Acute Heart Failure, demonstrates a high utility in ensuring accurate diagnosis and proper classification of these patients.
Similar content being viewed by others
Background
Emergency Departments (ED) constitute one of the cornerstones of virtually any healthcare system when it comes to managing a high volume of patients and are also the source of most hospitalized patients, facing a very significant number of diverse pathologies on a daily basis, many of which are time-dependent and require a highly accurate diagnosis in a short time. Among them, Acute Heart Failure (AHF) is particularly prevalent, accounting for a high percentage of all ED visits due to shortness of breath or dyspnea [1], and also requiring an accurate and early diagnosis upon which its morbidity and mortality depend. To achieve this, Emergency Physicians have a diagnostic arsenal which, although it has been developing and increasing in recent years, is still not fully accurate. On one hand, the scarcity of time and the convergence of multiple patients simultaneously, combined with the limited accuracy of traditional medical history and physical examination, and the imprecision of standard complementary tests (ECG and chest X-ray) do not provide an ideal starting point. The emergence of biomarkers, especially NT-proBNP, has significantly improved the diagnosis, but its specificity for AHF diagnosis is also not optimal since it also elevates in multiple clinical scenarios. All of this makes the possibility of a misclassification of AHF patients in ED a very high percentage [2].
Furthermore, adding Clinical Ultrasound (CU) presents the advantage of being performed at the patient’s bedside, evaluating multi-organ function with the mission of revealing real-time pathophysiology underlying a patient whose symptoms or signs suggest AHF. Although the individual assessment of the heart, lungs, and inferior vena cava is fairly accurate, when performed collectively, the accuracy is optimized [3]. The aim of this study is to understand the impact that a dual-check protocol with CU has on patients who have been diagnosed with AHF through a “traditional” approach (medical history, examination, complementary tests, and biomarkers) in terms of detecting misclassified patients and the additional information revealed by CU that is relevant from a diagnostic and therapeutic standpoint.
Methods
This is a prospective analytical cross-sectional observational study conducted at a tertiary-level hospital. Following approval from the Ethics and Research Committee, adult patients were included who appeared during the morning shift rounds with a diagnosis of AHF made without POCUS using opportunistic sampling (when the expert, not part of the treating team, performing POCUS was present) and obtaining informed consent. Exclusion criteria included hemodynamic instability or cardiogenic shock, patient refusal, or AHF not being considered the primary diagnosis for their Emergency Department stay by the outgoing treating team.
After the ultrasound assessment, the evaluator communicated the findings to the treating team, discussing the images obtained, and the course of action was decided accordingly taking in consideration the whole clinical picture (ultrasound, clinical information, other tests). In order to ensure the accuracy of the diagnosis made by the protocol, the final diagnosis in the clinical ward (when the patient was eventually admitted) when available.
Ultrasound evaluation
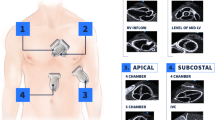
The study evaluators were certified Emergency Medicine experts by national and international scientific societies with over 10 years of experience. After collecting demographic parameters, a systematic evaluation was carried out using an ultrasound machine, including cardiac ultrasound with a phased-array probe, lung ultrasound, and ultrasound evaluation of the inferior vena cava using a curvilinear probe. Among the ultrasound parameters, left ventricular ejection fraction (LVEF) was measured using a biplane Simpson technique, categorized as < 40% or > 41%. Diastolic pattern was assessed using pulsed Doppler flow across the mitral valve and Tissue Doppler at the mitral annulus level. Valvular pathology was evaluated using continuous wave Doppler. For lung evaluation, a technique involving the division of the chest into 8 zones [4] was performed (anterior and laterals) using curvilinear probe and lung preset. The number of B-lines was counted using automatic calculation (auto B-lines) [5]. Similarly, for the assessment of the inferior vena cava, the automatic calculation tool for diameters and collapsibility percentage (auto IVC) was used, given its excellent correlation with expert visualization [6]. The number of B-lines per area (0 to 5) was used to calculate a lung score, [7] the arithmetic sum of these lines, ranging from 0 to 40, as a measure of lung aeration. Regarding the pulmonary diagnosis, a pneumonia was considered when a consolidation pattern or a focal B lines pattern were visualized, and respiratory infection with several and/or bilateral foci of B lines with subpleural consolidations appearances. Although the performance time was not originally recorded, evaluators indicated that entire protocol was performed in less than 15 min at the patient’s bedside.
Statistical analysis
Qualitative data were presented as absolute frequencies (n) and relative percentages (%), while quantitative data were presented as mean ± SD or as median [Interquartile Range] depending on the normality of distribution. Comparison between patients with and without an alternative diagnosis was performed with Wilcoxon rank sum test, Pearson’s Chi-squared test or Fisher’s exact test. Significance was considered when the p-value obtained was less than 0.05. The “cutpointr” package in R was used to obtain ROC curves and determine optimal cutoff points for maximum and minimum diameters and collapsibility coefficient of the inferior vena cava [8]. The optimal cutoff point was adjusted using the Youden Index, and diagnostic test parameters (Sensitivity, Specificity, ppv - Positive Predictive Value, and npv - Negative Predictive Value) were calculated. The decision tree for alternative diagnoses was obtained using the “rpart” and “rpart.plot” packages, with 80% of the samples used to determine the decision tree and the remaining 20% to validate it.
All analyses were performed using R software version 4.2.3.
Results
There were no losses due to participation rejection or absence of an ultrasound window, resulting in a total of 96 included patients. Demographic characteristics and ultrasound parameters are shown in Table 1.
The double-check protocol identified an alternative diagnosis in 32 of the included patients (33.3%) (Table 2), leading to therapeutic and/or clinical strategy changes (change of location, admission/discharge decision) in all of them. Among the 64 patients in whom the protocol reaffirmed the diagnosis of AHF, a clinically relevant echocardiographic finding was present in 47 (73.4%), including 17 (26.6%) with previously unknown LVEF < 40% and 42 (65.6%) with previously unknown or undescribed moderate or severe mitral, aortic, or tricuspid valve disease. Overall, the protocol’s application had an impact on 47% of all included patients, either through the identification of an alternative diagnosis or the discovery of additional/relevant pathology in patients with confirmed AHF diagnosis.
Analyzing each ultrasound examination separately, the accuracy of the inferior vena cava evaluation is shown in Fig. 1.
Regarding the lung pattern, the difference in the lung score was calculated not compared to all patients with an alternative diagnosis, but to patients diagnosed with pulmonary pathology (PE, respiratory infection, and pneumonia). As distinguishing a B-pattern between cardiogenic cause and others involves symmetry, a numerical asymmetry index was created, defined by the absolute value of the B-line difference between equivalent quadrants, with a correction factor involving a multiplication by 2 when there were two equivalent zones with a difference of 0, to accentuate the effect of asymmetry. Thus, the index ranged between 0 (completely symmetric) and 20 (completely asymmetric). The distribution of this lung asymmetry index between patients with and without an alternative diagnosis is shown in Fig. 1. Additionally, to observe the densitometric distribution of B-lines by regions, in addition to the lung score, a density map was created using the median of B-lines globally and adjusted for evolution time, as seen in Fig. 2.
With all variables included, a decision tree was constructed using the variables that our statistical model suggested were of greater significance in establishing a strategy for detecting an alternative diagnosis, shown in Fig. 3.
Regarding “particular” alternative diagnosis that could hypothetically be part of the AHF clinical spectrum, in our series, nor pericardial effusions nor the unilateral pleural effusion were associated to AHF in the final discharge medical report. In addition, the patient diagnosed as hypertensive emergency after the protocol application was discharged from the ED without diuretics, making the AHF diagnosis, at least, unlikely.
Discussion
In this study, we highlight the importance of adding POCUS evaluation to patients diagnosed with AHF in the ED. The proportion of misclassified patients is consistent with available literature [9] and presents a particularly concerning number, mainly because initiating specific AHF treatment can be detrimental for conditions detected as alternatives. Notably, the high number of significant pericardial effusions detected draws attention. This phenomenon was previously described by Blaivas [10], where misclassification as “classic AHF” and initiation of intensive diuretic treatment could potentially lead to hemodynamic instability.
This study also introduces two concepts not previously described in the literature: the asymmetry index and the temporal progression of B-lines adjusted for evolution time. The asymmetry index quantifies differences between interstitial pneumonia caused by SARS-CoV-2 and AHF. Since the distinguishing factor between them is the presence of a symmetric B-pattern [11], we sought to quantify symmetry, leading to the asymmetry index which, with significant implications, opens the potential for application in other patient groups. The index could be valuable in the differential diagnosis between asymmetric interstitial pathologies (interstitial pneumonia, ARDS) and symmetric interstitial or alveolar pathologies (pulmonary fibrosis or AHF), given that both circumstances exhibit a bilateral B-pattern, making B-lines indistinguishable. The second concept, the density of B-lines, offers relevant information, particularly when considering evolution time and the probable location of B-lines. Additionally, it indicates lung reaeration patterns with a centrifugal tendency, as observed in the graphs.
Regarding the evaluation of the inferior vena cava, the standalone precision values are consistent with other studies in the available literature, but we know that the precision is reinforced with multi-window evaluation, which forms the basis of the decision tree (Fig. 4).
With all these aspects considered, the decision tree suggested by our statistical model exhibits excellent classification ability. It’s worth noting that this model does not consider the presence of significant pericardial effusion initially. Clinically, the initial cardiac assessment appears necessary to exclude both pericardial effusion and acute cor pulmonale patterns, which if absent, would lead to subsequent pulmonary and inferior vena cava evaluations, as suggested. However, we are currently developing a “random forest” model that includes all variables, where both the asymmetry index and the presence of pericardial effusion are incorporated. Initial testing indicates perfect classification ability (100% sensitivity and specificity), though further studies are needed to confirm this.
Conceptually, the study highlights that the competencies required to find an alternative diagnosis don’t demand high expertise and should be part of basic ultrasound training for Emergency Medicine specialists [12]. This is where the study has significance in the day-to-day operations of an Emergency Department. On the other hand, in cases where an alternative diagnosis isn’t found, detecting relevant information for AHF management, such as valve pathology or non-preserved LVEF, requires quantitative measurements (Doppler quantification, biplane Simpson method, etc.) and, thus, more extensive training [13].
Limitations of this study include its single-center nature, conducted in a Level 3 hospital with a high-demand Emergency Department, potentially affecting external validity when applied to centers of different complexity or lower demand. Additionally, patients were already selected since they had been diagnosed with AHF by at least one treating team, rendering the protocol’s application invalid for patients with isolated clinical suspicion of AHF upon ED arrival. Nonetheless, the information derived from the early-stage B-line density map along with the asymmetry index could be applicable in these cases. Moreover, all diagnostic precision values need contextualization due to the highly selected patient population and the prevalence environment in which they are found. While the initial sample size calculation suggested around 87 patients for the main variable (detection of an alternative diagnosis), a larger sample size might be advisable to strengthen the description of the other variables or to include a larger cohort to test the protocol’s application. Finally, given the baseline characteristics of the patients included in this study always considering the main diagnosis, the possibility of the temporal coincidence of a respiratory infection and heart failure is not reflected, something that, according to some studies, is relatively common [14] an can be consider as a main limitation.
Conclusion
In a population of patients diagnosed with AHF in an Emergency Department, the application of POCUS in the form of a established double-check protocol aids in identifying misclassified patients and provides additional relevant clinical information for the remaining patients where AHF is confirmed. Furthermore, this study introduces novel statistically significant tools for evaluating various lung B-patterns, opening up possibilities for their utilization.
Data availability
Availability of data and supplemental material can be requested if needed.
References
Fabbri A, Marchesini G, Carbone G, Cosentini R, Ferrari A, Chiesa M, Bertini A, Rea F (2017) Italian Society of Emergency Medicine (SIMEU). Acute Heart failure in the Emergency Department: the SAFE-SIMEU Epidemiological Study. J Emerg Med 53(2):178–185
Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, Fermann GJ, deSouza I, Sinert R (2016) Diagnosing Acute Heart failure in the Emergency Department: a systematic review and Meta-analysis. Acad Emerg Med 23(3):223–242
Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ (2013) Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med 31(8):1208–1214
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW et al (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38(4):577–591
Gottlieb M, Patel D, Viars M, Tsintolas J, Peksa GD, Bailitz J (2023) Comparison of artificial intelligence versus real-time physician assessment of pulmonary edema with lung ultrasound. Am J Emerg Med 70:109–112
Tung-Chen Y, Villén-Villegas T, Herrero-Benito C, Vega-Vega L, Martín-Quirós A, Arenas-Berenguer I, Mayayo-Alvira R, Ruiz-Lopez D (2022) Reliability and consistency of point-of-care Ultrasonography for inferior vena cava measurement: visual versus automatic ultrasound systems. Acta Colomb Cuid Intensivo
Mongodi S, De Luca D, Colombo A, Stella A, Santangelo E, Corradi F et al (2021) Quantitative lung Ultrasound: technical aspects and clinical applications. Anesthesiology 134(6):949–965
Thiele C, Hirschfeld G, Cutpoint R (2021) Improved estimation and validation of optimal cutpoints in R. J Stat Softw 98(11):1–27
Llorens P, Javaloyes P, Martín-Sánchez FJ, Jacob J, Herrero-Puente P, Gil V, Garrido JM, Salvo E, Fuentes M, Alonso H, Richard F, Lucas FJ, Bueno H, Parissis J, Müller CE, Miró Ò, ICA-SEMES Research Group (2018) Time trends in characteristics, clinical course, and outcomes of 13,791 patients with acute heart failure. Clin Res Cardiol 107(10):897–913. https://doi.org/10.1007/s00392-018-1261-z
Blaivas M (2001) Incidence of pericardial effusion in patients presenting to the emergency department with unexplained dyspnea. Acad Emerg Med 8(12):1143–1146
Volpicelli G, Lamorte A, Villén T (2020) What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med 46(7):1445–1448. https://doi.org/10.1007/s00134-020-06048-9
Villén Villegas T, Campo Linares R, Alonso Viladot JR, Martínez Mas R, Luque Hernández MJ et al (2022) Description of basic competencies in clinical ultrasound imaging for emergency departments. Emergencias 34(5):377–387
Bahl A, Johnson S, Altwail M, Brackney A, Xiao J, Price J, Shotkin P, Chen NW (2021) Left ventricular ejection Fraction Assessment by Emergency Physician-Performed Bedside Echocardiography: a prospective comparative evaluation of multiple modalities. J Emerg Med 61(6):711–719
Bezati S, Velliou M, Ventoulis I, Simitsis P, Parissis J, Polyzogopoulou E (2023) Infection as an under-recognized precipitant of acute heart failure: prognostic and therapeutic implications. Heart Fail Rev 28(4):893–904
Acknowledgements
Not applicable.
Funding
For the development of this research no funding or grants have been received.
Author information
Authors and Affiliations
Contributions
All listed authors have made substantial contributions in this research, writing of this manuscript, read it, and approved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All recruited patients signed the approved consent by our institution Ethics Committee (HULP PI-3865).
Competing interests
All authors don’t disclose any competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Villén, T., Tung, Y., Llamas, R. et al. Results of the implementation of a double-check protocol with point-of-care ultrasound for acute heart failure in the emergency department. Ultrasound J 16, 25 (2024). https://doi.org/10.1186/s13089-024-00373-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-024-00373-6