Abstract
Rheumatoid arthritis (RA) is a chronic, systemic, autoimmune disease of unknown etiology with erosive, symmetric polyarthritis as the main clinical manifestations. Its basic pathological changes are the formation of synovitis, and patients gradually develop destruction of articular cartilage destruction and bone erosion, which eventually leads to joint deformity, disability, and various extra-articular manifestations. Clinical prediction models (CPMs), also known as risk prediction models or risk scores, are mathematical formulas used to estimate the probability that a given individual will have a disease or an outcome in the future. The models are mainly divided into two categories: diagnostic models and prognostic models, which can be used to provide information on disease diagnosis or prognosis to help make better medical decisions. Currently, there is no cure for RA, but effective early diagnosis and treatment are crucial for limiting the severity of the disease and preventing the occurrence and development of complications. This paper reviews the CPMs associated with RA and its related complications, including cardiovascular disease (CVD) and interstitial lung disease (ILD), in order to provide reference and evidence for the early diagnosis and treatment of these diseases and personalized medicine for patients. In addition, the possible pathogenesis and risk factors of these comorbidities are summarized, and possible directions for future related research are prospected.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic, systemic, autoimmune disease of unknown etiology with erosive, symmetric polyarthritis as the main clinical manifestations [1]. Its basic pathological changes are the formation of synovitis, and patients gradually develop destruction of articular cartilage destruction and bone erosion, which eventually leads to joint deformity, disability, and various extra-articular manifestations [2]. Chronic, persistent, and systemic inflammation in RA is characterized by an increase in specific inflammatory mediators, cytokines, and related antibodies, and a combination of genetic and environmental factors predisposes patients to different comorbidities and increases the risk of disability and death [3]. It is estimated that comorbidities are present in nearly 80% of inpatients with RA [4], such as cardiovascular disease (CVD), respiratory diseases including interstitial lung disease (ILD), infectious diseases, psychiatric diseases, gastrointestinal diseases, malignancies, chronic kidney disease, and osteoporosis (OP) et al. [3, 5] (Fig. 1). CVD is a prevalent complication of RA and represents the leading cause of mortality for patients [3]. Additionally, ILD is both the most common and severe manifestation of RA-related lung diseases [3]. The occurrence of these comorbidities will not only aggravate the condition of RA, but also further reduce the quality of life of patients and lead to a shortened life expectancy [2]. Currently, there is no cure for RA, and the goal of treatment is to maximize remission [6, 7]. Effective early diagnosis and treatment are of great significance to limit disease severity and prevent the occurrence and development of complications [3]. Therefore, in addition to new drug development and mechanism research, it is equally important to predict the effective response of RA patients to therapeutic drugs and early identification of patients who are prone to various complications.
Schematic illustration of systemic complications of rheumatoid arthritis. The most frequent comorbidities of patients with rheumatoid arthritis include cardiovascular disease, respiratory diseases, infectious diseases, psychiatric diseases, gastrointestinal diseases, malignancies, chronic kidney disease, and osteoporosis
When the medical model develops from empirical medicine through evidence-based medicine to precision medicine, the acquisition, storage, analysis, and prediction technology of medical data has developed rapidly, making the vision of personalized medicine more and more possible [8]. Clinical prediction models (CPMs), also known as risk prediction models or risk scores, are mathematical formulas used to estimate the probability that a given individual will have a disease or an outcome in the future [9], mainly divided into diagnostic models and prognostic models, which can be used to provide information on disease diagnosis or prognosis to help make better medical decisions. In recent years, there have been several studies reporting on CPMs related to RA, RA-CVD, and RA-ILD. However, there is a lack of comprehensive summaries regarding these models. This paper reviews the CPMs related to RA, RA-CVD, and RA-ILD, in order to provide reference and evidence for the early diagnosis and treatment of these diseases and personalized medicine for patients, and the possible pathogenesis and risk factors of RA-CVD and RA-ILD are summarized, and possible directions for future-related research are prospected.
RA
Models predicting the risk of RA
Due to the characteristics of RA that cannot be cured at present, treatment should be initiated as soon as possible once RA is diagnosed, as early treatment can significantly slow disease progression and prevent irreparable joint damage and disability [2, 10]. Therefore, identifying individuals at high risk for RA and making an early diagnosis are particularly important.
Karlson et al. [11] developed predictive models for RA (Table 1). These models were constructed using 8 human leucocyte antigen (HLA) alleles, 14 single nucleotide polymorphisms (SNPs), and clinical factors and generated an integrated, weighted genetic risk score (GRS) calculated as the product of individual-locus odds ratios. The model including genetic variables has a higher predictive ability than the model containing only clinical factors. The group went on to extend this research by incorporating 17 newly validated RA risk alleles into the GRS and assessing the GRS in relation to the more specific phenotypes of RA along the severity continuum [12] (Table 1). New models were able to forecast seronegative, seropositive, erosive and seropositive, and erosive RA, achieving area under the curve (AUC) values of 0.56, 0.65, 0.64, and 0.712 respectively. The results indicate that the GRS has virtually no ability to distinguish between the control group and seronegative RA, and the addition of 17 new alleles does not improve the predictive capability of the GRS. In contrast, this also suggests that seropositive RA and seronegative RA have distinct genetic foundations. Therefore, conducting separate studies on these two phenotypes in future research would provide a deeper comprehension of the genetic and functional composition of the disease.
Several other studies [10, 13, 14] (Table 1) have conducted comparable predictive analyses, utilizing a blend of clinical and genetic risk factors to devise models with good discriminative ability. A study [15] (Table 1) introduced a novel modeling approach, with model development facilitated by an R package [33]. This program incorporates published gene environment risk factor and disease statistics to categorize risk using a confidence interval (CI)-based approach within a simulated population. This study found that HLA and smoking status can be used to predict the risk of younger and older onset RA, respectively.
The involvement of genetic variables remains a double-edged sword, as it can enhance predictive capability on the one hand, but on the other hand, its difficulty in acquisition can hinder clinical application. A model devoid of genetic variables for RA has been developed, which solely utilizes common risk factors as predictors, including comorbidities, demographic, socioeconomic, and behavioral risk factors [16] (Table 1). In addition to delivering high predictive accuracy, the model has the ability to capture the impacts of individual variables along with the crucial higher-order interactions among them. For instance, age not only serves as a crucial predictor for RA, but it also exhibits strong interactive effects with variables such as smoking and depression.
Recently, an optimized polygenic risk score calculator using machine learning (ML) for RA was developed based on 9 ML-identified SNPs [17] (Table 1), which can be accessed through this link: https://xistance.shinyapps.io/prs-ra/ [17]. This model has extremely high predictive capability (AUC > 0.9), and it is very user-friendly. However, the fact that the derivation and validation data are both derived from the Singaporean Chinese underscores the need for continuous validation across different regions and ethnicities.
Models predicting insufficient response to methotrexate (MTX)
Currently, methotrexate (MTX) is recommended as a first-line treatment for RA [6]; however, approximately one-third of patients do not respond sufficiently to this medication [34]. Identifying who are likely to have a suboptimal response to MTX treatment prior to initiating therapy could potentially lead to better initial treatment decisions for patients with RA.
A clinical pharmacogenetic model was to predict the efficacy of MTX in RA [18] (Table 1). A scoring system ranging from 0 to 11.5 has been developed for convenient clinical use. The model combines clinical and genetic variables and demonstrates good discrimination with an AUC of 85%. Removing the genetic variables results in a decrease in discriminative ability, as evidenced by an AUC of 0.79. This study demonstrates that it is possible to predict the response to MTX therapy in patients with recent-onset RA. Since patients in the model are treated with MTX monotherapy only, this may not be consistent with current principles of combination therapy, and subsequent studies have shown that it has an inadequate performance for the prediction of nonresponse to MTX in RA patients treated with combination therapies [35].
A similar predictive analysis was conducted in a study that established a discriminative model with good performance by combining genetic, metabolic, clinical, and lifestyle variables [19] (Table 1). The AUC of the model was 0.8 in both the derivation and validation cohorts. Another study not only predicted the efficacy of MTX in patients with RA, but also predicted its hepatotoxicity [20] (Table 1). The model showed moderate diagnostic accuracy for MTX efficacy (AUC = 0.84) and high diagnostic accuracy for liver toxicity (AUC = 0.91). However, there is currently a lack of external validation.
While the above models have indicated that genetic variables contribute to the improvement of the models’ discrimination, their involvement may indeed make the routine use of the models challenging. Taking this into account, Gosselt et al. [21] enhanced the applicability of the original model [19] by removing genetic variables and validating the model in cohort data from different regions (Table 1). The simplified model has an AUC of 0.75 and is successfully integrated in an online tool “Evidencio,” which can be available by https://www.evidencio.com/models/show/2191 [21]. The updated model is user-friendly and can be further validated and utilized in clinical practice to identify individuals who are insufficient responders to MTX. The goal is to promptly initiate additional biologic or JAK pathway inhibitor therapies for these individuals in order to minimize disease activity and slow disease progression.
A model involving imaging variables has been established [22] (Table 1). The study investigated if magnetic resonance imaging (MRI) or ultrasound (US) examination is useful in anticipating poor response to MTX, or future structural damage progression. The results indicate that the detection of inflammation by MRI or US is unrelated to predicting MTX response, but is rather associated with elements related to future disease progression.
Models predicting insufficient response to tumor necrosis factor inhibitors (TNFi)
Upon conventional synthesis DMARDs (csDMARDs) such as MTX failure or loss of efficacy, the patients are switched to biologic DMARDs (bDMARDs), such as necrosis factor inhibitors (TNFi), for further treatment [6], but 30% of patients do not respond well to their initial TNFi therapy [36]. Therefore, the development of tools that can assist in providing practical guidance for the selection of candidate drugs for anti-tumor necrosis factor therapy is crucial.
A model was established to predict treatment response of RA patients to golimumab, a monoclonal anti-TNFα antibody [23] (Table 1). The AUC of this model is 0.648–0.809, when predicting 1-, 3-, and 6-month low disease activity or remission. A series of prediction matrix tools were created to facilitate the use of the model, which can be available at Rheumatology Online [23]. Although the model lacked external validation when it was published, follow-up research examined these tools in real-world RA patients undergoing anti-TNFα therapy and corroborated their effectiveness [37]. The data sources for establishing the model are large-sample studies across multiple countries, so they have great representativeness. Moreover, the readily accessible predictive factors facilitate the practical application of the model.
However, the study did not elucidate the biological mechanisms underlying this differential response to golimumab. Tao et al. [24] investigated the mechanisms of how RA patients respond differently to adalimumab or etanercept by analyzing gene expression and DNA methylation data, and established machine learning models to predict which therapy is effective for which patients before commencing therapy (Table 1). Adalimumab represents the initial fully human therapeutic monoclonal anti-TNFα antibody, whereas etanercept is a recombinant human TNF receptor (p75)–Fc fusion protein that functions as a competitive inhibitor of TNF [38]. This study suggests that response towards these two classes of TNFi is defined by the genetic and epigenetic differences between individual patients. However, whether the differential response to different drugs of monoclonal TNFi antibody or the inter-individual variability in response to a single drug is also determined by distinct genetic signatures remains a question that should be addressed in future studies.
Models predicting insufficient response to rituximab or tocilizumab
Rituximab, anti-CD20 antibody, has been approved for use in RA patients who have failed or appeared intolerant to TNFi therapy [39]; however, approximately 30–40% of RA patients display a poor response to rituximab therapy [40]. A model composed of disease activity score (DAS) in 28 joints, interferon score, and DMARDs use was developed to predict non-response to rituximab in RA and exhibited an AUC of 0.82 [25] (Table 1). The use of prednisolone had a significant impact on the predictive performance of the model, which could be due to the impact of prednisolone on the interferon score. The mechanism underlying the association between a high interferon score and poor response to rituximab is yet to be elucidated. Future studies could optimize the model by elucidating this impact and its mechanism.
Another study established models for predicting treatment response to rituximab (AUC = 0.74), as well as response to tocilizumab, an anti-IL6R monoclonal antibody (AUC = 0.68), and multidrug resistance (AUC = 0.69), through in-depth histological and molecular analyses of synovial biopsies in RA patients [26] (Table 1). The post-treatment modifications in synovial gene expression and cell infiltration have revealed significant differences in the response/non-response mechanisms between rituximab and tocilizumab. The discovery of genes and cell types related to multidrug resistance is a significant development that could facilitate the creation of novel drugs for refractory patients who are unresponsive to available medications targeting conventional immune pathways. Further research can be conducted to elucidate the biological mechanisms underlying the differential response of patients to rituximab, tocilizumab, or multidrug resistance and to improve the performance of the model by optimizing the genetic variables.
RA-CVD
The possible pathogenesis and risk factors of RA-CVD
CVD is one of the most common complications of RA and the leading cause of mortality for patients [3], accounting for 30–40% of deaths [41], affecting approximately 2.4 to 18.6% of patients with RA [42]. Patients with RA have approximately 50% greater risk for CVD compared to the general population [43]. The main clinical manifestations of CVD are ischemic cardiomyopathy and congestive heart failure (CHF). CHF and myocardial infarction (MI) may occur twice as often in RA patients compared to the general population [44]. Due to the increased risk of MI, heart sudden death and stroke in patients with RA have been estimated to be twofold and 1.7-fold, respectively [45]. The pathogenesis of RA-ILD has not been fully elucidated, which may be associated with endothelial dysfunction (ED) and atherosclerosis due to inflammation-associated loss of elasticity of the vascular wall [46] (Fig. 2). Compared with the matched healthy control group, the levels of peripheral endothelial progenitor cells (EPCs) are lower in RA patients [47]. However, the lower the EPCs’ number, the worst the endothelial function [48], which could partly explain the ED observed in patients with RA. C-reactive protein (CRP) can inhibit EPCs differentiation, survival, and function, which eventually leads to ED [49]. The endothelium plays a central role in atherosclerosis because it produces vasoactive substances including nitric oxide (NO) that acts on the vascular tone and affects homeostasis between the circulating blood cells and the vessel wall [3]. Inflammation is the common link between atherosclerosis and RA, which can alter the balance between the production of NO and other vasoactive substances, causing ED and consequently promoting atherosclerosis [50]. The endothelial-activating cytokines presumably synovitis-derived, including interleukins (IL)-6 and TNF-α, play important roles in endothelial damage since they inhibit the production of NO, which, in turn, are responsible for maintaining a healthy endothelium [46]. In addition, an association has been found between ED and HLA-DRB1*04 shared epitope [51], the strongest genetic risk factor for RA. ACPA positivity also can contribute to the development of CVD and may induce subclinical atherosclerotic damage [52]. All of these factors, coupled with traditional risk factors for CVD such as hypertension, hyperlipidemia, diabetes mellitus, and smoking [53], may underlie pro-atherogenic and pro-thrombotic changes, the promotion of cardiac remodeling, alterations in lipid blood profiles, and changes to the morphology of red blood cells, which favor accelerated development of CVD in patients with RA [5, 46].
Schematic illustration of the pathogenesis of cardiovascular disease in rheumatoid arthritis. Levels of peripheral endothelial progenitor cells (EPCs) in RA are inhibited compared with general population, which could trigger the endothelial dysfunction (ED). C-reactive protein (CRP) can inhibit EPCs’ differentiation, survival, and function, which further leads to ED. The endothelial-activating cytokines presumably synovitis-derived, including interleukins (IL)-6 and necrosis factor inhibitors (TNF)-α, play important roles in endothelial damage since they inhibit the production of nitric oxide (NO), which, in turn, are responsible for maintaining a healthy endothelium. In addition, RA susceptibility genes human leucocyte antigen (HLA)-DRB1*04 and anti-citrullinated protein antibodies (ACPA) positivity also can contribute to ED. The endothelium plays a central role in atherosclerosis because it produces vasoactive substances including NO that act on the vascular tone and affects homeostasis between the circulating blood cells and the vessel wall. All of these factors, coupled with traditional risk factors for CVD such as hypertension, hyperlipidemia, diabetes mellitus, and smoking, may underlie pro-atherogenic and pro-thrombotic changes, the promotion of cardiac remodeling, alterations in lipid blood profiles, and changes to the morphology of red blood cells, which favor accelerated development of CVD in patients with RA
Traditional CVD risk prediction models are not suitable for RA patients
Compared with traditional risk factors for CVD, patients with RA are more likely to cause CVD due to disease activity, ESR, CRP, RF, and ACPA [41, 54] (Table 2). Therefore, current methods for assessing CVD risk tend to underestimate the risk when applied to patients with RA. When the Framingham risk score and systematic coronary risk evaluation (SCORE) were applied to patients with RA, up to twofold risk underestimation was observed [55]. The risk of excess CVD is still attributed to inflammation, and current methods of assessing CVD risk do not account for RA patients who are chronically exposed to inflammatory environments [54]. To consider the effect of systemic inflammation of RA on CV risk, EULAR has suggested that the SCORE scoring system risk value be multiplied by 1.5 in RA patients who show at least two of the following: (1) RA disease of more than 10 years, (2) positive RF, (3) positive ACPA, and (4) presence of extraarticular manifestations [56]. It is, however, possible that even with the modified SCORE, a large number of RA patients still may not be identified and are at high risk for CVD [57]. The QRISK-2 scoring system includes RA as a risk factor for CVD, so there exist also expert consensus to recommend the use of QRISK-2 as a calculator for estimating the 10-year CVD risk of RA patients [58]. The study has shown cardiovascular risk age model and vascular age mode developed based on the SCORE model also has good performance when used in RA patients [59]. A limitation of these methods is that it treats all RA patients the same, regardless of the level of disease activity; therefore, there exists an urgent need for risk prediction models for CVD in RA patients.
CPMs of CVD for RA patients
An expanded risk score model for CVD in RA (ERS-RA) derived to predict 10-year probability of a CV event, such as MI, stroke, or CV-related death [27] (Table 1). To facilitate the use of the ERS-RA, a risk score calculator has been developed which can be downloaded at https://www.verityresearch.org/cvd-risk-calculator/ [27]. Although the model development data were derived from the cohort study in the USA, follow-up studies demonstrated the effectiveness of the ERS-RA in the European RA population [60]. The large sample size of the model’s data source and its validation in populations from different regions make the model highly reliable. Future research should focus on validating and continuously updating the model in populations of different races and regions.
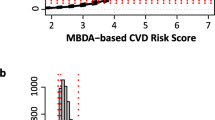
A study [28] conducted a similar predictive analysis, establishing a prognostic model for CVD in RA patients by integrating clinical variables, laboratory indicators, and the multi-biomarker disease activity (MBDA) score (Table 1). The MBDA score evaluates the disease activity of rheumatoid arthritis by measuring 12 serum protein biomarkers and is associated with the risk of CVD in RA patients [61]. This may partially explain the additional CVD risk in RA patients caused by inflammation. Another study [29] reported a model to predict the occurrence of coronary heart disease (CHD) in RA patients (Table 1). This model differs from the above prognostic models in that it has the potential to screen out RA patients with concomitant CHD. It demonstrates superior performance in predicting RA-CHD compared to the Framingham risk score. The AUC for the model was 0.77, along with a 63.9% sensitivity and 77.2% specificity. However, its retrospective design and use of data from a single center highlight the need for continuous validation before its clinical use.
RA-ILD
The possible pathogenesis and risk factors of RA-ILD
The second major cause of death in patients with RA is respiratory disease, which occurs in 30–40% of patients [62]. ILD is the most common and severe manifestation of RA lung diseases [3], affecting approximately 2.2 to 10% of patients with RA [63, 64], and median survival after diagnosis keeps approximately 7 years [65]. Compared with general people, patients with RA have a much higher probability of developing ILD [66], but the possible pathogenesis of RA-ILD has not been fully elucidated, which can be summarized as the consequence of a combination of genetic, environmental, and autoimmune factors [67] (Fig. 3). The interaction of these factors contributes to the aberrant tissue response in the alveolar wall and pulmonary parenchyma, which include airways and alveolar epithelial cells, lung fibroblasts, and components of extracellular matrix [67]. MUC5B promoter variant rs35705950 [68] and rs12702634 at RPA3-UMAD1 [69] lead to genetic susceptibility in the West and East Asian populations, respectively. Smoking keeps the most significant risk factor for the development of ILD in patients with RA. Alveolar epithelium injury from cigarette smoking characterized by cellular infiltration and release of pro-fibrotic cytokines including IL-17, IL-13, and transforming growth factor (TGF)-β, chemokines, and growth factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) that promote lung fibroblast proliferation and differentiation to myofibroblasts [70]. Smoking also leads to the generation of citrullinated proteins in lung alveolar cells [71], which means higher levels of RF or ACPA can be found in the affected lungs of RA patients in genetically susceptible individuals. Mechanistic study demonstrated ACPA is pathogenic and induces the release of neutrophil extracellular traps (NETs) which trigger activation of lung fibroblasts to differentiate into myofibroblast, eventually leading to lung fibrosis formation [72, 73]. In addition, other risk factors including males, elder, and longer duration of RA can also contribute to the development of RA-ILD [64, 70] (Table 2).
Schematic illustration of the pathogenesis of interstitial lung disease in rheumatoid arthritis. Alveolar epithelium injury from cigarette smoking characterized by cellular infiltration and release of pro-fibrotic cytokines including interleukins (IL)-17, IL-13, and transforming growth factor (TGF)-β, chemokines, and growth factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) that promote lung fibroblast proliferation and differentiation to myofibroblasts. Smoking also leads to the generation of citrullinated proteins in lung alveolar cell, which means higher levels of rheumatoid factor (RF) or anti-citrullinated protein antibodies (ACPA) can be found in the affected lungs of RA patients in the genetically susceptible individuals. Mechanistic study demonstrated ACPA is pathogenic and induces the release of neutrophil extracellular traps (NETs) which trigger the activation of lung fibroblasts to differentiate into myofibroblast, eventually leading to lung fibrosis formation. In addition, other risk factors including males, elder, and longer duration of RA, can also contribute to the development of RA-ILD. All this eventually leads to the development of lung fibrosis in patients with RA
CPMs related to RA-ILD
The diagnosis of RA-ILD proves difficult, because approximately 5–10% of patients have significant clinical signs, and an additional 20–30% may have subclinical RA-ILD [66]. High-resolution computed tomography (HRCT) represents the gold standard for diagnosing the disease [74], but ILD can appear in any stage of RA, entailing the need for a systematic assessment of lung involvement. It is not advisable to use routinely HRCT for screening programs because of both high cost and X-ray exposure [75], and therefore, there exists an urgent need for a way to screen patients with RA who may develop ILD to target HRCT to patients who need it more. Lung auscultation represents an economical and radiation-free screening method for RA-ILD; the detection of the velcro crackle (VC) in lung sounds can effectively raise the suspicion of an ILD and speed up diagnosis [30]. However, this task largely relies on the experience of physicians and requires standardization in clinical practice.
Pancaldi et al. [30] investigated the problem of the automatic detection of VC in lung sounds and developed an algorithm called velcro sound detector (VECTOR) to detect the presence of VC in lung sounds recorded by electronic stethoscope to infer the presence of ILD in RA patients (Table 1). The VECTOR demonstrates higher accuracy than clinical physicians in diagnosing RA-ILD. When VECTOR was validated in different populations, it showed a diagnostic accuracy of 83.9% and a sensitivity and specificity of 93.2 and 76.9%, respectively [76]. In general, the identification of VC has always been qualitative and subjective, but the proposal of VECTOR has the potential to transform it into a quantitative and objective process. Because the auscultation of lung sounds is inexpensive and non-invasive, VECTOR can be used as a routine screening tool for RA-ILD.
A study [31] analyzed the influencing factors of RA-ILD and constructed a diagnostic model with good discriminative ability (Table 1). The study included traditional Chinese medicine (TCM) variables as predictors, meaning that variables from complementary and alternative medicine may also contribute to model development. Another study [32] reported a prognostic model for RA-ILD (Table 1). Unlike the RA-ILD screening model mentioned above, this prognostic model provides a predicted probability of death after 90 days of acute exacerbation (AE)-RA-ILD. This study identified forced vital capacity (FVC) within the 12 months preceding AE and the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) during AE onset as independent predictive factors for mortality, which may contribute to the prognostic management of RA-ILD.
Discussion
This review aims to summarize CPMs related to RA, RA-CVD, and RA-ILD, in order to provide reference and evidence for the early diagnosis and treatment of these diseases and personalized medicine for patients. Moreover, the pathogenesis and risk factors of RA-CVD and RA-ILD are summarized. Recently, some literature has provided separate overviews of the risk factors and pathogenesis of RA-CVD [77] and RA-ILD [78], which bear similarities to our research. However, in addition to this, our study highlights the development of predictive models for these diseases. Interestingly, these studies have mentioned the necessity of screening RA patients for CVD or ILD, but currently, there is a lack of effective screening methods or tools in routine clinical practice, which is the problem our study aims to address.
The pathogenesis of RA-CVD and RA-ILD have not been fully elucidated, and genetic characteristics and inflammation may play an essential role in these processes [3]. Disease activity and systemic inflammation are the most common implicated non-traditional cardiovascular risk factors in inflammatory joint diseases [79]. Research by Solomon et al. [80] has proved that there was a 21% reduction in CVD risk for every 10-point reduction of the Clinical Disease Activity Index (CDAI) in patients with RA. Similar to RA-CVD, RA-ILD risk increased by 35% for each additional unit of DAS28 [81]. ACPA, as the most representative autoimmune antibody for RA, also seems to be involved in the development of various comorbidities. ACPA can lead to the development of CVD by contributing to ED in RA patients [29, 49] (Fig. 2). In the process of RA-ILD, NETs were released by the impact of ACPA, which trigger the activation of lung fibroblasts to differentiate into myofibroblast, eventually leading to lung fibrosis formation [72, 73] (Fig. 3).
CPMs use information about a patient at baseline to predict the risk of a current (diagnostic) or future (prognostic, e.g., non-response/adverse events) clinical event [82], which can not only provide high-quality evidence for evidence-based medicine [83], but also serve as a favorable tool for the application and popularization of precision medicine. With the advent of the era of precision medicine, clinical prediction models are increasingly used in medical diagnosis and treatment decisions, patient prognosis management, and public health resource allocation, so their value is becoming more and more important [9].
At present, the CPMs that predict drug response in the treatment of RA mainly concentrate on MTX and bDMARDs. It is worth noting that genetic variations have a certain impact on the therapeutic response to MTX [18,19,20], adalimumab [24], etanercept [24], rituximab [26], or tocilizumab [26]. The high cost of genetic testing may present a challenge for the routine use of the models. In future studies, it would be of interest to perform comprehensive cost–benefit analyses, examining the cost of genetic testing in relation to long-term medical treatment expenses and clinical and functional outcomes. There is currently no model that is effective in predicting the treatment response of JAK inhibitors (such as tofacitinib, baricitinib, and upadacitinib). JAK inhibitors are new targeted synthetic DMARDs used in the treatment of RA and are an important approach for treating the condition. However, their safety has been the subject of controversy [84]. Therefore, future research should not only focus on predicting the therapeutic response of these drugs but also consider their potential side effects and make predictions accordingly.
CVD is the most urgent and serious complication of RA because it is strongly associated with an increased risk of death [44]. In addition to traditional and RA-specific risk factors for CVD, biomarkers of cardiac dysfunction, including N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiac troponin T, have also been reported to predict CVD risk and mortality in RA patients [46]. It is noteworthy that although ED plays a crucial role in the development of RA-CVD [46], it has not been included in the current models as a predictive factor. This may be attributed to the fact that current research mainly focuses on traditional CVD risk factors and additional risk factors caused by systemic inflammation in RA, without delving into the underlying mechanisms of RA-CVD. This may explain why these models only have moderate discriminative ability, with an AUC of less than 0.8 [27,28,29]. Currently, there are several feasible approaches to assess ED, such as non-invasive examinations (flow-mediated dilation, subcutaneous adipose tissue thickness, and carotid intima-media thickness) as well as biomarkers (ischemia-modified albumin, pentraxin-3, E-selectin, endothelin-1, von Willebrand factor, endothelial microparticles, and EPCs) [85]. Among them, EPCs, E-selectin, and von Willebrand factor have been measured in RA patients and are associated with RA-CVD [86]. Identifying the optimal method for measuring endothelial function, which can be used to predict the risk of RA-CVD, is a crucial area for future research.
In 20–30% of patients with RA, a pulmonary complication is the first manifestation, rather than joint symptoms [87]. Therefore, some scholars also proposed another possible pathogenesis of RA-ILD that idiopathic pulmonary fibrosis-like pathology triggers an immune response to citrullinated proteins that promotes articular disease indicative of RA [88]. Interestingly, although RA is more common in females, with a female-to-male sex ratio ranging as high as 4:1 [89], RA-ILD is more prevalent in males, with a male-to-female ratio of 2:1 [67]. Therefore, men with RA should be highly alert for the development of ILD, and smoking cessation should be put on the agenda as early as possible. Smoking as a common risk factor for RA and RA-ILD, recent studies have shown that smoking may exhibit a threshold effect in its relationship with RA-ILD that smoking 30 pack-years or more was associated with a sixfold increase in RA-ILD risk, whereas smoking under this threshold was not associated with increased risk [90]. Therefore, it is not enough to focus on whether patients smoke, and future studies should further explore the relationship between the number of cigarettes smoked and RA-ILD. Several new biomarkers can enhance the detection of RA-ILD, including matrix metalloproteinase, surfactant protein D, and pulmonary and activation-regulated chemokine [91], which may be promising for the development of new predictors in future research.
It is noteworthy that only a few models have been updated in subsequent clinical practices in this review [11, 19]. Therefore, in addition to the development of new predictors and models, validation and updates of existing models should also be an area of future research focus. Every study has limitations; this study is no exception. Firstly, the comorbidities only focus on CVD and ILD and were not all-inclusive; some important comorbidities such as osteoporosis depression and malignancies were not included. Secondly, we only evaluated the predictive ability of the models and did not assess whether their methods are reliable.
Conclusions
In summary, the pathogenesis of RA-CVD and RA-ILD prove undoubtedly complex. Inflammation, disease activity, and specific autoimmune antibody are all inextricably associated with the development of these complications. We attempt to summarize the possible pathogenesis of these diseases that the combination of inflammation, autoimmune response, disease activity, and related traditional risk factors under the impact of susceptibility genes can lead to ED, and maturation of myofibroblasts, and ultimately to the occurrence in RA patients of CVD and ILD, respectively.
CPMs have the advantage of early detection of complications and prediction of drug response even in RA with complex pathological mechanisms. Therefore, in addition to new drug development, it is equally important to predict the effective response of RA patients to therapeutic drugs and early identification of patients who are prone to various complications. We hope that the future development of CPMs will take us from the current trial-and-error drug prescribing and into an emerging era where the selection of the optimal drug is based on pre-treatment predictions.
Availability of data and materials
Not applicable.
Abbreviations
- ACPA:
-
Anti-citrullinated protein antibodies
- ACR:
-
American College of Rheumatology
- APC:
-
Antigen-presenting cells
- AUC:
-
Area under the curve
- cfPWV:
-
Carotid-femoral pulse wave velocity
- CHD:
-
Coronary heart disease
- CPMs:
-
Clinical prediction models
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DAS:
-
Disease activity score
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ECG:
-
Electrocardiographic
- ED:
-
Endothelial dysfunction
- EPCs:
-
Endothelial progenitor cells
- ESR:
-
Erythrocyte sedimentation rate
- FVC:
-
Forced vital capacity
- GC:
-
Glucocorticoid
- HAQ:
-
Health assessment questionnaire
- HDL:
-
High-density lipoprotein cholesterol
- HRCT:
-
High-resolution computed tomography
- IL:
-
Interleukins
- ILD:
-
Interstitial lung disease
- MBDA:
-
Multi-biomarker disease activity
- MI:
-
Myocardial infarction
- MMP:
-
Matrix metalloproteinase
- MTX:
-
Methotrexate
- NETs:
-
Neutrophil extracellular traps
- NR:
-
Not reported
- OP:
-
Osteoporosis
- PGA:
-
Patient global assessment
- RA:
-
Rheumatoid arthritis
- LDL:
-
Low-density lipoprotein cholesterol
- RAI:
-
Ritchie articular index
- RF:
-
Rheumatoid factor
- SCORE:
-
Systematic coronary risk evaluation
- SJC:
-
Swollen joint count
- TG:
-
Triglyceride
- TGF:
-
Transforming growth factor
- TJC:
-
Tender joint count
- TNFi:
-
Tumor necrosis factor inhibitors
- US7:
-
7-Joint ultrasonic erosions score
- VC:
-
Velcro crackle
- VECTOR:
-
Velcro sound detector
- VEGF:
-
Vascular endothelial growth factor
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72.
Figus FA, Piga M, Azzolin I, McConnell R, Iagnocco A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20(4):102776.
Parodi M, Bensi L, Maio T, Mela G, Cimmino M. Comorbidities in rheumatoid arthritis: analysis of hospital discharge records. Reumatismo. 2005;57:154–60.
Taylor PC, Atzeni F, Balsa A, Gossec L, Müller-Ladner U, Pope J. The key comorbidities in patients with rheumatoid arthritis: a narrative review. J Clin Med. 2021;10(3):509.
Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–23.
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Chow N, Gallo L, Busse JW. Evidence-based medicine and precision medicine: complementary approaches to clinical decision-making. Precis Clin Med. 2018;1(2):60–4.
Hongqiu G, Zhirui Z, Zhongheng Z, Quan Z. Clinical prediction models: basic concepts, application scenarios, and research strategies. Chin J Evid Based Cardiovasc Med. 2018;10:12.
Sparks JA, Chen CY, Jiang X, Askling J, Hiraki LT, Malspeis S, Klareskog L, Alfredsson L, Costenbader KH, Karlson EW. Improved performance of epidemiologic and genetic risk models for rheumatoid arthritis serologic phenotypes using family history. Ann Rheum Dis. 2015;74(8):1522–9.
Karlson EW, Chibnik LB, Kraft P, Cui J, Keenan BT, Ding B, Raychaudhuri S, Klareskog L, Alfredsson L, Plenge RM. Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Ann Rheum Dis. 2010;69(6):1077–85.
Chibnik LB, Keenan BT, Cui J, Liao KP, Costenbader KH, Plenge RM, Karlson EW. Genetic risk score predicting risk of rheumatoid arthritis phenotypes and age of symptom onset. PLoS One. 2011;6(9):e24380.
Yarwood A, Han B, Raychaudhuri S, Bowes J, Lunt M, Pappas DA, Kremer J, Greenberg JD, Plenge R, Worthington J, et al. A weighted genetic risk score using all known susceptibility variants to estimate rheumatoid arthritis risk. Ann Rheum Dis. 2015;74(1):170–6.
Karlson EW, Ding B, Keenan BT, Liao K, Costenbader KH, Klareskog L, Alfredsson L, Chibnik LB. Association of environmental and genetic factors and gene-environment interactions with risk of developing rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(7):1147–56.
Scott IC, Seegobin SD, Steer S, Tan R, Forabosco P, Hinks A, Eyre S, Morgan AW, Wilson AG, Hocking LJ, et al. Predicting the risk of rheumatoid arthritis and its age of onset through modelling genetic risk variants with smoking. PLoS Genet. 2013;9(9):e1003808.
Lufkin L, Budišić M, Mondal S, Sur S. A Bayesian model to analyze the association of rheumatoid arthritis with risk factors and their interactions. Front Public Health. 2021;9:693830.
Lim AJW, Tyniana CT, Lim LJ, Tan JWL, Koh ET, Chong SS, Khor CC, Leong KP, Lee CG. Robust SNP-based prediction of rheumatoid arthritis through machine-learning-optimized polygenic risk score. J Transl Med. 2023;21(1):92.
Wessels JA, van der Kooij SM, le Cessie S, Kievit W, Barerra P, Allaart CF, Huizinga TW, Guchelaar HJ. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum. 2007;56(6):1765–75.
de Rotte M, Pluijm SMF, de Jong PHP, Bulatović Ćalasan M, Wulffraat NM, Weel A, Lindemans J, Hazes JMW, de Jonge R. Development and validation of a prognostic multivariable model to predict insufficient clinical response to methotrexate in rheumatoid arthritis. PLoS One. 2018;13(12):e0208534.
Onishi A, Kamitsuji S, Nishida M, Uemura Y, Takahashi M, Saito T, Yoshida Y, Kobayashi M, Kawate M, Nishimura K, et al. Genetic and clinical prediction models for the efficacy and hepatotoxicity of methotrexate in patients with rheumatoid arthritis: a multicenter cohort study. Pharmacogenom J. 2020;20(3):433–42.
Gosselt Helen R, Verhoeven Maxime MA, de Rotte Maurits CFJ, Pluijm Saskia MF, Muller Ittai B, Gerrit J, Janneke T, Maja B, Heil SG, Lafeber FPJG, et al. Validation of a prognostic multivariable prediction model for insufficient clinical response to methotrexate in early rheumatoid arthritis and its clinical application in Evidencio. Rheumatol Ther. 2020;7(prepublish).
Sundin U, Sundlisater NP, Aga AB, Sexton J, Nordberg LB, Hammer HB, van der Heijde D, Kvien TK, Haavardsholm EA, Lillegraven S, et al. Value of MRI and ultrasound for prediction of therapeutic response and erosive progression in patients with early rheumatoid arthritis managed by an aggressive treat-to-target strategy. RMD Open. 2021;7(1):e001525.
Vastesaeger N, Kutzbach AG, Amital H, Pavelka K, Lazaro MA, Moots RJ, Wollenhaupt J, Zerbini CA, Louw I, Combe B, et al. Prediction of remission and low disease activity in disease-modifying anti-rheumatic drug-refractory patients with rheumatoid arthritis treated with golimumab. Rheumatology (Oxford). 2016;55(8):1466–76.
Tao W, Concepcion AN, Vianen M, Marijnissen ACA, Lafeber F, Radstake T, Pandit A. Multiomics and machine learning accurately predict clinical response to adalimumab and etanercept therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2021;73(2):212–22.
de Jong TD, Sellam J, Agca R, Vosslamber S, Witte BI, Tsang ASM, Mantel E, Bijlsma JW, Voskuyl AE, Nurmohamed MT, et al. A multi-parameter response prediction model for rituximab in rheumatoid arthritis. Joint Bone Spine. 2018;85(2):219–26.
Rivellese F, Surace AEA, Goldmann K, Sciacca E, Cubuk C, Giorli G, John CR, Nerviani A, Fossati-Jimack L, Thorborn G, et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat Med. 2022;28(6):1256–68.
Solomon DH, Greenberg J, Curtis JR, Liu M, Farkouh ME, Tsao P, Kremer JM, Etzel CJ. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: a Consortium of Rheumatology Researchers of North America Registry Study. Arthritis Rheumatol. 2015;67(8):1995–2003.
Curtis JR, Xie F, Crowson CS, Sasso EH, Hitraya E, Chin CL, Bamford RD, Ben-Shachar R, Gutin A, Flake DD 2nd, et al. Derivation and internal validation of a multi-biomarker-based cardiovascular disease risk prediction score for rheumatoid arthritis patients. Arthrit Res Ther. 2020;22(1):282.
Wei T, Yang B, Liu H, Xin F, Fu L. Development and validation of a nomogram to predict coronary heart disease in patients with rheumatoid arthritis in northern China. Aging (Albany NY). 2020;12(4):3190–204.
Pancaldi F, Sebastiani M, Cassone G, Luppi F, Cerri S, Della Casa G, Manfredi A. Analysis of pulmonary sounds for the diagnosis of interstitial lung diseases secondary to rheumatoid arthritis. Comput Biol Med. 2018;96:91–7.
Ge XQ, Zhang JS, Zhang ZC. Influencing factors and construction of risk prediction nomogram model of rheumatoid arthritis patients complicated with interstitial lung disease. Pract J Card Cereb Pneumal Vasc Dis. 2021;29(09):53–8.
Hozumi H, Kono M, Hasegawa H, Kato S, Inoue Y, Suzuki Y, Karayama M, Furuhashi K, Enomoto N, Fujisawa T, et al. Acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: mortality and its prediction model. Respir Res. 2022;23(1):57.
Crouch DJ, Goddard GH, Lewis CM. REGENT: a risk assessment and classification algorithm for genetic and environmental factors. Europ J Hum Genet. 2013;21(1):109–11.
Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12(12):731–42.
Eektimmerman F, Allaart CF, Hazes JM, Madhar MB, den Broeder AA, Fransen J, Swen JJ, Guchelaar HJ. Validation of a clinical pharmacogenetic model to predict methotrexate nonresponse in rheumatoid arthritis patients. Pharmacogenomics. 2019;20(2):85–93.
Callaghan C, Boyter A, Mullen A, McRorie E. Biological therapy for rheumatoid arthritis: is personalised medicine possible? Eur J Hosp Pharm-S P. 2014;21(4):229–37.
Ganhão S, Lucas R, Fonseca JE, Santos MJ, Gonçalves DR, Madeira N, Silva C, Dourado E, Freitas R, Rodrigues J, et al. Remission and low disease activity matrix tools: results in real-world rheumatoid arthritis patients under anti-TNF therapy. Acta Reumatol Port. 2020;45(4):245–52.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis. 2010;69(9):1629–35.
DeMizio DJ, Geraldino-Pardilla LB. Autoimmunity and inflammation link to cardiovascular disease risk in rheumatoid arthritis. Rheumatol Ther. 2020;7(1):19–33.
Pappas DA, Nyberg F, Kremer JM, Lampl K, Reed GW, Horne L, Ho M, Onofrei A, Malaviya AN, Rillo OL, et al. Prevalence of cardiovascular disease and major risk factors in patients with rheumatoid arthritis: a multinational cross-sectional study. Clin Rheumatol. 2018;37(9):2331–40.
Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7.
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–32.
Ku IA, Imboden JB, Hsue PY, Ganz P. Rheumatoid arthritis: model of systemic inflammation driving atherosclerosis. Circ J. 2009;73(6):977–85.
Meyer PW, Anderson R, Ker JA, Ally MT. Rheumatoid arthritis and risk of cardiovascular disease. Cardiovasc J Afr. 2018;29(5):317–21.
Adawi M, Pastukh N, Saaida G, Sirchan R, Watad A, Blum A. Inhibition of endothelial progenitor cells may explain the high cardiovascular event rate in patients with rheumatoid arthritis. QJM. 2018;111(8):525–9.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New Engl J Med. 2003;348(7):593–600.
Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109(17):2058–67.
Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111(3):363–8.
Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, Vidan J, Hajeer AH, Ollier WE, Mattey DL, Gonzalez-Gay MA. HLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritis. Am J Med. 2003;114(8):647–52.
Bartoloni E, Shoenfeld Y, Gerli R. Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res (Hoboken). 2011;63(2):178–83.
Holthuis EI, Visseren FLJ, Bots ML, Peters SAE. Risk factor clusters and cardiovascular disease in high-risk patients: the UCC-SMART study. Glob Heart. 2021;16(1):85.
Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med. 2017;27(2):136–40.
Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4.
Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, Dougados M, Radner H, Atzeni F, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28.
Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford). 2014;53(12):2143–54.
Yu KH, Chen HH, Cheng TT, Jan YJ, Weng MY, Lin YJ, Chen HA, Cheng JT, Huang KY, Li KJ, et al. Consensus recommendations on managing the selected comorbidities including cardiovascular disease, osteoporosis, and interstitial lung disease in rheumatoid arthritis. Medicine (Baltimore). 2022;101(1):e28501.
Wibetoe G, Sexton J, Ikdahl E, Rollefstad S, Kitas GD, Van Riel P, Gabriel S, Kvien TK, Douglas K, Sandoo A, et al. Prediction of cardiovascular events in rheumatoid arthritis using risk age calculations: evaluation of concordance across risk age models. Arthrit Res Ther. 2020;22(1):1.
Ljung L, Ueda P, Liao KP, Greenberg JD, Etzel CJ, Solomon DH, Askling J. Performance of the Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis in a geographically distant National Register-based cohort: an external validation. RMD Open. 2018;4(2):e000771.
Curtis JR, Xie F, Chen L, Saag KG, Yun H, Muntner P. Biomarker-related risk for myocardial infarction and serious infections in patients with rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2018;77(3):386–92.
Pinheiro FA, Souza DC, Sato EI. A study of multiple causes of death in rheumatoid arthritis. J Rheumatol. 2015;42(12):2221–8.
Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, Ellingsen T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017;76(10):1700–6.
Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology (Oxford). 2014;53(9):1676–82.
Hyldgaard C, Ellingsen T, Hilberg O, Bendstrup E. Rheumatoid arthritis-associated interstitial lung disease: clinical characteristics and predictors of mortality. Respiration. 2019;98(5):455–60.
Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Gabriel SE, Matteson EL. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91.
Kadura S, Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev. 2021;30(160):210011.
Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, Kannengiesser C, Ottaviani S, Oka S, Tohma S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. New Engl J Med. 2018;379(23):2209–19.
Shirai Y, Honda S, Ikari K, Kanai M, Takeda Y, Kamatani Y, Morisaki T, Tanaka E, Kumanogoh A, Harigai M, et al. Association of the RPA3-UMAD1 locus with interstitial lung diseases complicated with rheumatoid arthritis in Japanese. Ann Rheum Dis. 2020;79(10):1305–9.
Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol. 2018;70(10):1544–54.
Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, Grunewald J, Skold CM, Klareskog L, Catrina AI. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67(10):1488–92.
Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, Sivridis E, Koffa M, Giatromanolaki A, Boumpas DT, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol. 2014;233(3):294–307.
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra140.
Balbir-Gurman A, Guralnik L, Yigla M, Braun-Moscovici Y, Hardak E. Imaging aspects of interstitial lung disease in patients with rheumatoid arthritis: Literature review. Autoimmun Rev. 2018;17(2):87–93.
Paschalaki KE, Jacob J, Wells AU. Monitoring of lung involvement in rheumatologic disease. Respiration. 2016;91(2):89–98.
Manfredi A, Cassone G, Cerri S, Venerito V, Fedele AL, Trevisani M, Furini F, Addimanda O, Pancaldi F, Della Casa G, et al. Diagnostic accuracy of a velcro sound detector (VECTOR) for interstitial lung disease in rheumatoid arthritis patients: the InSPIRAtE validation study (INterStitial pneumonia in rheumatoid ArThritis with an electronic device). BMC Pulm Med. 2019;19(1):111.
Dijkshoorn B, Raadsen R, Nurmohamed MT. Cardiovascular disease risk in rheumatoid arthritis Anno 2022. J Clin Med. 2022;11(10):2704.
Bendstrup E, Moller J, Kronborg-White S, Prior TS, Hyldgaard C. Interstitial lung disease in rheumatoid arthritis remains a challenge for clinicians. J Clin Med. 2019;8(12):2038.
Castañeda S, Vicente-Rabaneda EF, García-Castañeda N, Prieto-Peña D, Dessein PH, González-Gay MA. Unmet needs in the management of cardiovascular risk in inflammatory joint diseases. Expert Rev Clin Immunol. 2020;16(1):23–36.
Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, Harrold LR, Hochberg MC, Tsao P, Greenberg JD. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015;67(6):1449–55.
Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, Gill RR, Hatabu H, Nishino M, Murphy DJ, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol. 2019;71(9):1472–82.
Gehringer CK, Martin GP, Hyrich KL, Verstappen SMM, Sergeant JC. Clinical prediction models for methotrexate treatment outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2022;56:152076.
Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):Itc1–16.
Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–43.
Balta S. Endothelial dysfunction and inflammatory markers of vascular disease. Curr Vasc Pharmacol. 2021;19(3):243–9.
Khan F. Assessment of endothelial function as a marker of cardiovascular risk in patients with rheumatoid arthritis. Int J Rheum Dis. 2010;13(3):189–95.
Alunno A, Gerli R, Giacomelli R, Carubbi F. Clinical, epidemiological, and histopathological features of respiratory involvement in rheumatoid arthritis. Biomed Res Int. 2017;2017:7915340.
Paulin F, Doyle TJ, Fletcher EA, Ascherman DP, Rosas IO. Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev Invest Clin. 2015;67(5):280–6.
Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, Alpizar-Rodriguez D, Lauper K. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602.
Kronzer VL, Huang W, Dellaripa PF, Huang S, Feathers V, Lu B, Iannaccone CK, Gill RR, Hatabu H, Nishino M, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol. 2021;48(5):656–63.
Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, Golzarri MF, Traslosheros A, Chu SG, Frits ML, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015;191(12):1403–12.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation (81920108032), Innovation team of TCM scientific research project of Shanghai Municipal Health Commission (2022CX001), Shanghai "Science and Technology Innovation Action Plan" Medical Innovation Research Special Project (21Y11921400), Shanghai Hospital Traditional Chinese Medicine Preparation Industry Transformation Collaborative Innovation Center, Shanghai top priority clinical medical center (2022ZZ01009), Special project of emerging cross-field research of Shanghai Municipal Commission of Health and Health (2022JC005) and Shanghai Hospital Traditional Chinese Medicine Preparation Industry Transformation Collaborative Innovation Center.
Author information
Authors and Affiliations
Contributions
YS, HZ, QS, YW and QL: conceptualization. YS: writing-original draft preparation and drawing. HZ and QS: revising. YW and QL: supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao, Y., Zhang, H., Shi, Q. et al. Clinical prediction models of rheumatoid arthritis and its complications: focus on cardiovascular disease and interstitial lung disease. Arthritis Res Ther 25, 159 (2023). https://doi.org/10.1186/s13075-023-03140-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-023-03140-5