Abstract
Background
To evaluate the efficacy and safety of upadacitinib monotherapy versus methotrexate (MTX) monotherapy over 5 years among MTX-naïve patients with moderately to severely active rheumatoid arthritis (RA) in the long-term extension (LTE) of the phase 3 SELECT-EARLY trial.
Methods
Patients were randomized to receive upadacitinib 15 mg or 30 mg or MTX. Patients who did not achieve CDAI remission and had < 20% improvement in tender and swollen joint counts at week 26 received rescue therapy (addition of MTX in the upadacitinib group and addition of upadacitinib in the MTX group). Efficacy assessments were evaluated over 5 years and are reported as observed (AO) for patients who received continuous monotherapy with upadacitinib 15/30 mg or MTX and by randomized group applying non-responder imputation (NRI). Treatment-emergent adverse events (TEAEs) per 100 patient-years were summarized over 5 years.
Results
Of 945 patients randomized and treated, 775 (82%) completed week 48 and entered the LTE on study drug. Higher proportions of patients consistently achieved disease activity targets over 5 years with upadacitinib than MTX. In AO analyses, 53%/59% of patients attained CDAI remission with upadacitinib 15/30 mg versus 43% with MTX at week 260. NRI analyses showed better CDAI, DAS28(CRP), and ACR responses with upadacitinib relative to MTX at week 260 (all comparisons, nominal P < .001). Upadacitinib treatment also resulted in numerically greater inhibition of structural joint progression through week 260 compared to MTX. Most TEAEs, serious AEs, and AEs leading to discontinuation were numerically higher in patients receiving upadacitinib 30 mg. Rates of serious infections, herpes zoster, creatine phosphokinase elevation, nonmelanoma skin cancer, and neutropenia were numerically higher with upadacitinib than MTX. The observed safety profile of upadacitinib over 5 years was consistent with earlier trial results and integrated phase 3 safety analyses.
Conclusions
Upadacitinib showed better clinical responses versus MTX in patients with RA throughout the 5-year trial. Higher rates of several AEs were observed with upadacitinib, especially in the 30 mg group, compared to MTX. When used as monotherapy in MTX-naïve patients, the approved upadacitinib 15 mg dose showed better long-term efficacy versus MTX and an overall favorable benefit-risk profile.
Trial registration
NCT02706873.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease that can cause impaired functional ability, chronic pain, and increased mortality rates if not properly treated [1]. Methotrexate (MTX) is the most commonly used first-line therapeutic choice for RA due to its well-known safety profile and demonstrated effectiveness in reducing disease activity and preventing joint damage [2,3,4]. However, not all patients respond well to MTX or can tolerate its side effects, highlighting the need for alternative therapeutic options [5,6,7]. Inhibition of Janus kinase (JAK)-mediated pathways has emerged as one such alternative mechanism of action for the treatment of RA [8, 9].
The JAK inhibitor upadacitinib has been extensively evaluated as part of the phase 3 SELECT RA clinical program, which involves six trials comprised of approximately 4800 patients with moderately to severely active RA, including those who have not responded adequately to prior conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) or biologic DMARDs (bDMARDs) [8, 10,11,12,13,14,15]. In the SELECT-EARLY trial, which focused on MTX-naïve patients, once daily treatment with upadacitinib 15 mg or 30 mg as monotherapy led to significant improvements in clinical, functional, and patient-reported outcomes over a 24-week period compared to MTX monotherapy [12]. In terms of safety, the rates of adverse events were slightly higher with upadacitinib 30 mg compared to upadacitinib 15 mg or MTX through 24 weeks. However, considering the chronic nature of RA and ongoing treatment requirements for most patients, it is crucial to assess the long-term safety of any therapeutic intervention. Thus, our objective in this analysis was to examine the efficacy and safety of upadacitinib over a 5-year period in the long-term extension (LTE) of the SELECT-EARLY trial.
Methods
Patients
Study eligibility criteria and baseline demographics for SELECT-EARLY have been previously described [12]. In brief, patients were ≥ 18 years old, exhibited symptoms consistent with RA for ≥ 6 weeks, and fulfilled the 2010 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) classification criteria for RA. Additional eligibility criteria included ≥ 6 swollen joints (based on 66 joint counts), ≥ 6 tender joints (based on 68 joint counts), and high-sensitivity C-reactive protein ≥ 5 mg/L (upper limit of normal 2.87 mg/L). Eligible patients also had ≥ 1 bone erosion on radiography or both positive rheumatoid factor (RF) and positive anti–citrullinated protein antibodies (ACPA) at screening. Patients were naïve to MTX, or if already on MTX, had received ≤ 3 weekly MTX doses with a requirement to complete a 4-week MTX washout before the first dose of study drug. Patients with prior exposure to csDMARDs other than MTX may have been enrolled if they completed a pre-defined washout period. Patients were excluded if they were intolerant to MTX, had prior exposure to any JAK inhibitor or any bDMARD, had a history of any arthritis with onset prior to age 17 years, or a current diagnosis of inflammatory joint disease other than RA.
The study was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines, applicable regulations, and the Declaration of Helsinki. All study-related documents were approved by independent ethics committees and institutional review boards. All patients provided written informed consent.
Study design and treatments
SELECT-EARLY (clinical trial number: NCT02706873) included a 48-week double-blind treatment period followed by an open-label LTE period for up to an additional four years (Supplemental Fig. 1). Patients were randomized 1:1:1 to receive once daily upadacitinib 15 mg or 30 mg or MTX (titrated up to 20 mg/week by week 8). A Japan substudy was also conducted in which patients were randomized 2:1:1:1 to receive once daily upadacitinib 7.5 mg, 15 mg, 30 mg or MTX; however, data from the 7.5 mg dose are not presented here. Treatment with background non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, oral glucocorticoids (equivalent to prednisone ≤ 10 mg/day), or inhaled glucocorticoids was allowed but must have been at a stable dose ≥ 1 week prior to the first dose of study drug. Optimization of some background RA medications was permitted from week 12 to week 24 (including NSAIDs, glucocorticoids, and/or low-potency analgesics). At week 26, patients who did not achieve Clinical Disease Activity Index (CDAI) remission (≤ 2.8) and at least 20% improvement from baseline in tender and swollen joint counts received rescue therapy (addition of MTX in the upadacitinib groups and addition of upadacitinib 15/30 mg [by re-randomization] in the MTX group). From week 36 to week 40, further optimization of background RA medications was allowed (including NSAIDs, glucocorticoids, low-potency analgesics, and/or csDMARD [only 1 of the following: sulfasalazine, hydroxychloroquine, or chloroquine]). Starting at week 48, initiation of or change in background RA medication(s), including glucocorticoids, NSAIDs, acetaminophen/paracetamol, and csDMARDs (≤ 2 csDMARDs except the combination of MTX and leflunomide) was allowed according to local label. Per protocol amendment, all patients receiving upadacitinib 30 mg were later switched to the approved 15 mg dose. Most patients who received upadacitinib 30 mg switched to 15 mg between weeks 158 to 192, with the earliest switch occurring at week 108.
Efficacy assessments
Efficacy assessments included the proportions of patients attaining clinical remission (defined by CDAI ≤ 2.8) or low disease activity (LDA; defined by CDAI ≤ 10) [16], 28-joint Disease Activity Score based on C-reactive protein (DAS28(CRP) < 2.6 or ≤ 3.2) [17, 18], ACR20/50/70 response criteria [19], and the 2010 definition of ACR/EULAR Boolean remission [20]. Additionally, changes from baseline in ACR components such as Health Assessment Questionnaire-Disability Index (HAQ-DI) [21] and patient’s assessment of pain were examined, along with the severity and duration of morning stiffness. Radiographic assessments were completed at weeks 24, 96, 192, and 260 and included the proportion of patients with no radiographic progression (defined as modified Total Sharp Score [mTSS] ≤ 0) [22, 23] and change from baseline in mTSS, joint space narrowing, and erosion scores. All other efficacy assessments were performed every 12 weeks during the LTE.
Safety assessments
Data on safety outcomes were collected from all patients who received upadacitinib or MTX, with adverse event (AE) assignment determined based on the treatment at the time of the event. All treatment-emergent adverse events (TEAEs) were summarized using the Medical Dictionary for Regulatory Activities (MedDRA) (version 25.0) primary system organ class and preferred term. TEAEs were defined as any events that occurred after the first dose of study drug, but no later than 30 days after the last dose of study drug. All presented AEs were treatment-emergent, with the exception of mortality assessments, which also included deaths that were not treatment-emergent and occurred more than 30 days after the last dose of study drug.
Safety assessments were performed as previously described [12]. Major adverse cardiovascular events (MACE) and venous thromboembolism (VTE) events were adjudicated by an independent Cardiovascular Adjudication Committee (CAC) in a blinded manner. MACE were defined as either cardiovascular (CV) death, non-fatal myocardial infarction (MI), or non-fatal stroke. VTE was defined as deep vein thrombosis (DVT) or pulmonary embolism (PE). Gastrointestinal (GI) perforations were adjudicated by an internal independent committee of gastroenterologists. Laboratory parameters were assessed up to week 260, including the percentage of patients experiencing potentially clinically significant (grade 3 or 4) changes during the treatment period. Per protocol, study investigators evaluated the severity of TEAEs according to the Rheumatology Common Toxicity Criteria (CTC) version 2.0 (Outcome Measures in Rheumatology [OMERACT]). For creatine phosphokinase (CPK) and creatinine, however, National Cancer Institute (NCI) CTC version 4.0 criteria were used.
Statistical analysis
Efficacy assessments were evaluated through 5 years and are reported as observed (AO) for patients who received continuous monotherapy with upadacitinib 15 mg or 30 mg or MTX. Efficacy outcomes are also reported by randomized group for all randomized and dosed study participants, applying NRI for patients who were rescued or discontinued. Treatment comparisons for NRI analyses were made using the Cochran-Mantel-Haenszel test, adjusting for the stratification factor of geographic region. Continuous endpoints were evaluated using AO and the mixed model for repeated measures (MMRM) analysis for patients receiving continuous monotherapy. Given the small sample size of patients who received rescue therapy (i.e., upadacitinib 15 mg or 30 mg plus MTX), efficacy is not reported separately for these patients. For inclusion in radiographic analyses, patients were required to have X-ray images at both week 192 and week 260. Data collected after dose switch from upadacitinib 30 mg to the approved 15 mg dose following protocol amendment continued to be summarized under the original treatment sequences, regardless of dose switch. A separate evaluation of CDAI responses before and after the dose switch was also conducted.
TEAEs per 100 patient-years were summarized through 5 years for patients receiving either dose of upadacitinib monotherapy or MTX monotherapy, as well as patients who switched from upadacitinib monotherapy at 30 mg to 15 mg for the post-switch period. Upadacitinib or MTX monotherapy exposure was censored at time of rescue to upadacitinib plus MTX/csDMARD. In addition, upadacitinib 30 mg exposure was censored at the time of dose switch from 30 mg to 15 mg. All safety data are reported as exposure-adjusted event rates (EAERs), defined as events per 100 patient-years (E/100 PY).
Results
Patients
Baseline characteristics were generally balanced across all treatment groups (Supplemental Table 1), as previously reported [12]; 75% of patients had no exposure to any csDMARD prior to enrollment. Most patients had early RA (median disease duration of 0.5 years) and risk factors for structural progression, as demonstrated by positivity for RF and ACPA and/or at least one bone erosion. Of the 945 patients randomized and treated, 775 (82%) completed week 48 and entered the LTE on study drug (Fig. 1). Of these 775 patients, 255 (33%) discontinued study drug during the LTE. The most common primary reason for discontinuation was TEAE (11%), followed by withdrawal of consent (8%), lack of efficacy (3%), lost to follow-up (3%), or other reasons (8%). Higher proportions of patients randomized to MTX (11%) received rescue therapy versus those who initially received either dose of upadacitinib (3–6%). Similarly, higher proportions of patients randomized to upadacitinib 15 mg (61%) or 30 mg (54%) completed 5 years of continuous treatment relative to those who started with MTX (39%).
During the study, approximately half of patients used concomitant glucocorticoids (55%, 53%, and 59% for upadacitinib 15 mg, upadacitinib 30 mg, and MTX monotherapy groups, respectively). Among patients with glucocorticoid use at baseline, the percentages of patients who discontinued their use by week 260 were as follows: 36% (n = 38/107) for upadacitinib 15 mg, 48% (n = 41/86) for upadacitinib 30 mg, and 39% (n = 28/71) for MTX.
Disposition of Patients Through 5 Years in SELECT-EARLY. AE, adverse event; CDAI, Clinical Disease Activity Index; D/C, discontinued; L/C, logistical constraints; LoE, lack of efficacy; MTX, methotrexate; QD, once daily; UPA, upadacitinib; W, week. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 mg dose per protocol amendment. The switch occurred at different visits across the patient population, with the earliest switch occurring at the week 108 visit. bAt week 26, patients who did not achieve CDAI remission and at least 20% improvement from baseline in tender and swollen joint counts received rescue therapy (addition of MTX to insufficient responders in the UPA groups and addition of UPA 15/30 mg to insufficient responders in the MTX group)
Efficacy
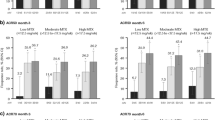
Patients receiving upadacitinib consistently demonstrated higher achievement of disease activity targets over 5 years compared with those receiving MTX. In AO analyses, 53%/59% of patients attained CDAI remission with upadacitinib 15/30 mg monotherapy versus 43% with MTX monotherapy at week 260 (Fig. 2). Similarly, 91%/88% achieved CDAI LDA with upadacitinib 15/30 mg compared to 80% with MTX at week 260; attainment of DAS28(CRP) < 2.6 and ≤ 3.2 with upadacitinib 15/30 mg was 78%/81% and 89%/92% compared to 60% and 76% with MTX, respectively (AO; Fig. 2). Among patients randomized to upadacitinib 30 mg, those who switched to the approved 15 mg dose, per protocol amendment, maintained their CDAI responses (Supplemental Fig. 2). NRI analyses also showed better CDAI and DAS28(CRP) responses with upadacitinib 15/30 mg relative to MTX at week 260 (all comparisons from week 8 to week 260, nominal P < .001) (Fig. 3). Higher proportions of patients also attained the stringent Boolean-based definition of remission with upadacitinib compared to MTX (Figs. 2 and 3). Based on NRI analyses at week 260, 23%/25% of patients attained Boolean remission with upadacitinib 15/30 mg treatment versus 12% with MTX (both comparisons, nominal P < .001 (Fig. 3).
Proportions of Patients Achieving CDAI, DAS28(CRP) Disease Activity States and Boolean Remission Through 5 Years (AO). AO, as observed; CDAI, Clinical Disease Activity Index; DAS28(CRP), 28-joint Disease Activity Score based on C-reactive protein; LDA, low disease activity; mono, monotherapy; MTX, methotrexate; QD, once daily; UPA, upadacitinib. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 dose mg per protocol amendment. The switch occurred at different visits across the patient population, with the earliest switch occurring at the week 108 visit. Thresholds for CDAI were ≤ 2.8 for remission and ≤ 10 for LDA
Proportions of Patients Achieving CDAI, DAS28(CRP) Disease Activity States and Boolean Remission Through 5 Years (NRI). CDAI, Clinical Disease Activity Index; DAS28(CRP), 28-joint Disease Activity Score based on C-reactive protein; LDA, low disease activity; MTX, methotrexate; NRI, non-responder imputation; QD, once daily; UPA, upadacitinib. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 mg dose per protocol amendment. The switch occurred at different visits across the patient population, with the earliest switch occurring at the week 108 visit. Indicated assessments from period 1 (at weeks 4, 12, 24, 36, and 48) are summarized here along with all assessments from the long-term extension. Nominal ***P < .001, **P < .01, and *P < .05 for upadacitinib 15 mg versus MTX. Nominal ###P < .001, ##P < .01, and #P < .05 for upadacitinib 30 mg versus MTX. Thresholds for CDAI were ≤ 2.8 for remission and ≤ 10 for LDA
Numerically greater proportions of patients achieved ACR20/50/70 responses with upadacitinib 15 mg or 30 mg compared to MTX over 5 years (AO; Fig. 4). When analyzing the results by NRI, more patients randomized to upadacitinib 15 mg or 30 mg met ACR20/50/70 criteria at week 260 than those randomized to MTX (upadacitinib 15 mg: 56%/51%/44%; upadacitinib 30 mg: 50%/46%/36%; MTX: 33%/30%/23%; all comparisons, nominal P < .001) (Fig. 5). Additionally, patients receiving upadacitinib 15 mg or 30 mg demonstrated greater numerical improvements from baseline in HAQ-DI and pain at week 260 compared to those treated with MTX monotherapy (HAQ-DI: -1.13/-1.03 with upadacitinib 15/30 mg versus − 0.89 with MTX; pain: -51.2/-49.1 with upadacitinib 15/30 mg versus − 41.9 with MTX; AO) (Supplemental Fig. 3). Consistent results were observed for other ACR core components over 5 years, except for 68-tender joint count, 66-swollen joint count, and physician’s global assessment in which responses were generally similar between groups. Treatment with both upadacitinib doses also led to improvements in morning stiffness relative to MTX (Supplemental Fig. 4).
Proportions of Patients Achieving ACR Response Criteria Through 5 Years (AO). ACR20/50/70, ≥ 20%/50%/70% improvement in American College of Rheumatology response criteria; AO, as observed; mono, monotherapy; MTX, methotrexate; QD, once daily; UPA, upadacitinib. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 mg dose per protocol amendment. The switch occurred at different visits across the patient population, with the earliest switch occurring at the week 108 visit
Proportions of Patients Achieving ACR Response Criteria Through 5 Years (NRI). ACR20/50/70, ≥ 20%/50%/70% improvement in American College of Rheumatology response criteria; mono, monotherapy; MTX, methotrexate; NRI, non-responder imputation; QD, once daily; UPA, upadacitinib. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 mg dose per protocol amendment. The switch occurred at different visits across the patient population, with the earliest switch occurring at the week 108 visit. Indicated assessments from period 1 (at weeks 4, 12, 24, 36, and 48) are summarized here along with all assessments from the long-term extension. Nominal ***P < .001, **P < .01, and *P < .05 for upadacitinib 15 mg versus MTX. Nominal ###P < .001, ##P < .01, and #P < .05 for upadacitinib 30 mg versus MTX
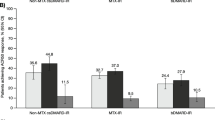
Regarding structural joint damage, 87%/86% of patients receiving upadacitinib 15/30 mg monotherapy had no radiographic progression (defined as a change from baseline in mTSS ≤ 0) at week 260 compared to 70% of those receiving MTX monotherapy (Fig. 6). The change from baseline in mTSS, erosion score, and joint space narrowing was numerically lower with upadacitinib 15 mg and 30 mg relative to MTX at all radiographic assessments through week 260.
Radiographic Progression Through 5 Years (AO). AO, as observed; BL, baseline; mono, monotherapy; mTSS, modified Total Sharp Score; MTX, methotrexate; QD, once daily; UPA, upadacitinib. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 mg dose per protocol amendment. The switch occurred at different visits across the patient population, with the earliest switch occurring at the week 108 visit. Patients were required to have radiographic images at both week 192 and week 260 for inclusion in these analyses. Error bars represent the 95% confidence interval
Safety
The mean duration of exposure was 3.4 years with upadacitinib 15 mg monotherapy, 2.4 years with upadacitinib 30 mg monotherapy, and 2.7 years with MTX monotherapy. Most TEAEs, including serious AEs, were more frequent in patients receiving upadacitinib 30 mg monotherapy than those receiving upadacitinib 15 mg or MTX monotherapy, and patients who switched from upadacitinib 30 mg to the approved 15 mg dose per protocol amendment had similar event rates post-switch compared to those initially randomized to upadacitinib 15 mg (Table 1). Across treatment groups, the majority of TEAEs (~ 95%) were mild to moderate in severity. The rates of TEAEs leading to discontinuation of study drug were comparable in the upadacitinib 15 mg group and the MTX group, but higher in the upadacitinib 30 mg group. The most frequently reported TEAEs (≥ 5 E/100 PY in any dose group) for patients receiving upadacitinib were elevated CPK, upper respiratory tract infection, and urinary tract infection (Supplemental Table 2).
Adverse events of special interest (AESIs) were consistent with the established safety profile of upadacitinib, and no new safety issues were identified from the long-term study. The most frequently reported AESIs (> 5 E/100 PY in any treatment group) were hepatic disorder, neutropenia, and CPK elevation (Fig. 7). EAERs of CPK elevation, neutropenia, serious infections, herpes zoster, and nonmelanoma skin cancer (NMSC) were numerically higher with upadacitinib 15 mg or 30 mg than MTX. Rates of CPK elevation appeared to be dose-dependent with upadacitinib (6.4 and 14.3 E/100 PY for upadacitinib 15 mg and 30 mg groups, respectively) and lower with MTX (1.4 E/100 PY). Most CPK events were mild to moderate in severity, asymptomatic, and transient; there were no reports of rhabdomyolysis. Two events of CPK elevation were serious (one each in the upadacitinib 15 mg and 30 mg plus MTX rescue group), one of which led to discontinuation of study drug. Rates of neutropenia were higher in the upadacitinib 30 mg monotherapy group (5.5 E/100 PY) than in the upadacitinib 15 mg monotherapy group (3.2 E/100 PY); rates in both upadacitinib groups were higher than in the MTX monotherapy group (1.7 E/100 PY). Most cases of neutropenia were mild to moderate in severity.
Exposure-Adjusted Event Rates for Adverse Events of Special Interest Through 5 Years. AE, adverse event; AESI, adverse event of special interest; CPK, creatine phosphokinase; DMARD, disease-modifying antirheumatic drug; GI, gastrointestinal; MACE, major adverse cardiovascular event; mono, monotherapy; MTX, methotrexate; NMSC, nonmelanoma skin cancer; PY, patient-years; QD, once daily; TB, tuberculosis; UPA, upadacitinib; VTE, venous thromboembolism. Treatment-emergent adverse event is defined as any adverse event with an onset date that is after the first dose of study drug, and no more than 30 days after the last dose of study drug. Data include patients receiving UPA or MTX monotherapy, censored at either time of rescue to UPA + MTX or with addition of background conventional synthetic DMARD. aPatients in the UPA 30 mg treatment group were later switched to the approved UPA 15 mg dose per protocol amendment. Upadacitinib 30 mg QD exposure was censored at the time of dose switch from 30 mg QD to 15 mg QD. bOpportunistic infections exclude herpes zoster and TB. cDefined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. dIncludes pulmonary embolism and deep vein thrombosis
Four treatment-emergent adjudicated GI perforations were reported among three patients in the upadacitinib 30 mg monotherapy group, all of which were serious events, including diverticular perforation, peritonitis and gastric ulcer perforation, and large intestine perforation. Confounding factors for these patients included gastroesophageal reflux disease, hypothyroidism, osteoporosis, hypertension, gastritis, obesity, and diverticulosis; one patient was also a former smoker (1 pack per day for 40 years). No GI perforation events were reported in patients receiving upadacitinib 15 mg or MTX.
Consistent with the known increased risk of herpes zoster with JAK inhibitor treatment [24,25,26], rates of herpes zoster events were higher in the upadacitinib 15 mg and 30 mg monotherapy groups (3.9 E/100 PY and 4.5 E/100 PY, respectively) compared to the MTX monotherapy group (0.8 E/100 PY), with the highest rate in the upadacitinib 30 mg monotherapy group. Most herpes zoster events were nonserious and involved only 1 or 2 dermatomes. Five herpes zoster events were reported as having ophthalmic involvement (2 with upadacitinib 15 mg + MTX, 1 with upadacitinib 30 mg monotherapy, and 2 in the upadacitinib 30 mg switched to upadacitinib 15 mg monotherapy group). One patient receiving upadacitinib 30 mg + MTX had herpes zoster meningitis, which led to discontinuation of study drug, and the patient subsequently recovered.
Rates of serious infection were higher with upadacitinib 15 mg (3.2 E/100 PY) and 30 mg (4.5 E/100 PY) compared to MTX (1.9 E/100 PY). Pneumonia was the most frequently reported serious infection among upadacitinib monotherapy and MTX monotherapy groups, except the upadacitinib 30 mg switched to 15 mg group, where COVID-19 pneumonia was the most frequently reported serious infection. Treatment-emergent COVID-19 infection or COVID-19-related AEs were comparable across the upadacitinib 15 mg monotherapy and MTX monotherapy groups. Consistent with the timing of the transition of patients to the approved 15 mg dose from the 30 mg dose with the onset and course of the COVID-19 pandemic, rates of COVID-19-related AEs were lower in patients receiving upadacitinib monotherapy and higher in the upadacitinib 30 mg switched to 15 mg group.
EAERs of MACE, VTE, and malignancy excluding NMSC were generally comparable across treatment groups (Fig. 7). Rates of MACE were 0.3, 0.3, and 0.5 E/100 PY in the upadacitinib 15 mg, 30 mg, and MTX monotherapy groups, respectively; rates of VTE were 0.6, 0.3, and 0.5 E/100 PY; rates of malignancies excluding NMSC were 0.9, 0.6, and 1.1 E/100 PY. Additional details regarding these AESIs are reported in the Supplemental Materials text. Most patients who experienced MACE had at least 1 CV risk factor at baseline. Among the 24 cases of malignancies excluding NMSC that were reported among monotherapy groups, there was no clear pattern in the types of malignancies that were observed and most occurred in a single patient. Although the rates of any malignancy other than NMSC were similar between treatment groups, the rate of NMSC was higher in the upadacitinib 30 mg monotherapy group compared to the upadacitinib 15 mg group (1.1 and 0.4 E/100 PY, respectively), and there were no events of NMSC in the MTX monotherapy group.
EAERs of anemia and hepatic disorders were also generally similar across treatment groups. Most cases of anemia were mild to moderate in severity, and the majority of hepatic disorders events were mild, asymptomatic transaminase elevations (further detailed in Supplemental Materials text). Rates of lymphopenia were numerically lower in the upadacitinib 15 mg group (1.6 E/100 PY) compared to upadacitinib 30 mg or MTX groups (3.1 and 3.3 E/100 PY, respectively). Most lymphopenia events had a mild to moderate severity.
Rates of death were similar between upadacitinib 15 mg and MTX (0.6 and 0.9 E/100 PY, respectively) but numerically higher with upadacitinib 30 mg (1.2 E/100 PY). Of the 31 deaths reported during the study, 8 were adjudicated by the CAC as cardiovascular in nature, 7 were COVID-19-related, and 4 had unknown/undetermined causes (Supplemental Materials text). All 7 COVID-19-related deaths occurred in patients treated with upadacitinib 15 mg or 30 mg. Common underlying risk factors included hypertension, obesity, tobacco use, and/or diabetes.
There were no notable mean changes in laboratory parameter values (hematology, clinical chemistry, and urinalysis) from baseline. However, higher proportions of patients with grade 3/4 CPK elevations were reported with upadacitinib than MTX, with the highest proportions occurring in the upadacitinib 30 mg monotherapy group (Supplemental Table 3). Among patients with an increase in blood CPK values that were grade 3/4, most were asymptomatic. The percentage of patients with a grade 3 decrease in neutrophils also occurred more frequently with either dose of upadacitinib than MTX; however, most instances were isolated events, occurring at only one time point.
Discussion
The management of RA involves a multi-faceted approach, with the goals of sustained disease remission, or at least LDA, and prevention of long-term structural joint damage [3, 4]. MTX has long been considered the cornerstone of RA treatment, but the development of newer targeted therapies over the last decade has expanded the treatment options available to patients, particularly in those who do not respond adequately to MTX or are intolerant of its side effects. Our study provides valuable insights into the head-to-head efficacy and safety of upadacitinib monotherapy versus MTX monotherapy in patients with early RA and risk factors for structural joint progression. Throughout the 5-year study, upadacitinib 15 mg and 30 mg once daily continued to be effective in treating the signs and symptoms of RA, resulting in better long-term efficacy versus MTX. Higher rates of several AEs, including serious infection, herpes zoster, and CPK elevation were observed with upadacitinib, particularly in the 30 mg dose group, compared to MTX. No new safety issues emerged from the long-term study, and our findings are consistent with the known safety profile of upadacitinib [24, 27].
Although many patients were able to achieve disease activity targets with MTX monotherapy, as expected in this mostly (~ 93%) MTX naïve population, those receiving upadacitinib 15 mg or 30 mg monotherapy showed consistently better efficacy responses. In terms of clinical remission, the primary treatment target for this population, 53% and 59% of patients achieved CDAI remission with upadacitinib 15 mg and 30 mg, respectively, versus 43% with MTX (AO). Similarly, greater proportions of patients achieved CDAI LDA, DAS28(CRP), and ACR20/50/70 targets with either upadacitinib 15 mg or 30 mg versus MTX. These results are further supported by NRI analyses, indicating that upadacitinib 15 mg and 30 mg outperformed MTX across all evaluated efficacy endpoints at week 260 (all comparisons, nominal P < .001). Over the 5-year study, notable improvements from baseline were observed for physical function and pain across all groups, with upadacitinib 15 mg or 30 mg treatment resulting in numerically greater improvements relative to MTX. Morning stiffness, a key symptom of RA that can greatly impact a patient’s daily life and work [28], also showed reduced severity and duration with upadacitinib treatment compared to MTX. Additionally, there was no apparent loss of benefit in patients who switched from upadacitinib 30 mg to the approved 15 mg dose following protocol amendment.
Treatment with either upadacitinib or MTX demonstrated an inhibitory effect on structural joint damage in patients with RA. However, a larger percentage of patients receiving upadacitinib 15 mg and 30 mg monotherapy showed no radiographic progression, as determined by a change from baseline to week 260 in mTSS ≤ 0, compared to those receiving MTX monotherapy. Changes from baseline in mTSS, as well as joint erosion and narrowing scores, were also numerically lower with upadacitinib 15 mg and 30 mg relative to MTX at all radiographic assessments through week 260. Ultimately, however, both upadacitinib and MTX appeared to successfully slow joint damage progression, and there was effectively no clinically meaningful difference between the treatment arms in this regard.
The safety profile of upadacitinib over 5 years was generally consistent with earlier results from this trial and integrated phase 3 safety analyses [12, 24, 27, 29]. Most TEAEs were more frequent in patients receiving upadacitinib 30 mg compared to upadacitinib 15 mg or MTX. Moreover, patients who switched from upadacitinib 30 mg to 15 mg had similar event rates post-switch compared to those initially randomized to upadacitinib 15 mg. The rates of CPK elevation, serious infections, herpes zoster, NMSC, and neutropenia were also numerically higher with upadacitinib, especially in the 30 mg treatment group, compared to MTX. It is important to note, however, that the majority of CPK events were asymptomatic and transient; moreover, most herpes zoster events were nonserious and limited to only 1 or 2 dermatomes. Rates of MACE and VTE were comparable across treatment groups. Malignancies excluding NMSC also occurred at similar rates between upadacitinib 15 mg or 30 mg and MTX and were generally consistent with the rates expected in RA populations based on real-world data [30,31,32]. Rates of death were similar between upadacitinib 15 mg and MTX, but numerically higher with upadacitinib 30 mg.
Limitations of this study include potential biases inherent to LTE studies, an example of which is that they tend to overestimate treatment efficacy because patients who continue participating in the trial over the long term are typically those who respond well to the treatment and tolerate it without issues. To address this potential bias, we included more conservative estimates based on NRI in addition to AO data. Another limitation is that although most patients had a relatively short duration of RA, a proportion had been living with the disease for a longer period but had not undergone treatment with MTX. Lastly, the trial was restricted to patients who had risk factors for radiographic progression, which may not be representative of all MTX-naïve patients with RA. Thus, caution should be exercised when extrapolating these findings to other patient populations. However, despite these limitations, results from this 5-year study provide important insights into the long-term benefit-risk of upadacitinib versus MTX in a clinically controlled setting. Additionally, with a total of 945 patients treated, SELECT-EARLY stands as one of the largest, global double-blind trials ever conducted in MTX-naïve patients.
Conclusions
In summary, upadacitinib 15 mg or 30 mg showed better clinical, radiographic, and patient-reported outcomes versus MTX in patients with RA throughout the 5-year trial. Higher rates of serious infection, herpes zoster, and neutropenia were observed with upadacitinib compared to MTX, especially in the 30 mg group; higher rates of CPK elevation was also observed with both upadacitinib doses but without clinical consequences. When used as monotherapy in MTX-naïve patients, treatment with upadacitinib 15 mg demonstrated better long-term efficacy versus MTX and an overall favorable benefit-risk profile. These findings support the use of the 15 mg daily dose for RA, as has been approved by the US Food and Drug Administration and the European Medicine Agency.
Data availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home.”
Abbreviations
- ACPA:
-
Anti–citrullinated protein antibodies
- ACR:
-
American College of Rheumatology
- AE:
-
Adverse event
- AESI:
-
Adverse event of special interest
- AO:
-
As observed
- bDMARD:
-
Biologic disease-modifying antirheumatic drug
- CAC:
-
Cardiovascular Advisory Committee
- CDAI:
-
Clinical Disease Activity Index
- CPK:
-
Creatine phosphokinase
- csDMARD:
-
Conventional synthetic disease-modifying antirheumatic drug
- CTC:
-
Common Toxicity Criteria
- CV:
-
Cardiovascular
- DAS28(CRP):
-
28-joint Disease Activity Score based on C-reactive protein
- DVT:
-
Deep vein thrombosis
- E:
-
Event
- EAER:
-
Exposure-adjusted event rate
- EULAR:
-
European Alliance of Associations for Rheumatology
- HAQ-DI:
-
Health Assessment Questionnaire-Disability Index
- JAK:
-
Janus kinase
- LDA:
-
Low disease activity
- LTE:
-
Long-term extension
- MACE:
-
Major adverse cardiovascular events
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- MI:
-
Myocardial
- MMRM:
-
Mixed model for repeated measures
- mTSS:
-
Modified Total Sharp Score
- MTX:
-
Methotrexate
- NMSC:
-
Nonmelanoma skin cancer
- NRI:
-
Non-responder imputation
- OMERACT:
-
Outcome Measures in Rheumatology
- PY:
-
Patient-years
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- TEAE:
-
Treatment-emergent adverse event
- VTE:
-
Venous thromboembolism
References
Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. https://doi.org/10.1038/nrdp.2018.1.
Pincus T, Gibson KA, Castrejon I. Update on methotrexate as the anchor drug for rheumatoid arthritis. Bull Hosp Jt Dis (2013) 2013;71 Suppl 1:S9-19.
Smolen JS, Landewe RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2022. https://doi.org/10.1136/ard-2022-223356.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924–39. https://doi.org/10.1002/acr.24596.
Fatimah N, Salim B, Nasim A, et al. Frequency of methotrexate intolerance in rheumatoid arthritis patients using methotrexate intolerance severity score (MISS questionnaire). Clin Rheumatol. 2016;35(5):1341–5. https://doi.org/10.1007/s10067-016-3243-8.
Ling S, Bluett J, Barton A. Prediction of response to methotrexate in rheumatoid arthritis. Expert Rev Clin Immunol. 2018;14(5):419–29. https://doi.org/10.1080/1744666X.2018.1465409.
Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–82. https://doi.org/10.1016/S0140-6736(08)61000-4.
Tanaka Y. A review of upadacitinib in rheumatoid arthritis. Mod Rheumatol. 2020;30(5):779–87. https://doi.org/10.1080/14397595.2020.1782049.
Cohen S, Reddy V. Janus kinase inhibitors: efficacy and safety. Curr Opin Rheumatol. 2023;35(6):429–34. https://doi.org/10.1097/BOR.0000000000000972.
Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–24. https://doi.org/10.1016/S0140-6736(18)31116-4.
Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–12. https://doi.org/10.1016/S0140-6736(18)31115-2.
van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of Upadacitinib Monotherapy in Methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a Multicenter, Multi-country, Randomized, Double-Blind, active Comparator-Controlled Trial. Arthritis Rheumatol. 2020;72(10):1607–20. https://doi.org/10.1002/art.41384.
Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib Versus Placebo or Adalimumab in patients with rheumatoid arthritis and an inadequate response to Methotrexate: results of a phase III, Double-Blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–800. https://doi.org/10.1002/art.41032.
Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N Engl J Med. 2020;383(16):1511–21. https://doi.org/10.1056/NEJMoa2008250.
Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303–11. https://doi.org/10.1016/S0140-6736(19)30419-2.
Aletaha D, Smolen J. The simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8.
Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. https://doi.org/10.1002/art.1780380107.
Wells G, Becker JC, Teng J, et al. Validation of the 28-joint disease activity score (DAS28) and European League against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954–60. https://doi.org/10.1136/ard.2007.084459.
Felson DT, Anderson JJ. Methodological and statistical approaches to criteria development in rheumatic diseases. Baillieres Clin Rheumatol. 1995;9(2):253–66. https://doi.org/10.1016/s0950-3579(05)80189-x.
Bykerk VP, Massarotti EM. The new ACR/EULAR remission criteria: rationale for developing new criteria for remission. Rheumatology (Oxford). 2012;51(Suppl 6):vi16–20. https://doi.org/10.1093/rheumatology/kes281.
Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–93.
van der Heijde D. How to read radiographs according to the Sharp/van Der Heijde method. J Rheumatol. 1999;26(3):743–5.
van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35(1):26–34. https://doi.org/10.1002/art.1780350105.
Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-218510.
Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76(7):1253–62. https://doi.org/10.1136/annrheumdis-2016-210457.
Smolen JS, Genovese MC, Takeuchi T, et al. Safety Profile of Baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol. 2019;46(1):7–18. https://doi.org/10.3899/jrheum.171361.
Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1). https://doi.org/10.1136/rmdopen-2022-002735.
Bacci ED, DeLozier AM, Lin CY, et al. Psychometric properties of morning joint stiffness duration and severity measures in patients with moderately to severely active rheumatoid arthritis. Health Qual Life Outcomes. 2017;15(1):239. https://doi.org/10.1186/s12955-017-0813-7.
van Vollenhoven R, Takeuchi T, Aelion J, et al. POS0655 long-term safety and efficacy of upadacitinib in patients with rheumatoid arthritis: 3-year results from SELECT-EARLY study [Abstract]. Ann Rheum Dis. 2021;80:568–69.
Askling J, Berglind N, Franzen S, et al. How comparable are rates of malignancies in patients with rheumatoid arthritis across the world? A comparison of cancer rates, and means to optimise their comparability, in five RA registries. Ann Rheum Dis. 2016;75(10):1789–96. https://doi.org/10.1136/annrheumdis-2015-208105.
Gross RL, Schwartzman-Morris JS, Krathen M, et al. A comparison of the malignancy incidence among patients with psoriatic arthritis and patients with rheumatoid arthritis in a large US cohort. Arthritis Rheumatol. 2014;66(6):1472–81. https://doi.org/10.1002/art.38385.
Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–95. https://doi.org/10.1002/art.22864.
Acknowledgements
AbbVie and the authors thank the patients, trial sites, and investigators who participated in this clinical trial. AbbVie was the trial sponsor, contributed to trial design, data collection, analysis and interpretation, and to writing, reviewing, and approval of final version. No honoraria or payments were made for authorship. Medical writing support was provided by Matthew Eckwahl, PhD, of AbbVie. Editorial support was provided by Angela T. Hadsell of AbbVie.
Funding
AbbVie funded the study and had a role in the study design, data collection, data analysis, data interpretation and writing of the report. All authors had access to the relevant data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
RvT, TT, and VS contributed to study conception and design. JA, TT, NC, PMW, AS, and JS participated in data acquisition. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Studies were conducted per the International Conference on Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki. Study-related documents were approved by independent ethics committees and institutional review boards. All patients provided written, informed consent.
Consent for publication
Not applicable.
Role of authors and Sponsor
AbbVie Inc. was the trial sponsor, and the trial was designed by AbbVie Inc., the authors, and investigators. Clinical data were collected by the investigators, their teams, and AbbVie Inc. AbbVie Inc. was involved in data analysis, the interpretation of results and the preparation, review and approval of the final version of this report. All the authors had access to the data, reviewed and approved the final version, made the decision to submit the manuscript for publication, and attest to the accuracy and completeness of the data. The corresponding author had access to all relevant data and the final responsibility to submit for publication. A medical writer, employed by AbbVie Inc., assisted with preparing an initial draft under the direction of the authors.
Disclosure
Some of the results reported in this manuscript were originally presented at the American College of Rheumatology 2023 Congress, as follows: van Vollenhoven R et al., Upadacitinib Monotherapy versus Methotrexate in Patients with Rheumatoid Arthritis: Efficacy and Safety Through 5 Years in the SELECT-EARLY Randomized Controlled Trial. Arthritis Rheumatol. 2023; 75 (suppl 9); https://acrabstracts.org/abstract/upadacitinib-monotherapy-versus-methotrexate-in-patients-with-rheumatoid-arthritis-efficacy-and-safety-through-5-years-in-the-select-early-randomized-controlled-trial/.
Competing interests
R van Vollenhoven: Research Support (institutional grants): BMS, UCB; support for educational programs (institutional grants): AstraZeneca, Galapagos, MSD, Novartis, Pfizer, Roche, Sanofi, UCB; consultancy, for which institutional and/or personal honoraria were received: AbbVie, AstraZeneca, Biogen, BMS, Galapagos, Janssen, Pfizer, RemeGen, UCB; speaker, for which institutional and/or personal honoraria were received: AbbVie, AstraZeneca, BMS, Galapagos, GSK, Janssen, Pfizer, UCB.V Strand: Consultant for AbbVie, Alpine Immune Sciences, Alumis, Amgen, AstraZeneca, Bayer, Blackrock, BMS, Boehringer Ingelheim, Celltrion, Ermium, Genentech/Roche, GlaxoSmithKline, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Merck, MiMedx, Novartis, Omeros, Pfizer, R-Pharm, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix, and Urica.T Takeuchi: Grant/research support from AbbVie and Eisai; consulting fees from AbbVie, Astellas Pharma, Eli Lilly Japan, and Gilead Sciences; speaker/honoraria from AbbVie, Astellas Pharma, Eisai, Eli Lilly Japan, Gilead Sciences, and Pfizer Japan.J Aelion: Grant or research support: AbbVie, Acceleron, Acelyrin, Aclaris Therapeutics, Alpine Immune Sciences, Amgen, AstraZeneca, Biogen, BMS, Eli Lilly, Galapagos, GSK, Horizon, Janssen, Novartis, Selecta, UCB, Ventyx; consultancy for AbbVie, Amgen, BMS, Janssen, Novartis; speaker for AbbVie and Amgen. N Chávez: Speakers bureau for AbbVie, Janssen, and Pfizer; consultant of AbbVie, Janssen, and Pfizer; grant/research support from AbbVie, Galapagos, Gilead, Pfizer, and Sanofi. P Mannucci Walter: consulting fees from AbbVie; grant/research support from AbbVie, Bristol-Myers Squibb, Lilly, Genentech/Roche, GSK, Janssen, and UCB. Atul Singhal: No conflicts of interest to declare. J Swierkot: Speaker, research grants, and consulting fees from AbbVie, Amgen, Astra Zeneca, Celltrion, Eli Lilly, Novartis, Sandoz, Pfizer, Roche, BMS, UCB, MSD, Accord, and Janssen.N Khan, X Bu, Y Li, SK Penn, HS Camp: Employee of AbbVie and may hold stock or options.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Vollenhoven, R., Strand, V., Takeuchi, T. et al. Upadacitinib monotherapy versus methotrexate monotherapy in patients with rheumatoid arthritis: efficacy and safety through 5 years in the SELECT-EARLY randomized controlled trial. Arthritis Res Ther 26, 143 (2024). https://doi.org/10.1186/s13075-024-03358-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-024-03358-x