Abstract
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease, characterized by chronic and systemic inflammation. Besides, it is known that RA patients may present several comorbidities, such as sarcopenia, a condition where patients present both muscle mass and muscle quality impairment. RA treatment is mostly pharmacological and consists in controlling systemic inflammation and disease activity. Despite that, the effect of pharmacological treatment on sarcopenia is not well characterized.
Objective
To summarize the effects of disease-modifying anti-rheumatic drugs (DMARDs) on skeletal muscle tissue in rheumatoid arthritis (RA) patients.
Methods
A systematic review of randomized clinical trials and observational studies was conducted using MEDLINE, Embase, Cochrane Library, and Web of Science. We selected studies with rheumatoid arthritis patients treated with disease-modifying anti-rheumatic drugs (DMARDs) that analyzed muscle mass parameters such as lean mass and appendicular lean mass. Methodological quality was assessed using the Newcastle-Ottawa Quality Assessment Scale. Standardized mean difference (SMD) and 95% confidence intervals (CI) were set. A meta-analysis of observational studies was performed using the R software, and we considered significant statistics when p < 0.05.
Results
Nine studies were included in this systematic review. In the meta-analysis, DMARD treatment had no positive difference (p = 0.60) in lean mass. In the same way, in the appendicular lean mass parameter, our results showed that DMARDs did not have changes between baseline and post-treatment analysis (p = 0.93).
Conclusion
There is no evidence of a significant effect of DMARD therapy, either synthetic or biological, on muscle mass. However, this association should be investigated with more studies.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune disease characterized by systemic inflammation that affects mainly the joints [1]. In addition, RA leads to several comorbidities, such as cardiovascular disease and metabolic syndrome [2, 3]. Furthermore, RA patients are often associated with changes in body composition [3, 4] such as reduced skeletal muscle mass [5], decreased muscle strength [6], and poor physical function [4, 5, 7, 8]. The alterations in RA patients regarding body composition can be a description of a sarcopenic patient that carries the risk of physical incapacity, low quality of life, and death [9, 10]. The prevalence of sarcopenia ranges from 25.9 to 43.3% between cohort studies, a wide variation due to the differences in sample, age, gender, race, and definitions and methods of diagnosing sarcopenia [9, 11,12,13].
Muscle impairment in RA and during sarcopenia is associated with several mechanisms triggered by inflammatory signaling [14]. Inflammatory mediators, such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β), are pointed out as triggers of catabolic effects in muscle tissue [14]. Thus, interleukin 6 (IL-6) has a role in driving catabolism in muscle mass and anabolism in fat mass [15, 16].
The available treatments for RA aim to attenuate disease activity by blocking inflammatory mediators and their signaling or inducing anti-inflammatory and regulatory pathways [17]. Disease-modifying anti-rheumatic drugs (DMARDs) significantly improve disease activity and prevent joint damage in RA by targeting the key inflammatory pathways [17, 18]. The classification of therapeutic drugs are as follows: conventional synthetic DMARDs (csDMARDs), which comprehend methotrexate, leflunomide, sulfasalazine, and hydroxychloroquine; biological DMARDs (bDMARDs); and targeted synthetic DMARDs (tsDMARDs) [19, 20].
bDMARDs include targeting monoclonal antibodies against TNF (infliximab, adalimumab, certolizumab, and golimumab) and IL-6 (tocilizumab and sarilumab), soluble receptor for TNF (etanercept), an inhibitor of T-cell co-stimulation (abatacept), and anti-CD20 B-cell depleting monoclonal antibody (rituximab) [21]. The tsDMARDs are inhibitors of the Janus tyrosine kinase family (JAK), which targets intracellular signaling of type I and II cytokines (tofacitinib, baricitinib, upadacitinib, and filgotinib). Thus, tsDMARD effects are T cell reduction and decreased leukocyte recruitment to joint, resulting in less synovial inflammation and prevented joint damage in RA patients [22, 23]. In a recent narrative review published by our group [8], we found that csDMARDs, tsDMARDs, and bDMARDS have no benefits on muscle mass when used to treat RA patients. However, tocilizumab, an IL-6 inhibitor, may improve muscle mass by increasing appendicular lean mass and total lean mass in RA patients [8]. Also, glucocorticoids (CG) that can control disease activity in RA have known negative effects on the skeletal muscle in RA patients [24, 25]. Targeting inflammatory cytokines seems to have a positive role in muscle wasting. Data from many studies have shown that cytokine inhibition has been effective at preventing or treating muscle wasting. TNF-α blockade can partially revert muscle atrophy by suppression of the NF-κB pathway in several animal models and can prevent survival in aging mice [26,27,28]. Additionally, IL-6 have been independently implicated in some forms of muscle atrophy, and its deficiency attenuates atrophy in sepsis, diabetes melitus, and Duchenne muscular dystrophy [16, 29,30,31].
Nevertheless, sDMARDs and bDMARDs can control disease activity by blocking inflammatory signaling, but their effect on skeletal muscle tissue in RA patients remains unclear. Thus, this systematic review aims to summarize the current evidence on the effect of pharmacological treatment on skeletal muscle tissue in RA patients.
Materials and methods
We conducted this systematic review in accordance with the PRISMA [32] guidelines after registering the protocol with the PROSPERO platform (CRD42021279386).
PICOS/PECOS format
This systematic review with meta-analysis was based on a focused question described in a PICO/PECO format [33]. We established the following: Patient/Problem/Population= Rheumatoid arthritis patients, Intervention/Exposure= Chronic treatment with biological and synthetic DMARD and glucocorticoids, Comparison= Baseline and post treatment, Outcomes= Muscle mass parameters, such as muscle mass, fat-free mass, appendicular lean mass and lean mass; and Study= Randomized clinical trials and Observational studies.
Data sources
The electronic databases used were Cochrane Library, PubMed, Embase, and Web of Science (DATA). We used a comprehensive search strategy tailored to each database. We contacted the authors, when necessary, for more information on the statistical methodology of the articles chosen as a reference. However, in some cases, we have not received any feedback.
Search terms
Keywords and medical subject headings (MeSH) for the following terms,: “Rheumatoid arthritis,”, “Antirheumatic agents,”, “Methotrexate,”, “Leflunomide,”, “Sulfonamides,”, “Hydroxychloroquine,”, “Glucocorticoids,”, “Tumor necrosis factor,”, “Interleukin-6,”, “Janus Kinases,”, “Muscle,”, “Skeletal,”, “Body composition,”, “Cachexia,”, “Sarcopenia,” and related terms were selected. The term OR was used for Union of MeSH terms and "“entry terms",” and the term AND was used to attach the terms. The cComplete search is available below.
(Arthritis Rheumatoid [mh] OR Rheumatoid Arthritis [all fields] OR RA [all fields]) AND (Antirheumatic Agents [mh] OR Antirheumatic*[all fields] OR Anti-rheumatic*[all fields] OR DMARD [all fields] OR Methotrexate [mh] OR Methotrexate [all fields] OR Leflunomide [mh] OR Leflunomide [all fields] OR Sulfonamides [mh] OR Abatacept [all fields] OR rituximab [all fields] OR Sulfonamides [all fields] OR Hydroxychloroquine [mh] OR Hydroxychloroquine [all fields] OR Glucocorticoids [mh] OR Glucocorticoid*[all fields] OR Tumor Necrosis Factor-alpha [mh] OR Tumor Necrosis Factor-alpha [all fields] OR TNFalpha [all fields] OR TNF-alpha [all fields] OR Interleukin-6[mh] OR Interleukin-6[all fields] OR Janus Kinases [mh] OR Janus Kinases [all fields]) AND (Muscle Skeletal [mh] OR Muscle mass [all fields] OR Body Composition [mh] OR Body Composition [all fields] OR Cachexia [mh] OR Cachexia [all fields] OR Sarcopenia [mh] OR Sarcopenia [all fields])
Inclusion/exclusion criteria
Randomized clinical trials and observational studies with patients diagnosed with RA that were treated with bDMARD, tsDMARD, and csDMARD that analyzed muscular parameters, and articles that were written in the English language were included. No restrictions about the year of the studies were applied. Articles that reported data from RA patients < 18 years old, clinical trials, experimental studies, reviews, meta-analyses, studies of patients without RA, and studies that proposed acute treatment were excluded.
Study selection and data extraction
Title, abstract, and full-text screening were performed in pairs by two independent reviewers (Hein, TR, and Bartikoski, BJ). The reviewers extracted the data from the studies independently, using a pre-established data sheet, which is available upon request. All data from the study were screened using a bibliographic management program (Mendeley®, version1.17.9). Disagreements about data abstraction were resolved by a discussion between the two reviewers. If no agreement could be reached, a third reviewer (Santo, RCE) provided the final decision. The information extracted during the data abstraction included authors’ names, date of publication, journal of publication, number of participants in the study, the age group of the population, type of population, type of treatment, duration of treatment, treatment posology, and results obtained for lean mass and appendicular lean mass. After the authors’ agreement, nine studies were included in this review. The baseline mean and after-treatment mean were extracted and converted and the delta of the mean (difference of final mean and baseline mean) for meta-analysis. In one study [34], we estimated the baseline mean from graph bars with the ImageJ software.
Methodological quality assessment
Methodological quality was assessed by the Newcastle-Ottawa Scale for cohort studies or for randomized clinical trials [35,36,37] by two independent reviewers (Santos, LP, and Portes, JKS). In these scales, each study was judged by questions about groups of criteria: selection of cohort, comparability of the study, and ascertainment of the outcomes for cohort studies and selection, comparability, and exposure for randomized clinical trials. For each item, in the selection, outcome, and exposure groups, a maximum of one star can be assigned, and for the comparability group, a maximum of two stars can be assigned. So, the maximum possible score was 9 stars. Based on the scale, studies with scores of 3 or 4 in the selection, 1 or 2 scores in comparability, and 2 or 3 in outcome or exposure were classified as good quality studies. On the other hand, studies with 2 stars in the selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcome and exposure were classified as fair-quality studies. Finally, studies with scores of 0 or 1 in the selection, 0 scores in comparability, or 0 or 1 score in outcome or exposure were classified as poor-quality studies.
Risk of bias assessment
The risk of bias in the randomized clinical trials was assessed using the Risk of Bias Tool 2.0 (RoB2) from Cochrane to randomized clinical trials [38]. The evaluators examined the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Thus, the studies were classified into low, moderate, or high risk of bias.
Statistical analysis
The meta-analysis was conducted using the meanchange and SDchange from each study. All outcome measures were continuous variables. A meta-analysis, representing the effects of interventions, was performed: the random-effects model with the mean difference (MD) MD was performed when studies reported outcomes using the same assessment scale or assessment instrument.
The 95% confidence intervals (CI) were used, and the heterogeneity of the studies included in the meta-analysis was assessed using the inconsistency test (I2). We considered low, moderate, and high inconsistency in the approximated values of 25%, 50%, and 75%, respectively [39, 40]. The software used for statistical analysis was RevMan (Review Manager 5.4.1, The Cochrane Collaboration, 2020), and we considered it significant statistically when p < 0.05.
Results
Search strategy
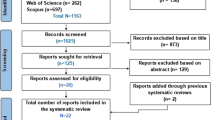
We identified 1123 possible studies (134 duplicate publications) based on our search items. First, the title and abstract of the 1244 studies were screened. After this process, 32 articles were included for the full-text screening. Finally, after the full-text reading, we included nine studies: Engvall et al. [41] and Marcora et al. [42] as randomized clinical trials and Al Khayyad et al. [43], Vial et al. [44], Tournadre et al. [45], Toussirot et al. [46], Ferraz-Amaro et al. [47], Metsios et al. [48], and Chikugo et al. [34] as observational studies. The search and inclusion/exclusion criteria are described in Fig. 1.
Characteristics of the studies
Of the nine studies included, four of them were performed in France [42, 44,45,46], one of them in the UK [48], one in Spain [47], one in Japan [34], one in Sweden [41], and one in Italy [43]. Studies included were published between 2007 and 2020. Only one study was performed using female patients [34] while the other four were performed with male and female patients [45,46,47,48]. Included papers reported sample size ranged from 8 to 146 subjects, patients’ age means from 50 to 61 years. Studies also showed baseline DAS-28 ranged from 3.0 to 6.1 [34, 45,46,47,48]. Characteristics of the included studies are described in Tables 1 and 2.
Characteristics of treatments
Among the nine papers included, the treatments used in the studies were tocilizumab [45, 46], anti-TNF [47, 48], JAKi [34], rituximab [43], bDMARDs [44], etanercept [42], methotrexate [41, 42], sulfasalazine [41], and hydroxychloroquine [41].
Methods of assessment of the muscle mass and treatment effect
Three of nine studies (33%) used bioimpedance as a measurement method [34, 47, 48], while the other six (66%) used dual-energy X-ray absorptiometry (DEXA) [41,42,43,44,45,46]. Despite being different methods of assessing muscle mass, studies have shown that these methods have good validity and agreement [49, 50]. Toussirot et al. used lean mass, and the proposed treatment showed significant improvement in this parameter (3.3%) [46]. Tournadre et al. analyzed parameters lean mass and appendicular lean mass showing significant benefits in both parameters after one year of treatment with tocilizumab (2.6% in lean mass and 5.6% in appendicular lean mass) [45]. Ferraz-Amaro et al. (5.9%) and Metsios et al. (0.39%) used lean mass as a parameter, but both showed no significant improvement after anti-TNF treatment [47, 48]. Chikugo et al. used appendicular lean mass as a parameter and showed no significant changes in this parameter after JAKi treatment (0.49%) [34]. Al Khayyat et al. [43] used both lean mass and appendicular lean mass as parameters and showed a decrease (3.3%) in lean mass and an increase (10%) in appendicular lean mass. Vial el al [44]. used lean mass as a parameter and showed an improvement of lean mass in the TNFi group (2.2%) and a decrease (0.7%) of lean mass in the non-TNFi group.
Methodological quality of the studies
In the methodological quality assessment of the nine studies, eight studies [34, 41,42,43,44,45, 47, 48] were classified as good-quality studies, and one was classified as a poor-quality study [46]. Data were described in Table 3.
Risk of bias of studies
In the risk of bias analysis, two of the two studies [41, 42] were classified with a high risk of bias. Data was described in Table 4.
Meta-analysis of lean mass
Four out of seven observational studies performed lean mass measures [45,46,47,48]. About this outcome, we performed two different methods in our meta-analysis: a general analysis comparing the four studies, and a subgroup analysis comparing types of treatment. Two studies used tocilizumab as treatment, and the other two used anti-TNF therapy. Despite the lack of significant difference, in the general analysis, five [44,45,46, 48] of eight groups analyzed have shown a positive delta of lean mass, and three [43, 44, 47] groups have shown a negative delta. In general analysis, the treatment with DMARD was not able to increase lean mass in patients (mean = 0.47; 95% CI [− 0.92 to 1.87]; I2, 0% p = 0.91) (Fig. 2). In the subgroup analysis, tocilizumab treatment (mean = 1.32; 95% CI [− 0.87 to 3.52;] I2, 0%; p = 0.95) and TNFi treatment (mean = 0.21; 95% CI [− 2.63 to 3.04;] I2, 0%; p = 0.47) had positive mean (Fig. 3). In rituximab (mean = − 1.30) and bDAMRD nTNFi (mean = − 0.30) treatment, the mean was negative.
Meta-analysis of appendicular lean mass
Regarding appendicular lean mass, three studies have measured this outcome. Still, one of these studies has performed a trial with two groups of treatment: one group treated with tofacitinib, and one group treated with other bDMARDs [34]. Regardless of the increase of mean appendicular lean mass, treatment with DMARD showed no significant change in appendicular lean mass delta (mean = 1.11, 95% CI [− 0.58 to 2.79]; I2, 0%; p = 0.91) (Fig. 4).
Discussion
As a result of this systematic review with meta-analysis, we found that DMARD treatment did not appear to induce significant muscle mass changes in RA patients. Still, regarding lean mass measurement, we described in subgroup analysis that anti-IL-6 and anti-TNF treatments were more related to the gain of lean mass than other DMARD therapies. Besides, considering the slight mass gain in both lean mass and appendicular lean mass and the small number of studies, we cannot exclude the possibility of a beneficial effect, particularly in anti-IL6 and anti-TNF therapy. This systematic review with meta-analysis is the first to verify the effect of DMARD treatment and its subclasses in muscle mass parameters.
Considering the muscle mass loss present in RA sarcopenia [10], DMARD treatment not only prevented this but also showed a trend of a slight gain of muscle mass in analyzed parameters. Dao et al. (2021) [51] investigated the associations between RA treatment and sarcopenia prevalence. Inherently, the authors showed that RA patients on csDMARD treatment had a lower prevalence of sarcopenia compared to RA patients on bDMARD. tsDMARD treatment had no association with sarcopenia. As we also saw in our review, Dao et al. emphasized the small number of papers in the literature and pointed out that it could be the reason for the lack of associations.
In our review, studies showed that IL-6 inhibition tended to be related to slight lean mass gain. IL-6 can bind the membrane IL-6 (IL-6R) and induce intracellular signals [52]. Still, IL-6 can also bind to soluble receptors (sIL-6R) creating a complex able to stimulate cells that do not have the membrane receptor [53]. Due to these mechanisms, the IL-6 effect in cells can be dualistic, being either inflammatory or anti-inflammatory [16]. Indeed, in an acute exercise setting, IL-6 secreted by muscle cells can drive muscle growth signaling, muscular regeneration, and activation of muscle stem cells [54]. On the other hand, chronic expression of IL-6 by inflammatory and immune cells is related to the induction of muscle atrophy and protein degradation [55, 56]. These effects occur by IL-6R binding leading to activation of the JAK/STAT complex [57] and signaling the increased expression of catabolic genes, such as muscle RING-finger protein-1 (MURF1), ubiquitin-proteasome subunits, caspases and cathepsins [14]. Thus, we consider that anti-IL6 therapy could have a positive effect on muscle mass in conditions of chronic inflammation based on its influence on important routes of inflammatory signaling and on its role as a locally secreted myokine [58]. Differently from other proinflammatory cytokines, which are mostly secreted by inflammatory cells and their action is generally systemic, IL-6 is secreted by muscle cells for paracrine communication leading to potent local signaling [59].
TNF-α is another key factor in muscle impairment in RA [60]. TNF-α inhibition therapy also seemed to have a positive effect on lean mass in AR patients. At a molecular level, TNF-α is the main responsible for the NFκꞵ activation pathway [61, 62], a transcript factor known to drive the subsequent expression of inflammatory mechanisms [63]. With the meta-analysis results, we speculate that despite its approved effect against RA disease activity, blocking systemic inflammation, anti-TNF treatment tended to have a local effect to block TNF downstream in the muscle being able to prevent AR muscle loss [64]. Interestingly, in both randomized clinical trials mentioned in our review, Marcora et al. and Engvall et al. did not present, in their results, significant change in both lean mass and appendicular lean mass, when patients were treated with anti-TNF drug [41, 42].
JAKi treatment, a more recent approach, has been demonstrated to be effective against RA inflammation [65]. The JAK/STAT pathway is known for acting together with cytokine receptors carrying the intracellular signaling through the phosphorylation of STATs [57, 66, 67]. For example, JAK/STATs are attached to IL-6 membrane receptors and are responsible for activating the transcription of inflammatory genes [68]. In our review, we showed that JAKi treatment did not present a significant effect on appendicular lean mass. Still, its effect was similar to DMARD treatment performed in the same study [34]. We believe that JAKi analysis was limited by the lack of studies and the study sample size.
In this review, we used Newcastle Ottawa to describe the quality of each study included in our systematic review with meta-analysis. The majority of studies were identified with good quality. Finally, this systematic review with meta-analysis has some limitations. First, there were a small number of studies included. Furthermore, the studies included were performed by enrolling both male and female patients, and it is known that men have higher muscle mass than women.
We conclude that DMARDs have no effect on muscle mass parameters in rheumatoid arthritis patients. Indeed, we showed that DMARD treatment was not able to have a positive effect both in lean mass (total lean mass including trunk) and appendicular lean mass (lean mass of arms and legs only), results that coincide with clinical trials available in the literature. However, this review could be a path to better understanding the treatment of RA muscle loss, being the first to systematically analyze the literature about it. We believe that the limitations found in our review, such as the small number of studies and sample size, may have been relevant for not having found differences in our analyses. Emphasizing this is important to drive and induce researchers to develop investigations about it. In addition, the enlightenment of how DMARDs act in muscle mass is important for the formulation of treatment protocols that can treat not only autoimmune and inflammatory diseases but also muscle-wasting conditions such as sarcopenia and cachexia. Finally, by summarizing and qualifying the data about the relationship between DMARDs and muscle mass in RA, this systematic review is crucial to enlighten the evidence presented in the literature.
Conclusion
We conclude that this review was the first to summarize the data about the relationship between DMARDs and muscle mass. In addition, we have that DMARD treatment has no positive effect on rheumatoid arthritis muscle mass loss. With this review, we contribute to enlightenment in DMARD treatment in rheumatoid arthritis once it does not have any approved pharmacological therapy for comorbidities such as muscle loss.
Availability of data and materials
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
Abbreviations
- RA:
-
Rheumatoid arthritis
- MD:
-
Mean difference
- SMD:
-
Standardized mean difference
- CI:
-
Confidence interval
- DMARD:
-
Disease-modifying anti-rheumatic drug
- bDMARD:
-
Biological disease-modifying anti-rheumatic drug
- tsDMARD:
-
Target synthetic disease-modifying anti-rheumatic drug
- csDMARD:
-
Conventional synthetic disease-modifying anti-rheumatic drug
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-6:
-
Interleukin 6
- sIL-6R:
-
Soluble interleukin 6 receptor
- IL-1β:
-
Interleukin 1 beta
- JAK:
-
Janus kinase
- IL-2:
-
Interleukin 2
- IL-7:
-
Interleukin 7
- DEXA:
-
Dual-energy X-ray absorptiometry
- BIA:
-
Bioimpedance
- MuRF1:
-
Muscle RING-finger protein 1
- TGF-β:
-
Transforming growth factor-beta
- JAK/STAT:
-
Janus kinase/signal transducer and activator of transcription
- MTX:
-
Methotrexate
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Prim. 2018; [cited 2022 Jun 16];4. Available from: https://pubmed.ncbi.nlm.nih.gov/29417936/.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med [Internet]. 2011;365:2205–19 [cited 2019 Jun 17]Available from: http://www.ncbi.nlm.nih.gov/pubmed/22150039.
Masuko K. Rheumatoid cachexia revisited: a metabolic co-morbidity in rheumatoid arthritis. Front Nutr. 2014;1 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/25988122/.
de Souza MPGU, Guimarães NS, de Resende Guimarães MFB, de Souza VA, Kakehasi AM. Effect of biological disease-modifying antirheumatic drugs on body composition in patients with rheumatoid arthritis: a systematic review and meta-analysis. Adv Rheumatol. 2022;62 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/35606888/.
Wysham KD, Shoback DM, Imboden JB, Katz PP. Association of high anti–cyclic citrullinated peptide seropositivity and lean mass index with low bone mineral density in rheumatoid arthritis. Arthritis Care Res. 2018;70:961–9 K.D. Wysham, University of California, San Francisco, CA, United States. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L618910640&from=export.
Weber D, Long J, Leonard MB, Zemel B, Baker JF. Development of novel methods to define deficits in appendicular lean mass relative to fat mass. PLoS One. 2016;11 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/27723820/.
Lemmey AB. Rheumatoid cachexia: the undiagnosed, untreated key to restoring physical function in rheumatoid arthritis patients? Rheumatology (Oxford) [Internet]. Rheumatology (Oxford). 2016;55:1149–50 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/26672906/.
Cavalheiro R, Hein T, Xavier RM. The effect of pharmacological treatment on rheumatoid arthritis related sarcopenia: an integrative review. Curr Rheumatol Res. 2021;2. https://doi.org/10.46439/rheumatology.2.011.
Lian L, Wang JX, Xu YC, Zong HX, Teng YZ, Xu SQ. Sarcopenia may be a risk factor for osteoporosis in Chinese patients with rheumatoid arthritis. Int J Gen Med. 2022;15:2075–85 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/35237070/.
Santo RC, Silva JM, Lora PS, Moro ALD, Freitas EC, Bartikoski BJ, et al. Cachexia in patients with rheumatoid arthritis: a cohort study. Clin Rheumatol. 2020;39:3603–13 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/32447598/.
Tada M, Yamada Y, Mandai K, Hidaka N. Matrix metalloprotease 3 is associated with sarcopenia in rheumatoid arthritis - results from the CHIKARA study. Int J Rheum Dis. 2018;21:1962–9.
Torii M, Hashimoto M, Hanai A, Fujii T, Furu M, Ito H, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29:589–95 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/30092163/.
Ceyhan Dogan S, Hizmetli S, Hayta E, Kaptanoglu E, Erselcan T, Guler E. Sarcopenia in women with rheumatoid arthritis. Eur J Rheumatol. 2015;2:57–61 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/27708927/.
Webster JM, Kempen LJAP, Hardy RS, Langen RCJ. Inflammation and Skeletal Muscle Wasting During Cachexia. Front Physiol. 2020;11:597675. https://doi.org/10.3389/fphys.2020.597675.
Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev [Internet]. Physiol Rev. 2008;88:1379–406 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/18923185/.
Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–48 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/23663276/.
Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77 [cited 2019 Jun 17]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28264816.
Buttgereit F. Views on glucocorticoid therapy in rheumatology: the age of convergence. Nat Rev Rheumatol. 2020;16:239–46 [cited 2022 Jun 16] Available from: https://pubmed.ncbi.nlm.nih.gov/32076129/.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:S685–99 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/31969328/.
Henrique M, da Mota L, Afonso Cruz B, Viegas Brenol C, Alves Pereira I, Stange Rezende Fronza L, et al. Consensus of the Brazilian Society of Rheumatology for diagnosis and early assessment of rheumatoid arthritis. Rev Bras Reum. 2011;2011(51):199–219.
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. 2018 [cited 2022 Jun 16]; Available from: https://jamanetwork.com/
Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–81 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/22006202/.
Lundquist LM, Cole SW, Sikes ML. Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop. 2014;5:504–11 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/25232526/.
Tong J-J, Xu S-Q, Wang J-X, Zong H-X, Chu Y-R, Chen K-M, et al. Interactive effect of sarcopenia and falls on vertebral osteoporotic fracture in patients with rheumatoid arthritis. Arch Osteoporos. England. 2021;16:145.
Yamada Y, Tada M, Mandai K, Hidaka N, Inui K, Nakamura H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis: from the CHIKARA study. Clin Rheumatol. Germany. 2020;39:1757–64.
Johnen H, Lin S, Kuffner T, Brown DA, Tsai VWW, Bauskin AR, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–40 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/17982462/.
Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. United States. 2000;35:537–44.
Sciorati C, Gamberale R, Monno A, Citterio L, Lanzani C, De Lorenzo R, et al. Pharmacological blockade of TNFα prevents sarcopenia and prolongs survival in aging mice. Aging (Albany NY). 2020;12:23497–508 [cited 2022 Jun 16] Available from: https://pubmed.ncbi.nlm.nih.gov/33260150/.
Yang B, Yang X, Sun X, Shi J, Shen Y, Chen R. IL-6 deficiency attenuates skeletal muscle atrophy by inhibiting mitochondrial ROS production through the upregulation of PGC-1 α in septic mice. Oxid Med Cell Longev. 2022;2022:9148246 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/35528525/.
Tsai CH, Huang PJ, Lee IT, Chen CM, Wu MH. Endothelin-1-mediated miR-let-7g-5p triggers interlukin-6 and TNF-α to cause myopathy and chronic adipose inflammation in elderly patients with diabetes mellitus. Aging (Albany NY). 2022;14:3633–51 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/35468098/.
Pelosi L, Berardinelli MG, De Pasquale L, Nicoletti C, D’Amico A, Carvello F, et al. Functional and morphological improvement of dystrophic muscle by interleukin 6 receptor blockade. EBioMedicine. 2015;2:285–93 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/26137572/.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12 [cited 2022 Jun 16] Available from: https://pubmed.ncbi.nlm.nih.gov/19631508/.
Mattos CT, Ruellas AC d O. Systematic review and meta-analysis: what are the implications in the clinical practice? Dental Press J Orthod. 2015;20:17–9 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/25741820/.
Chikugo M, Sebe M, Tsutsumi R, Iuchi M, Kishi J, Kuroda M, et al. Effect of Janus kinase inhibition by tofacitinib on body composition and glucose metabolism. J Med Invest. Japan. 2018;65:166–70.
Wells GA. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2015. Available: http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:1–11 [cited 2022 Jun 16]. Available from: https://mmrjournal.biomedcentral.com/articles/10.1186/s40779-020-00238-8.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/20652370/.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ [Internet]. BMJ. 2019;366 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/31462531/.
Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One. 2012;7(7):e39471. https://doi.org/10.1371/journal.pone.0039471. Epub 2012 Jul 25.
Julian T, Higgins AP, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. Rapid responses Topic collections. 2003;327:557–60 [cited 2022 Jun 16]. Available from: http://bmj.com/cgi/content/full/327/7414/557. http://bmj.com/cgi/content/full/327/7414/557#otherarticles. http://bmj.com/cgi/content/full/327/7414/557#BIBL. http://bmj.com/cgi/eletter-submit/327/7414/557. http://bmj.com/cgi/content/full/327/7414/557#responses.
Engvall I-L, Tengstrand B, Brismar K, Hafström I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomised study over 21 months. Arthritis Res Ther. 2010;12:R197.
Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr. United States. 2006;84:1463–72.
Al Khayyat SG, Falsetti P, Conticini E, DÁlessandro R, Bellisai F, Gentileschi S, et al. Bone-sparing effects of rituximab and body composition analysis in a cohort of postmenopausal women affected by rheumatoid arthritis – retrospective study. Reumatologia. 2021;59:206–10 S.G. Al Khayyat, University Hospital of Siena, Viale Mario Bracci, 16, Siena, Italy; . Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L2014444794&from=export.
Vial G, Lambert C, Pereira B, Couderc M, Malochet-Guinamand S, Mathieu S, et al. The effect of TNF and non-TNF-targeted biologics on body composition in rheumatoid arthritis. J Clin Med. 2021;10:487 Available from: https://www.mdpi.com/2077-0383/10/3/487.
Tournadre A, Pereira B, Dutheil F, Giraud C, Courteix D, Sapin V, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle. 2017;8:639–46.
Toussirot E, Marotte H, Mulleman D, Cormier G, Coury F, Gaudin P, et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: a 12-month multicentre study. Arthritis Res Ther. 2020;22(1):224. https://doi.org/10.1186/s13075-020-02297-7.
Ferraz-Amaro I, Arce-Franco M, Muñiz J, López-Fernández J, Hernández-Hernández V, Franco A, et al. Systemic blockade of TNF-α does not improve insulin resistance in humans. Horm Metab Res. 2011;43:801–8. https://doi.org/10.1055/s-0031-1287783.
Metsios GS, Stavropoulos-Kalinoglou A, Douglas KMJ, Koutedakis Y, Nevill AM, Panoulas VF, et al. Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: effects on components of rheumatoid cachexia. Rheumatology. 2007;46:1824–7.
Cheng KYK, Chow SKH, Hung VWY, Wong CHW, Wong RMY, Tsang CSL, et al. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J Cachexia Sarcopenia Muscle. 2021;12:2163–73 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/34609065/.
Spanjer MJ, Bultink IEM, de van der Schueren MAE, Voskuy AE. Prevalence of malnutrition and validation of bioelectrical impedance analysis for the assessment of body composition in patients with systemic sclerosis. Rheumatology. 2017;56:1008–12 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/28340004/.
Dao T, Kirk B, Phu S, Vogrin S, Duque G. Prevalence of sarcopenia and its association with antirheumatic drugs in middle-aged and older adults with rheumatoid arthritis: a systematic review and meta-analysis. Calcif Tissue Int. 2021;109:475–89.
Kyrana E, Briggs S, Dhawan A. Molecular mechanisms of cachexia in chronic disease. Expert Rev Endocrinol Metab. 2012;7:73–90 A. Dhawan, Paediatric Liver, GI and Nutrition Centre, King’s College Hospital, Denmark Hill, London, SE5 9RS, United Kingdom. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L363049494&from=export.
Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195:173–83 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/16741736/.
Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab [Internet]. Cell Metab. 2008;7:33–44 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/18177723/.
Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–5 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/8108469/.
Penna F, Costamagna D, Pin F, Camperi A, Fanzani A, Chiarpotto EM, et al. Autophagic degradation contributes to muscle wasting in cancer cachexia. Am J Pathol. 2013;182:1367–78 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/23395093/.
Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/22669242/.
Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev [Internet]. Physiol Rev. 2013;93:23–67 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/23303905/.
Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97:244–9 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/8550842/.
Patel HJ, Patel BM. TNF-α and cancer cachexia: molecular insights and clinical implications. Life Sci. 2017;170:56–63 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/27919820/.
Thoma A, Lightfoot AP. NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol [Internet]. Adv Exp Med Biol. 2018;1088:267–79 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/30390256/.
Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21:223–44 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/21243012/.
Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324:242–6 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/19359585/.
Lightfoot AP, Sakellariou GK, Nye GA, McArdle F, Jackson MJ, Griffiths RD, et al. SS-31 attenuates TNF-α induced cytokine release from C2C12 myotubes. Redox Biol. 2015;6:253–9 [cited 2022 Jun 16]Available from: https://pubmed.ncbi.nlm.nih.gov/26291279/.
Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/25549588/.
Moresi V, Adamo S, Berghella L. The JAK/STAT pathway in skeletal muscle pathophysiology. Front Physiol. 2019;10 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/31114509/.
Jang Y-N, Baik EJ. JAK-STAT pathway and myogenic differentiation. JAK-STAT. 2013;2:e23282 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/24058805/.
Bousoik E, Mahdipoor P, Alhazza A, Montazeri AH. Combinational silencing of components involved in JAK/STAT signaling pathway. Eur J Pharm Sci. 2022;175:106233 [cited 2022 Jun 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/35680032/.
Acknowledgements
We thank the Coordination for the Improvement of Higher Level Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)) and the Rio Grande do Sul State Research Foundation (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS)) for granting scholarships to the students that contributed to this study. Additionally, we thank the HCPA Department of Rheumatology and Biostatistics Service for the scientific support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
T.H. participated in the design of the study, carried out the initial selection of the studies, participated in the data analysis, drafted the manuscript, performed the meta-analysis, and prepared all the figures. L.P. participated in the design of the study, carried out a full-length selection of the studies, performed the methodological quality and risk of bias analysis, and helped in the meta-analysis. B.J. participated in the design of the study, carried out the initial selection of the studies, and participated in the data analysis. J.P. participated in the design of the study, carried out a full-length selection of the studies, and performed the methodological quality and risk of bias analysis. R.C. participated in the design of the study, helped in the selection of the studies, helped in the methodological quality and risk of bias analysis, helped in the meta-analysis, and helped in the preparation of the figures. R.M. participated in the design of the study, helped in the manuscript draft, and helped in the preparation of figures. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Where applicable, I confirm that all human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Where applicable, I confirm that all persons gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study have been omitted.
Consent for publication
All authors give their consent to the publication of this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hein, T.R., Peterson, L., Bartikoski, B.J. et al. The effect of disease-modifying anti-rheumatic drugs on skeletal muscle mass in rheumatoid arthritis patients: a systematic review with meta-analysis. Arthritis Res Ther 24, 171 (2022). https://doi.org/10.1186/s13075-022-02858-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02858-y