Abstract
Background
Subarachnoid hemorrhage (SAH) patients with cerebral autoregulation (CA) impairment at an early post-SAH period are at high risk of unfavorable outcomes due to delayed cerebral ischemia (DCI) or other complications. Limited evidence exists for an association between early-stage CA impairments and SAH patient outcomes. The objective of this prospective study was to explore associations between CA impairments detected in early post-SAH snapshot examinations and patient outcomes.
Methods
The pilot observational study included 29 SAH patients whose CA status was estimated 2–3 days after spontaneous aneurysm rupture and a control group of 15 healthy volunteers for comparison. Inflatable leg recovery boots (reboots.com, Germany) were used for the safe controlled generation of arterial blood pressure (ABP) changes necessary for reliable CA examination. At least 5 inflation‒deflation cycles of leg recovery boots with a 2–3 min period were used during examinations. CA status was assessed according to the delay time (∆TCBFV) measured between ABP(t) and cerebral blood flow velocity (CBFV(t)) signals during artificially induced ABP changes at boot deflation cycle. CBFV was measured in middle cerebral artery by using transcranial Doppler device.
Results
Statistically significant differences in ∆TCBFV were found between SAH patients with unfavorable outcomes (∆TCBFV = 1.37 ± 1.23 s) and those with favorable outcomes (∆TCBFV = 2.86 ± 0.99 s) (p < 0.001). Early assessment of baroreflex sensitivity (BRS) during the deflation cycle showed statistically significant differences between the DCI and non-DCI patient groups (p = 0.039).
Conclusions
A relatively small delay of ∆TCBFV <1.6 s between CBFV(t) and ABP(t) waves could be an early warning sign associated with unfavorable outcomes in SAH patients. The BRS during boot deflation can be used as a biomarker for the prediction of DCI.
Trial registration
ClinicalTrials.gov Identifier: NCT06028906. Registered 31 August 2023 - Retrospectively registered, https://www.clinicaltrials.gov/study/NCT06028906.
Similar content being viewed by others
Background
Spontaneous aneurysmal subarachnoid hemorrhage (SAH) is a severe hemorrhagic stroke associated with the early onset of multiple complications that can lead to poor outcomes [1, 2]. The mortality rate after SAH is up to 35%, and more than one-third of survivors remain severely disabled [3].
After SAH, breakdown products of hemorrhaged blood release bioactive compounds that trigger inflammatory reactions leading to serious complications [4]. Cerebral vasospasm (CV) and delayed cerebral ischemia (DCI) are typical phenomena that occur in the human brain within 4–21 days after SAH [4, 5]. In addition, other complications, such as hydrocephalus, edema, sepsis and even rebleeding, may occur as a result of inflammation and disrupted cerebrospinal fluid circulation [3, 6, 7].
There is an urgent need to predict the clinical course in the first 1–3 days after SAH to mitigate possible complications. Different measures related to cerebral blood flow autoregulation (CA)— invasive pressure reactivity index (PRx), non-invasive TCD-based mean flow index (Mx) or phase shift (PS) between slow waves vasogenic B-waves [8] of arterial blood pressure (ABP) and cerebral blood flow velocity (CBFV), and baroreflex sensitivity (BRS) index—have been suggested as possible predictive factors that are considered feasible for personalized treatment in SAH patients.
Although several pilot studies have shown that CA impairment assessed in the first 1–4 days after SAH (early prevasospasm period) by PRx, Mx, and PS is associated with outcome after SAH [9,10,11,12], there is still limited evidence about the association of early CA impairment with DCI or CV.
Studies show that early deterioration of CA status is strongly associated with DCI in patients with large-artery CV after SAH [9, 11], but associations with vasospasm are contradictory in different studies [11, 13, 14]. Some studies show that CA impairment is observed before appearing the symptoms of CV [11, 14], therefore, the CA assessment in the early period would be useful for predicting cerebral vasospasms and outcome. However, other authors demonstrate that early TCD-based examination of CA status can be used to predict unfavorable outcome or cerebral infarction but not CV [10, 12, 13]. Monitoring optimal cerebral perfusion pressure (CPP) has been suggested to individualize CPP regulation-based management to avoid regional hypoperfusion in patients with SAH [9, 15]. It was demonstrated that optimal CPP increased approximately 30 h before the onset of DCI [16].
Invasive PRx monitoring is suggested for long-term continuous CA assessment and personalized SAH management in the intensive care unit (ICU). However, it requires invasive intracranial pressure (ICP) monitoring, which is not always appropriate for agitated or semiconscious patients with SAH, particularly in 4–14 days after SAH when the risk of CV occurrence is high. Noninvasive assessment of CA based on cerebral blood flow velocity monitoring by using transcranial Doppler (TCD) and calculation of the Mx or PS could be a rational solution for short CA monitoring sessions lasting up to a few hours [10, 11, 13, 17, 18] but not for long-term daily bedside monitoring.
The key limitation of Mx- and PRx-based monitoring approaches is that for reliable CA assessment, the presence of slow B-waves is necessary [19]. As slow B-waves are intermittent, the absence of slow waves can increase variation in measured CA indices, thus distorting diagnostic information [8, 20]. The variation in Mx and other CA indices might also be because different methodologies of assessing CA indices and simplified quantifications of a complex physiological mechanism were applied [20].
Mechanical generation of ABP changes or slow ABP waves was introduced to reduce the time required for CA assessment as well as to reduce the variation in CA indices. Repeated body position changes [21], sit-stand maneuvers [22], transient hyperemic response tests [12, 14, 23], deep breathing [24], pressure-cuff release tests [25,26,27] or noradrenaline infusion [28] are used to assess the dynamic response of cerebral blood flow and CA status. However, the application of most of these tests is limited in clinical practice due to strong interactions in critically ill neurosurgical patients.
To solve the aforementioned problem, we suggested the use of a commercially available pressotherapy device with inflatable boots capable of generating safe repeatable changes in ABP necessary for rapid CA assessment. The proposed method provides a much slower boot deflation cycle (\(\sim\) 10 s) with a controlled ABP amplitude and therefore can overcome the disadvantages of the thigh cuff technique related to the risk of secondary brain injuries in critically ill patients.
The purpose of this observational clinical study was to explore the associations of CA impairments detected in the first 1–3 days after SAH with the clinical course of SAH patients by performing TCD-based snapshot CA examinations with ABP manipulations.
Methods
Design and setting of the study
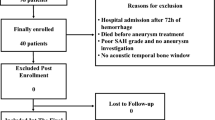
The pilot observational study was conducted at Center for Anesthesiology, Intensive Care and Pain Management at Vilnius University Hospital Santaros Clinics (Lithuania), from 29 June 2021 to 30 June 2023. The study involved the assessment of CA status in 29 patients with spontaneous SAH (after aneurysm clipping surgery) and 15 healthy volunteers (control group) for comparison. Patients’ inclusion criteria: patients after SAH who received routine nursing in ICU after aneurysm clipping surgery. All patients were older than 18 years. Patients received routine care in accordance with the Lithuanian Guidelines on the Diagnosis and Management of Head Injury (2010) and the National Methodical Recommendations for the Diagnosis and Treatment of Brain Injury (2017).
Ethical considerations
Clinical data collection was carried out in accordance with study protocols approved by the Vilnius Regional Biomedical Research Ethics Committees (Protocol No. 2021/6-1364-838). Participants and/or their legal guardians signed a written informed consent to participate in the study and use their anonymized clinical data for retrospective analysis. The study protocol was retrospectively registered at ClinicalTrials.gov Protocol Registration and Results System (Identifier: NCT06028906).
Assessment of cerebral blood flow autoregulation
Noninvasive CA status examination was performed in the early prevasospasm period: 2–3 days after aneurysm rupture, before the onset of CV or DCI. A pressotherapy device (leg recovery inflatable boots (Reboots, Germany) was used to generate artificial ABP changes required to assess CA dynamics (Fig. 1). The inflatable boots were placed on the patients’ legs (calf and thigh) to apply external pressure within safe controlled pressure limits not exceeding 200 mmHg during the maximal inflation phase. The boots were periodically inflated and deflated to generate artificial ABP changes: periodic slow ABP increase and fast 10 s drop during deflation by \(\sim\) 10 mmHg with a 2–3 min period.
Multi-DopT (Compumedics DWL GmbH, Germany) and Dolphin 4D (Viasonix Ltd, Israel) TCD monitors were used to record transient responses of cerebral blood flow velocity (CBFV(t)) measured in the middle cerebral artery (MCA) in relation to induced ABP changes. The CA status was estimated by measuring the time delay between ABP(t) and CBFV(t) changes (∆TCBFV) during boots deflation cycle assuming that observed ∆TCBFV values of 2…4 s reflect intact cerebral blood flow autoregulation, while lower values are associated with impaired CA [25, 26].
Assessment of baroreflex sensitivity
The baroreflex sensitivity (BRS) was assessed together with CA examinations while performing ABP manipulation with inflatable boots. The BRS indices, calculated as a ratio of heart interbeat interval (∆TIBI) and ABP changes [29], were analyzed to explore their associations with later development of DCI. The methodology of applying BRS indices for outcome prediction after SAH was previously tested using naturally spontaneous ABP waves [30,31,32,33]. In our study, we calculated BRS indices during the boot deflation cycle at the maximum slope moment of ABP drop (BRSdrop) and at the moment of ABP recovery after the nadir point (BRSrecovery).
Data collection
At least 5 inflation‒deflation cycles were used to estimate the average transient response of ABP, CBFV and heart rate (HR) data for each participant. Recorded responses of ABP, CBFV and HR were synchronized according to ABP nadir moments from each deflation cycle to calculate average values of ∆TCBFV and BRS indices (Figs. 1 and 2). The total duration of the examination did not exceed 20 min.
Example of ABP changes generated during deflation moments and registered responses in cerebral blood flow velocity (CBFV) and heart rate (HR). Dashed lines show the peak of ABP drop (nadir) moments during deflation cycle, which were used to synchronize responses from each boot deflation cycle in order to calculate average response of CBFV
In addition, the mean flow autoregulation index Mx, as a Pearson correlation coefficient between slow waves of ABP(t) and CBFV(t) within a 5-minute moving time window [34], was calculated during the examination sessions for comparison.
All monitoring data were collected using ICM + software (Cambridge Enterprise Ltd., UK). The sampling frequency of recorded ABP(t) and CBFV(t) data was 200 Hz. To eliminate instrumental delay, recorded CBFV(t) and ABP(t) data coincided in the time domain according to heartbeat variability. The CBFV(t) and ABP(t) data were filtered with a 5th order Butterworth low-pass filter with a cutoff frequency of 0.1 Hz and downsampled to 0.1 Hz before calculating the time delay ∆TCBFV.
Patient outcome assessment
The Glasgow Outcome Score (GOS) was assessed 30 days after ictus or at hospital discharge, classifying patients into the following categories: deceased (GOS = 1), vegetative (GOS = 2), severe disability (GOS = 3), moderate disability (GOS = 4) and good recovery (GOS = 5). Computed tomography (CT) and computed tomographic angiography (CTA) scans were performed routinely to evaluate CV/DCI occurrence at 7th day after SAH or when clinical symptoms together with TCD examination showed incidences of CV (moderate CV when CBFVMCA is 120–200 cm/s; critical CV when CBFVMCA > 200 cm/s). Additionally, SAH patients were classified into groups according to whether they had been diagnosed with CV or DCI. For each study participant, CA-related parameters (∆TCBFV, Mx, BRS) were calculated and compared between the SAH patients and control groups. SAH patients were divided into outcome groups of fatal (GOS = 1) vs. survivors (GOS = 2…5) and unfavorable (GOS = 1…3) vs. favorable (GOS = 4…5).
Statistical analysis
The Mann–Whitney U test or Fisher’s exact test were applied to estimate statistically significant differences in selected parameters between groups, which was indicated by p < 0.05. The threshold values of selected parameters that significantly separate outcome groups were identified according to the chi-square maximum value. The distribution of data among groups was expressed using either the mean and standard deviation (std) or the median and interquartile range (IQR). MatLab R2016a software was used for data processing and statistical analysis.
The Mann–Whitney U test (for ordinary variables) or Fisher’s exact test (for binary variables) were applied to estimate statistically significant differences in selected parameters between groups, which was indicated by p < 0.05.
The threshold values of parameters that significantly separate outcome groups were identified by forming series of 2 × 2 tables grouping the patients according to selected parameter into different outcome groups at different thresholds, and identifying the best discriminative threshold according to the highest chi-square value. The distribution of data among groups was expressed using either the mean and standard deviation (std) or the median and interquartile range (IQR). MatLab R2016a software was used for data processing and statistical analysis.
The sample size (29 SAH patients following aneurysm clipping surgery and 15 healthy volunteers) was impacted by project resource limitations, the constraints imposed by the pandemic and the limited study period (June 29, 2021 - June 30, 2023). Additionally, the modest sample size was influenced by the difficulties in obtaining high-quality signals during TCD examinations, by participant (or by their legal guardians) reluctance to participate in the study or their difficulties tolerating the pressure exerted by inflatable boots during the experiment. Accordingly, the non-parametric Mann–Whitney U test was chosen for its suitability with limited sample sizes and independence from the normal distribution of data, ensuring robust evaluation of statistically significant differences in selected parameters between groups.
Results
SAH patients’ demographic data, clinical data and estimated CA-related metrics are presented in Tables 1 and 2, grouping patients according to outcome into survival vs. fatal outcomes (Table 1) and into favorable vs. unfavorable outcomes (Table 2). From the study population (29 SAH patients), 8 patients had fatal outcomes (28%), 23 patients had unfavorable outcomes (79%, including patients with fatal outcomes), and 6 patients had good recovery (21%). CV was detected in 10 patients (34%) in the later phase of the clinical course (4–9 days after SAH) according to computed tomography angiography (CTA) examinations or clinical symptoms. DCI was detected in 8 SAH patients (28%) according to computed tomography (CT) scans. All patients with DCI diagnosis experienced CV. No one had symptoms of CV during CA examination in the early period (2–3 days after SAH).
Examples of transient responses of CBFV(t) and HR(t) caused by ABP(t) drop during the boot deflation cycle for healthy volunteers and SAH patients with favorable and unfavorable outcomes are shown in Fig. 3. Examples show that the CBFV(t) response overtook ABP(t) signal during deflation cycle due to active cerebral blood flow autoregulatory response [27], thus causing 2…4 s delay between CBFV(t) and ABP(t) signals. Examples in Fig. 3c and d show cases of disturbed cerebral blood flow autoregulation with baroreflex failure for patients with unfavorable outcomes: DCI and severe disability (Fig. 3c) and fatal outcome (Fig. 3d).
Examples of averaged transient responses of human cerebral blood flow autoregulation function in healthy volunteers (a) and SAH patients with favorable outcomes (b), severe disability with DCI (c) and fatal outcomes without DCI (d). The time moments at t = 0 show the peak of ABP drop (nadir) moments during the boot deflation cycle. The patients with later development of DCI are characterized by synchronous ABP and HR variation during the boot deflation phase (c)
Statistically significant differences in CA-related delay ∆TCBFV were found among the fatal outcome patient group (∆TCBFV = 0.74 ± 0.58 s), survival group (∆TCBFV = 2.05 ± 1.35 s) and healthy control group (∆TCBFV = 2.76 ± 0.75 s) (Fig. 4a and b). Statistically significant differences in delay ∆TCBFV were also found between the unfavorable outcome group (∆TCBFV = 1.37 ± 1.23 s) and the favorable outcome group (∆TCBFV = 2.86 ± 0.99 s). ∆TCBFV for the healthy control group also significantly differed from that of patients with unfavorable outcomes (p < 0.001) but not from that of patients with favorable outcomes (Fig. 4b).
Distribution of delay time between ABP(t) and CBFV(t) during deflation cycle (∆TCBFV) identified for SAH patients with fatal outcome (GOS 1), survivors (GOS > = 2) and healthy control group (a), and for SAH patients with unfavorable (GOS < = 3) and favorable (GOS > 3) outcomes and the healthy control group (b). Dashed lines show the threshold values that statistically significant separate fatal and survivors (a) and unfavorable and favorable patients groups (b)
The analyzed CA-related parameters, either Mx or ∆TCBFV, showed significant associations with CV and DCI occurrence. The BRS-related index at the boot deflation cycle (BRSrecovery) showed statistically significant differences between the patient group who experienced DCI and those without DCI (p = 0.039) (Fig. 5). However, BRSrecovery and BRSdrop did not show statistically significant associations with patient outcomes or CV patient groups.
Discussion
In this study, we investigated the impact of CA impairments detected in the early period on SAH patient outcomes by performing snapshot TCD examinations with artificial ABP manipulations. We chose the delay time between ABP(t) and CBFV(t) during induced ABP changes (∆TCBFV) as a CA-related predictor of SAH patient outcome. Higher ∆TCBFV values (2.86 ± 0.99 s) measured in the early period of the clinical course (2–3 days after SAH) were associated with favorable SAH patient outcomes, whereas lower values (∆TCBFV < 1.7 s) were observed for patients with unfavorable outcomes. We found no significant associations between ∆TCBFV and the occurrence of DCI or CV. In our study, not only DCI but also other complications, such as cerebral edema, hydrocephalus, sepsis, and pneumonia, were leading causes of unfavorable outcomes after SAH. The total number of unfavorable cases was 78%, compared to 29% of DCI cases.
A moderate correlation between ∆TCBFV and the noninvasive Mx index was found in the study population (r=-0.461, p = 0.012), confirming that both measures show an indirect association with CA status. However, the Mx index estimated in the early period of SAH patients’ treatment during the examination sessions with inflatable boots did not show a statistically significant association with patients’ outcome or DCI/CV events.
In this study, we applied a pressotherapy device capable of generating safe controlled changes in ABP instead of thigh cuff release methods, which are often used for dynamic CA assessment. The main disadvantage of the thigh cuff technique is the risk of secondary brain injuries in ischemic stroke patients when ABP drops by up to 30 mmHg during a short deflation cycle. Furthermore, the rapid decrease in ABP may induce additional responses due to baroreflex triggering or changes in arterial partial pressure of carbon dioxide (pCO2), which may independently affect blood flow and ABP changes [35]. In our study, we used a pressure-based therapeutic massage device (leg recovery device (Reboots), Germany) with a controlled boot inflation pressure (not exceeding 200 mmHg) and ABP drop amplitude (not exceeding 10…15 mmHg per 10 s deflation cycle). Similar devices are used in most ICUs to prevent deep vein thrombosis, venous thromboembolism, as well as to improve blood circulation for long-term patient treatment.
To explore the impact of baroreflex on cerebral blood flow regulation during artificially induced ABP changes, we also plotted averaged responses of HR, ABP and CBFV during the boot deflation cycle (Fig. 3). For healthy volunteers, an increase in HR is observed due to triggered baroreflex after a sudden fall in ABP, resulting in a regulation of CBFV, thus causing a leftward shift of the CBFV nadir peak with respect to the ABP nadir (Fig. 3a).
However, for SAH patients, the symptoms of baroreflex failure [36] were observed in almost all examination cases; the impact of cardiovascular response after the fall in ABP on CBFV regulation was not observed in either favorable or unfavorable outcome groups of SAH patients, despite the autoregulatory responses in CBFV. This allows us to hypothesize that a leftward shift in CBFV with respect to ABP during inflation is achieved through autoregulatory interactions of various neurogenic, hemodynamic, autoregulatory, and metabolic factors [37], whereas baroreflex is not the only factor affecting the autoregulatory blood flow response during the initial phase of the ABP drop [37, 38]. A decrease in ABP triggers an integrated physiological response that not only involves arterial baroreflex-mediated changes in HR and peripheral vasculature resistance but also induces a cerebrovascular autoregulatory response [36, 37].
Analysis of BRS-related factors did not reveal a significant association with the outcome of patients with SAH. However, a statistically significant association was observed between the baroreflex-related index BRSrecover (calculated as ∆TIBI/∆ABPrecover ratio during the recovery phase after ABP drop) and DCI events (Fig. 5; Table 3). Examples of averaged HR and CBFV responses for SAH patients who experienced DCI are shown in Fig. 3c in comparison with other SAH patients without DCI (Fig. 3b and d) and healthy volunteers (Fig. 3a).
The impact of BRS on SAH patients’ clinical course has been analyzed in several studies [30,31,32,33]. It was shown that an inverse compensatory correlation between BRS and CA is observed for SAH patients who avoided CV occurrence, whereas this relationship was lost in patients who experienced CV, both before and during vasospasm [30]. Decreased BRS within the first five days following SAH was found to be associated with regional cerebral deoxygenation [31] and consequently with poor outcomes [32, 33].
Limitations
The main limitation is the relatively small number of patients included in the study, which might affect the reliability of statistically significant factors. The obtained relationship between the CA-related biomarkers used in the study and patient outcomes might be affected by secondary complications following SAH, not DCI alone [39]. Additional factors - age and Glasgow coma scale (GCS) - also showed statistically significant associations with patient outcomes; therefore, a larger number of participants is necessary for the further development of more accurate multivariate outcome prediction models.
We limited our study to BRS and CA-related factors evaluated from short 20-minute examination cycles performed 2–3 days after SAH, while CV occurred in 4–9 days. More frequent examinations of SAH patient parameters would be more rational during the overall clinical course. However, CA assessment by using TCD and pressotherapy devices became complicated in semiconscious and disorientated patients after they awakened from surgery sedation. Moreover, TCD examinations were performed only on one intact side of the head after brain aneurysm surgery.
Some patients were sedated during examinations. Some patients in our study received administration of vasopressors at individually adjusted dosages to regulate ABP. Although, applied pharmaceutical treatment by anesthetics (mainly propofol) and vasopressors can affect both CA and BRS test results by blocking the cardiovascular reflex response [40] or affecting cerebrovascular reactivity [41, 42], however, recent study showed that propofol did not produce a significant alteration in the average heart rate, which serves as the basis for measuring baroreflex sensitivity [43].
Additional limitations are related to patient outcome assessment and CV/DCI diagnosis. Patients’ outcome data were available only at hospital discharge. CV and DCI were diagnosed according to clinical symptoms or CT/CTA scan examinations (data obtained from the hospital database). Therefore, CVs are clearly seen only in large cerebral arteries, while spasm detection in smaller arteries (with diameters < 3 mm) can be missed.
Conclusions
The proposed methodology based on TCD and inflatable leg recovery boots application allows safe assessment of CA status within a short period of time − 20 min. CA-related biomarker—delay between CBFV(t) and ABP(t) during the boot deflation cycle—measured during snapshot TCD examination in the early prevasospasm period showed a significant association with SAH patient outcome but not with CV and DCI. The BRS-related ratio between changes in the heartbeat period (∆TIBI) and ABP at the boot deflation cycle assessed in the early prevasospasm phase was significantly associated with later occurrence of DCI. Not only DCI but also other complications, such as cerebral edema, hydrocephalus, sepsis, and pneumonia may worsen clinical outcomes after SAH.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABP:
-
arterial blood pressure
- BRS:
-
baroreflex sensitivity
- CA:
-
cerebral blood flow autoregulation
- CBFV:
-
cerebral blood flow velocity
- Cont.:
-
control group
- CPP:
-
cerebral perfusion pressure
- CT:
-
computed tomography
- CTA:
-
computed tomography angiography
- CV:
-
cerebral vasospasm
- DCI:
-
delayed cerebral ischemia
- GOS:
-
Glasgow outcome scale
- GCS:
-
Glasgow coma scale
- ICU:
-
Intensive care unit
- IQR:
-
interquartile range
- MCA:
-
middle cerebral artery
- Mx:
-
mean flow autoregulation index
- pCO2:
-
arterial partial pressure of carbon dioxide
- PS:
-
phase shift
- PRx:
-
Pressure reactivity index
- SAH:
-
subarachnoid hemorrhage
- Surv.:
-
survivor group
- std:
-
standard deviation
- TCD:
-
transcranial Doppler
References
van Donkelaar CE, Bakker NA, Birks J, Veeger NJGM, Metzemaekers JDM, Molyneux AJ et al (2019) Prediction of Outcome after Aneurysmal Subarachnoid Hemorrhage. Stroke 50:837–844. https://doi.org/10.1161/STROKEAHA.118.023902
Roquer J, Cuadrado-Godia E, Guimaraens L, Conesa G, Rodríguez-Campello A, Capellades J et al (2020) Short- and long-term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology 95(13):e1819–e1829. https://doi.org/10.1212/WNL.0000000000010618
Danière F, Gascou G, Menjot de Champfleur N, Machi P, Leboucq N, Riquelme C et al (2015) Complications and follow up of subarachnoid hemorrhages. Diagn Interv Imaging 96:677–686. https://doi.org/10.1016/j.diii.2015.05.006
Dodd WS, Laurent D, Dumont AS, Hasan DM, Jabbour PM, Starke RM et al (2021) Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc 10(15):e021845. https://doi.org/10.1161/JAHA.121.021845
Ciurea AV, Palade C, Voinescu D, Nica DA (2013) Subarachnoid hemorrhage and cerebral vasospasm - literature review. J Med Life 6:120–125
Chen S, Luo J, Reis C, Manaenko A, Zhang J (2017) Hydrocephalus after Subarachnoid Hemorrhage: pathophysiology, diagnosis, and treatment. Biomed Res Int 2017:8584753. https://doi.org/10.1155/2017/8584753
Flinspach AN, Konczalla J, Seifert V, Zacharowski K, Herrmann E, Balaban Ü et al (2022) Detecting Sepsis in patients with severe subarachnoid hemorrhage during critical care. J Clin Med 11:4229. https://doi.org/10.3390/jcm11144229
Spiegelberg A, Preuß M, Kurtcuoglu V (2016) B-waves revisited. Interdiscip Neurosurg 6:13–17. https://doi.org/10.1016/j.inat.2016.03.004
Rasulo FA, Girardini A, Lavinio A, De Peri E, Stefini R, Cenzato M et al (2012) Are optimal cerebral perfusion pressure and cerebrovascular autoregulation related to long-term outcome in patients with aneurysmal subarachnoid hemorrhage? J Neurosurg Anesthesiol 24:3–8. https://doi.org/10.1097/ANA.0b013e318224030a
Calviere L, Nasr N, Arnaud C, Czosnyka M, Viguier A, Tissot B et al (2015) Prediction of delayed cerebral ischemia after subarachnoid hemorrhage using cerebral blood Flow velocities and Cerebral Autoregulation Assessment. Neurocrit Care 23:253–258. https://doi.org/10.1007/s12028-015-0125-x
Lang EW, Diehl RR, Mehdorn HM (2001) Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med 29:158–163. https://doi.org/10.1097/00003246-200101000-00031
Rynkowski CB, de Oliveira Manoel AL, Dos Reis MM, Puppo C, Worm PV, Zambonin D et al (2019) Early transcranial doppler evaluation of cerebral autoregulation independently predicts functional Outcome after Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 31:253–262. https://doi.org/10.1007/s12028-019-00732-5
Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D et al (2012) Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke 43:3230–3237. https://doi.org/10.1161/STROKEAHA.112.669788
Al-Jehani H, Angle M, Marcoux J, Teitelbaum J (2018) Early abnormal transient hyperemic response test can predict delayed ischemic neurologic deficit in subarachnoid hemorrhage. Crit Ultrasound J 10(1):1. https://doi.org/10.1186/s13089-017-0079-7
Johnson U, Engquist H, Lewén A, Howells T, Nilsson P, Ronne-Engström E et al (2017) Increased risk of critical CBF levels in SAH patients with actual CPP below calculated optimal CPP. Acta Neurochir (Wien) 159:1065–1071. https://doi.org/10.1007/s00701-017-3139-7
Weiss M, Albanna W, Conzen C, Megjhani M, Tas J, Seyfried K et al (2022) Optimal cerebral perfusion pressure during delayed cerebral ischemia after Aneurysmal Subarachnoid Hemorrhage. Crit Care Med 50:183–191. https://doi.org/10.1097/CCM.0000000000005396
Panerai RB (1998) Assessment of cerebral pressure autoregulation in humans–a review of measurement methods. Physiol Meas 19. https://doi.org/10.1088/0967-3334/19/3/001. :305 – 38
Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB, international Cerebral Autoregulation Research Network (CARNet) (2016) Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab 36:665–680. https://doi.org/10.1177/0271678X15626425
Deimantavicius M, Chaleckas E, Boere K, Putnynaite V, Tamosuitis T, Tamasauskas A et al (2022) Feasibility of the optimal cerebral perfusion pressure value identification without a delay that is too long. Sci Rep 12:17724. https://doi.org/10.1038/s41598-022-22566-6
Olsen MH, Riberholt CG, Mehlsen J, Berg RM, Møller K (2022) Reliability and validity of the mean flow index (Mx) for assessing cerebral autoregulation in humans: a systematic review of the methodology. J Cereb Blood Flow Metab 42:27–38. https://doi.org/10.1177/0271678X211052588
Stok WJ, Karemaker JM, Berecki-Gisolf J, Immink RV, van Lieshout JJ (2019) Slow sinusoidal tilt movements demonstrate the contribution to orthostatic tolerance of cerebrospinal fluid movement to and from the spinal dural space. Physiol Rep 7(4):e14001. https://doi.org/10.14814/phy2.14001
Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM (2021) Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 101:1487–1559. https://doi.org/10.1152/physrev.00022.2020
Smielewski P, Czosnyka M, Kirkpatrick P, Pickard JD (1997) Evaluation of the transient hyperemic response test in head-injured patients. J Neurosurg 86:773–778. https://doi.org/10.3171/jns.1997.86.5.0773
Lewis PM, Rosenfeld JV, Diehl RR, Mehdorn HM, Lang EW (2008) Phase shift and correlation coefficient measurement of cerebral autoregulation during deep breathing in traumatic brain injury (TBI). Acta Neurochir (Wien) 150:139 – 46. discussion 146-7 https://doi.org/10.1007/s00701-007-1447-z
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H (1989) Cerebral autoregulation dynamics in humans. Stroke 20:45–52. https://doi.org/10.1161/01.str.20.1.45
Parthasarathy AB, Gannon KP, Baker WB, Favilla CG, Balu R, Kasner SE et al (2018) Dynamic autoregulation of cerebral blood flow measured non-invasively with fast diffuse correlation spectroscopy. J Cereb Blood Flow Metab 38:230–240. https://doi.org/10.1177/0271678X17747833
Simpson DM, Payne SJ, Panerai RB (2022) The INfoMATAS project: methods for assessing cerebral autoregulation in stroke. J Cereb Blood Flow Metab 42(3):411–429. https://doi.org/10.1177/0271678X211029049
Olsen MH, Capion T, Riberholt CG, Bache S, Ebdrup SR, Rasmussen R et al (2023) Effect of controlled blood pressure increase on cerebralblood flow velocity and oxygenation in patients withsubarachnoid haemorrhage. Acta Anaesthesiol Scand 67:1054–1060. https://doi.org/10.1111/aas.14277
Swenne CA (2013) Baroreflex sensitivity: mechanisms and measurement. Neth Heart J 21:58–60. https://doi.org/10.1007/s12471-012-0346-y
Uryga A, Nasr N, Kasprowicz M, Budohoski K, Sykora M, Smielewski P et al (2022) Relationship between Baroreflex and cerebral autoregulation in patients with cerebral vasospasm after Aneurysmal Subarachnoid Hemorrhage. Front Neurol 12:740338. https://doi.org/10.3389/fneur.2021.740338
Uryga A, Nasr N, Kasprowicz M, Woźniak J, Goździk W, Burzyńska M (2022) Changes in autonomic nervous system during cerebral desaturation episodes in aneurysmal subarachnoid hemorrhage. Auton Neurosci 239:102968. https://doi.org/10.1016/j.autneu.2022.102968
Nellgard B, Rydenhag B, Nylén K, Terajima K (2007) Changes in baroreflex sensitivity in correlation to incidence of vasospasm and outcome in patients with subarachnoid hemorrhage. Acta Anaesthesiol Scand 51(suppl 118):31–32 Abstract
Nasr N, Gaio R, Czosnyka M, Budohoski K, Liu X, Donnelly J et al (2018) Baroreflex Impairment after Subarachnoid Hemorrhage is Associated with unfavorable outcome. Stroke 49:1632–1638. https://doi.org/10.1161/STROKEAHA.118.020729
Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD (1996) Monitoring of cerebral autoregulation in head-injured patients. Stroke 27(10):1829–1834. https://doi.org/10.1161/01.str.27.10.1829
Xiong L, Liu X, Shang T, Smielewski P, Donnelly J, Guo ZN et al (2017) Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry 88:520–531. https://doi.org/10.1136/jnnp-2016-314385
Farrell MC, Brenner AS, Shibao CA (2018) Diagnostic treatment dilemma: baroreflex failure or autoimmune autonomic ganglionopathy? Clin Auton Res 28:589–591. https://doi.org/10.1007/s10286-018-0556-5
Ogoh S, Tarumi T (2019) Cerebral blood flow regulation and cognitive function: a role of arterial baroreflex function. J Physiol Sci 69:813–823. https://doi.org/10.1007/s12576-019-00704-6
Fadel PJ, Stromstad M, Wray DW, Smith SA, Raven PB, Secher NH (2003) New insights into differential baroreflex control of heart rate in humans. Am J Physiol Heart Circ Physiol 284:H735–H743. https://doi.org/10.1152/ajpheart.00246.2002
Lantigua H, Ortega-Gutierrez S, Schmidt JM, Lee K, Badjatia N, Agarwal S, Claassen J et al (2015) Subarachnoid hemorrhage: who dies, and why? Crit Care 19:309. https://doi.org/10.1186/s13054-015-1036-0
Bischoff P, Rundshagen I (2011) Awareness under general anesthesia. Dtsch Arztebl Int 108:1–7. https://doi.org/10.3238/arztebl.2011.0001
Heming N, Mazeraud A, Azabou E, Moine P, Annane D (2020) Vasopressor Therapy and the brain: dark side of the Moon. Front Med (Lausanne) 6:317. https://doi.org/10.3389/fmed.2019.00317
Kecskés S, Menyhárt Á, Bari F, Farkas E (2023) Nimodipine augments cerebrovascular reactivity in aging but runs the risk of local perfusion reduction in acute cerebral ischemia. Front Aging Neurosci 15:1175281. https://doi.org/10.3389/fnagi.2023.1175281
Uryga A, Kasprowicz M, Burzyńska M, Kazimierska A, Czosnyka M, Nasr N (2023) Association between temporal patterns of baroreflex sensitivity after traumatic brain injury and prognosis: a preliminary study. Neurol Sci 44:1653–1663. https://doi.org/10.1007/s10072-022-06579-7
Acknowledgements
Not applicable.
Funding
This research was supported by the Research Council of Lithuania (Grant No. MIP-20–216) and the European Regional Development Fund under grant agreement with the Research Council of Lithuania (No. 01.2.2-LMT-K-718-03-0091).
Author information
Authors and Affiliations
Contributions
A.R., S.R. and V.Pe. initiated the research. S.R., and A.P., prepared study protocol and prepared documents to obtain ethical approval. I.L., A.P. and M.S performed clinical examinations on SAH patients, collected the clinical data and supervised patients during their treatment in ICU. L.B. and V.Pu. performed examinations on healthy control group and collected volunteers’ examination data. V.Pe, V.Pu. and E.C. prepared hardware and software for clinical data monitoring. E.C. and V.Pe processed and analyzed collected data. S.R. and A.R. supervised the analysis of study results and contributed to the final manuscript. All authors discussed the study results and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Clinical data collection was carried out in accordance with study protocols approved by the Vilnius Regional Biomedical Research Ethics Committees (Protocol No. 2021/6-1364-838). Participants and/or their legal guardians signed a written informed consent to participate in the study and use their anonymized clinical data for retrospective analysis.
Consent for publication
Written informed consent for publication of photography was obtained from the volunteer in Fig. 1. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
V.Pe., E.C., V.Pu., S.R. and M.S. received a research grant from the Research Council of Lithuania (Grant No. MIP-20-216). V.Pe., A.P., I.L., S.R. and M.S. received a research grant from the European Regional Development Fund (projects No 01.2.2-LMT-K-718-03-0091) under grant agreement with the Research Council of Lithuania (LMTLT). A.R. and L.B. declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaleckas, E., Putnynaite, V., Lapinskiene, I. et al. Impaired cerebral autoregulation detected in early prevasospasm period is associated with unfavorable outcome after spontaneous subarachnoid hemorrhage: an observational prospective pilot study. Ultrasound J 16, 24 (2024). https://doi.org/10.1186/s13089-024-00371-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-024-00371-8