Abstract
Background
Systemic lupus erythematosus (SLE) is characterized by loss of immune tolerance and imbalance of immune cell subsets. Natural killer (NK) cells contribute to regulate both the innate and adaptive immune response. In this study, we aimed to detect alterations of peripheral NK cells and explore intrinsic mechanisms involving in NK cell abnormality in SLE.
Methods
Blood samples from healthy controls (HCs) and patients with SLE and rheumatoid arthritis (RA) were collected. The NK count, NK subsets (CD56bright, CD56dimCD57−, and CD56dimCD57+), phenotypes, and apoptosis were evaluated with flow cytometer. Mitochondrial reactive oxygen species (mtROS) and total ROS levels were detected with MitoSOX Red and DCFH-DA staining respectively. Published data (GSE63829 and GSE23695) from Gene Expression Omnibus (GEO) was analyzed by Gene Set Enrichment Analysis (GSEA).
Results
Total peripheral NK count was down-regulated in untreated SLE patients in comparison to that in untreated RA patients and HCs. SLE patients exhibited a selective reduction in peripheral CD56dimCD57+ NK cell proportion, which was negatively associated with disease activity and positively correlated with levels of complement(C)3 and C4. Compared with HCs, peripheral CD56dimCD57+ NK cells from SLE patients exhibited altered phenotypes, increased endogenous apoptosis and higher levels of mtROS and ROS. In addition, when treated with hydrogen peroxide (H2O2), peripheral CD56dimCD57+ NK cell subset was more prone to undergo apoptosis than CD56dimCD57− NK cells. Furthermore, this NK cell subset from SLE patients exhibited impaired cytotoxicity in response to activated CD4+ T cells in vitro.
Conclusion
Our study demonstrated a selective loss of mature CD56dimCD57+ NK cell subset in SLE patients, which may caused by preferential apoptosis of this subset under increased oxidative stress in SLE. The attenuated in vitro cytotoxicity of CD56dimCD57+ NK cells may contribute to the impaired ability of eliminating pathogenic CD4+ T cells in SLE.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease driven by aberrant activation of autoreactive T and B cells and the production of autoantibodies [1]. Patients with SLE are known to exhibit diminished antiviral immunity and heightened risk for the development of severe opportunistic infections, which represents an important cause of mortality [2].
NK cells are innate lymphoid cells which mediate resistance against tumor cells and microbial pathogens, especially the virus, and also contribute to the regulation of adaptive immune response [3, 4]. NK cells exert cytotoxicity through expresssion of CD107a, production of lytic granules (e.g. perforin and granzyme B) and engagement of CD16. They also regulate the immune response by cytokine production, such as IFN-γ and IL-10. In humans, NK cells have long been classified into two well-characterized subsets, known as CD56dim NK cells and CD56bright NK cells. The former, with stronger cytotoxic activity, are more mature and predominant in the peripheral blood. The latter, with more cytokine production, are predominant in tissues [5]. Recent studies suggest that CD57 expression marks mature human NK cells and CD56dimCD57+ NK cell is a newly discovered important subgroup, which is characterized by enhanced lytic granule protein contents, including perforin and granzyme B [5,6,7]. Furthermore, transcriptome and protein expression data have displayed gradients in cell activities from CD56bright NK cells to CD56dimCD57− NK cells to CD56dimCD57+ NK cells [7].
NK cells have been shown to play a beneficial role in the regulation of pathogenic autoreactive T cell and B cell responses [3, 4]. NK cell depletion accelerates disease activity in lupus-prone mice, whereas NK cell transfer delays disease onset [8]. A transcriptome level study suggests that NK cell cytotoxicity transcript is negatively correlated with lupus disease activity [9]. Number reductions and phenotype alterations of NK cells in SLE patients have been described [10,11,12], but the results are not consistent. The discrepancies in the above studies may be explained by differences in treatment regimen and NK cell heterogeneity, as total NK cells rather than NK cell subsets were detected in most studies.
We conducted this study with the purpose of detecting the number and the subsets of NK cells in peripheral blood from SLE patients and their correlations with disease activity. Furthermore, we investigated their cytotoxic effect on activated CD4+ T cells, and explored the intrinsic mechanisms which may contribute to NK cell abnormality in SLE patients.
Methods
Study subjects
All the samples were obtained from individuals fulfilling the diagnosis of SLE or rheumatoid arthritis (RA) according to the American College of Rheumatology (ACR) criteria [13, 14] in Affiliated Hospital of Nantong University and The Second Hospital of Dalian Medical University (from 2017 to 2020). Patients complicated with tumor, infections or other autoimmune diseases were excluded. Healthy controls (HCs) were chosen from age and gender matched healthy volunteers. Our study was approved by the ethics committee of the Affiliated Hospital of Nantong University and Dalian Medical University.
Baseline characteristics
Related test results of the subjects enrolled were collected through review of medical records, including: age, gender, duration, white blood cell (WBC), hemoglobin (Hb), platelet (PLT), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complement C3 and C4, anti-double-stranded-DNA antibodies (anti-dsDNA antibodies), antinuclear antibodies (ANA), immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), and albumin (ALB). Disease activity was measured using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI) score for SLE patients [15]. Disease activity was measured using 28-joint disease activity score (DAS28) for RA patients. Lupus nephritis (LN) is defined as proteinuria (more than 0.5 g of protein per day or 3 + on dipstick) or the presence of cellular casts (either red blood cell, hemoglobin, granular, tubular or mixed) in urinary sediment or renal biopsy.
Total NK cell analysis in treatment-naive patients
We conducted 156 treatment-naive SLE patients and 22 treatment-naive RA patients with no history of glucocorticoids or immunosuppressive drugs. 30 age and gender matched HCs were enrolled. Blood cells were incubated with a panel of monoclonal antibodies (anti-human-CD3, anti-human-CD56). Trucount tubes were used to determine the absolute number. The count and percentage of CD3−CD56+ NK cells were measured.
NK subset analysis by flow cytometry
We conducted 37 untreated SLE patients and 30 HCs matched for age and gender. Blood samples were stained with fluorophores conjugated antibodies for phenotypic detecting. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation (400 g, 30 min) for cell stimulation. The NK cell subset gating strategies were depicted in Supplementary Fig. 1. All antibodies for staining were shown in Supplementary Table 1. Cell suspensions were surface-labeled with antibodies for 30 min. For intracellular staining, cell suspensions were firstly incubated with surface antibody, then fixed and permeabilized using the Transcription Factor Fix/Perm Buffer (eBioscience). For IFN-γ and IL-10 production assays, cells were stimulated with phorbol 12-myristate 13-acetate (PMA, 50 ng/ml) and ionomycin (1 µg/ml) for 4 h in the presence of Brefeldin A (10 µg/ml). All the cell samples were detected by BD FACSCanto™ II or ACEA NovoCyte and the acquired data were further analyzed with BD FACSDiva software or FlowJo software.
Apoptosis detection
Apoptosis of NK cell subsets was detected by staining fresh PBMCs with annexin V (Invitrogen) and 7-amino-actinomycin D (7-AAD) (BD Biosciences) in 19 SLE patients and 10 HCs.
Measurement of reactive oxygen species(ROS) levels
ROS levels were assessed by incubating cells with 10 µM 2',7'-Dichlorodihydrofluorescein diacetate (DCFH-DA) (AAT Bioquest) for 20 min at 37℃. Mitochondrial reactive oxygen species (mtROS) levels were assessed by incubating cells with 5 µM MitoSOX Red probe (invitrogen) for 15 min at 37℃. DCFH-DA and MitoSOX fluorescence intensities in NK cells were detected by flow cytometry as mean fluorescence intensity (MFI).
Isolation of NK cell subsets
Total NK cells (> 92% purity) were negatively isolated by magnet associated cell sorting (MACS) (Biolegend). CD56dimCD57+ NK cells and CD56dimCD57− NK cells (> 95% purity of each subset) were isolated from fresh PBMCs by fluorescence activated cell sorting (FACS) using a FACSAria II cell sorter (BD).
Detection of CD107a expression
MACS-sorted NK cells were stimulated with PMA (50 ng/ml) and ionomycin (1 µg/ml) for 6 h in the presence of Brefeldin A (10 µg/ml) and PE-conjugated anti-CD107a in RPMI 1640 medium (10% FBS) at 37℃ in a 5% CO2 atmosphere. The expressions of CD107a on CD56dimCD57+ NK cells and CD56dimCD57− NK cells were detected by Flow cytometry.
Cytoxicity assay
Purified CD4+ T cells (> 95% purity) (Biolegend) were labeled with 1 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) and rested or activated in RPMI 1640 medium (10% FBS) with human T-activator CD3/CD28 antibody (both 2 µg/ml, eBioscience) for 72 h. FACS-sorted NK cell subsets (CD56dimCD57+ NK cells and CD56dimCD57− NK cells) were cocultured with these CD4+ T cells at a ratio of 1:1 for 15 h in RPMI 1640 medium (10% FBS) at 37℃ in a 5% CO2 atmosphere. FACS analysis was performed after 15 h of coculture. Cytotoxicity was assessed by Fixable Viability Dyes (FVD) eFluor™ 780 (eBioscience). The percentage of FVD 780+ cells, gated on CFSE+ cells, was measured as the indicator of NK cell-mediated cytoxicity.
In vitro stimulation assays
PBMCs (1 × 106 cells/ml) and sorted NK cells were treated with hydrogen peroxide(H2O2) at concentrations of 20 µM, 40 µM and 80 µM, respectively, for 24 h culture in RPMI 1640 medium (10% FBS) at 37℃ in a 5% CO2 atmosphere. H2O2 was diluted in PBS and used within minutes of preparation.
T-distributed stochastic neighbour embedding (t-SNE) analysis
Samples (from four SLE patients with active disease activities and four HCs) were used for t-SNE analysis using R scripts based on the ‘flowCore’ package and ‘Rtsne’ package [16]. In R, all data were transformed using the logicleTransform function to roughly match scaling historically used in FlowJo.
Gene Set Enrichment Analysis (GSEA)
The gene expression datasets were attained from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/)(GSE63829 and GSE23695). And datasets were analyzed using R scripts based on the ‘clusterProfiler’ package [17]. Normalized enrichment score (NES) was calculated for every gene set (using p value < 0.05 and FDR < 0.25 as the threshold). The gene sets used in the GSEA belong to hallmark gene sets from Molecular Signature v7.2 Database (MSigDB).
Statistical analysis
Continuous variables were expressed as mean ± SD or median(interquartile range, IQR). Categorical variables were expressed as number(percent). The chi-square test or Fisher test was used to compare qualitative values according to distribution. The Student’s unpaired or paired t test was performed to compare two groups for parametric data, and the Mann–Whitney U test or Wilcoxon rank sum test was performed for nonparametric data. The Pearson test or Spearman was used to determine the correlation between variables. The statistical analyses were performed using R v.3.6.1 software. And the figures were drawn using GraphPad Software. P values less than 0.05 were considered significant.
Results
Peripheral NK cell deficiency was associated with disease activity in treatment-naive SLE patients
The present study included a total of 156 treatment-naive SLE patients (median age 33, IQR:26–47 years, 90.4% female), 22 treatment-naive RA patients (median age 37, IQR: 32–47 years, 90.9% female) and 30 HCs (median age 32, IQR:27–38 years, 90.0% female). The demographic and clinical manifestations of these participants were shown in Supplementary Table 2. The percentages and counts of NK cells were remarkably down-regulated in untreated SLE patients compared with that of untreated RA patients or HCs [NK cell percentage: 6.68 ± 5.17%,11.75 ± 8.46% and 12.72 ± 5.04% respectively; NK cell count: 50 (26–77) /µl, 104 (70–215) /µl and 266 (167–336) /µl respectively, Fig. 1A, B, all P < 0.001], suggesting that NK cell deficiency was associated with the disease itself regardless of treatment. While there was no significant difference in CD56 MFI of NK cells between SLE patients and HCs (Supplementary Fig. 2A).
Peripheral NK cell deficiency was associated with disease activity in treatment-naive SLE patients. A The percentage of NK cells in untreated SLE patients (n = 156), untreated RA patients (n = 22) and HCs (n = 30). B The absolute number of peripheral NK cells in untreated SLE patients (n = 156), untreated RA patients (n = 22) and HCs (n = 30). C Correlation of the NK count with the level of C3 in SLE patients. D Correlation of the NK count with the level of PLT in SLE patients. E Correlation of the NK count with SLEDAI score in SLE patients. F Comparison of the absolute number of peripheral NK cells in SLE patients with mild activity (SLEDAI < 10) (n = 97) and moderate-high activity (SLEDAI ≥ 10) (n = 59). G Comparison of the absolute number of peripheral NK cells in SLE patients with (n = 74) and without LN (n = 82). H Comparison of the absolute number of peripheral NK cells in SLE patients with (n = 112) and without hemocytopenia (n = 44). (* P < 0.05, **P < 0.01, ***P < 0.001). C3: serum concentrations of complement C3; PLT: blood platelet; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; LN: lupus nephritis
Correlation analysis showed that in treatment-naive SLE patients, the NK count showed weak positive correlations with the levels of complement C3 (r = 0.193, P = 0.017) (Fig. 1C) and PLT (r = 0.258, P = 0.001) (Fig. 1D) and a negative correlation with SLEDAI score (r = − 0.211, P = 0.008) (Fig. 1E). No significant correlations were found between peripheral NK count and the levels of anti-dsDNA antibody and complement C4 (Supplementary Fig. 2B, C). In addition, no significant correlation was found between peripheral NK count with the level of DAS28 in RA patients (r = 0.03, P = 0.89) (Supplementary Fig. 2D). SLE patients were grouped based on their SLEDAI score: mild activity (SLEDAI < 10) and moderate-high activity (SLEDAI ≥ 10). SLE patients in moderate-high activity group showed lower NK count [45 (21–72) /µl, n = 97 versus 59 (33–100) /µl, n = 59, P < 0.01, Fig. 1F]. Compared to SLE patients without LN [59 (33–93) /µl, n = 82], NK count was lower in SLE patients with LN [35 (21–72) /µl, n = 74] (P < 0.05) (Fig. 1G). NK count was lower in SLE patients with hemocytopenia [48 (26–72) /µl, n = 112] compared to SLE patients without hemocytopenia [61 (26–100) /µl, n = 44] (P < 0.05) (Fig. 1H). There were no statistic differences between two groups when grouped by rash, arthritis and central nervous system (CNS) involvement (Supplementary Fig. 2E-G).
Peripheral CD56dimCD57+ NK cells exhibited a selective reduction in SLE patients
We described a t-SNE map of CD45+ lymphocyte cells on four SLE patients with active disease activities and four HCs. The t-SNE map (Fig. 2A) and heatmap (Fig. 2B) visually showed an abnormal distribution of subpopulations [18] between SLE patients and HCs. Compared with HCs, SLE patients exhibited fewer NK cell subsets (Fig. 2A-B), especially for CD56dimCD57+ NK subgroup. To further confirm the above results and assess the relationship between NK subsets and disease activities, three NK cell subpopulations (CD56bright NK cells, CD56dimCD57− NK cells, and CD56dimCD57+ NK cells; gating strategy shown in Supplementary Fig. 1) were studied in 37 untreated SLE patients and 30 HCs (Fig. 2C). Our study indicated that SLE patients exhibited a selective reduction in CD56dimCD57+ NK cells (33.9 ± 21.5% versus 50.1 ± 15.8%, P < 0.01), but not CD56bright NK cells (6.09 ± 4.88% versus 3.57 ± 2.44%) or CD56dimCD57− NK cells (59.8 ± 20.2% versus 46.1 ± 15.5%) compared with HCs (Fig. 2C, middle panel). Instead, CD56dimCD57− NK cell proportion was significantly increased in SLE patients (P < 0.01). The proportions of three NK cell subsets in CD45+ lymphocytes from SLE patients were decreased (CD56dimCD57+ NK cell percentage: 9.01 ± 6.56% and 3.32 ± 3.73% for HCs and SLE respectively; CD56bright NK cell percentage: 0.49 ± 0.21% and 0.39 ± 0.39% for HCs and SLE respectively; CD56dimCD57− NK cell percentage: 7.60 ± 4.04% and 4.15 ± 3.38% for HCs and SLE respectively), and the two groups of CD56dim NK cells reached the statistically significant differences (P < 0.01) (Fig. 2C, right panel).
Peripheral CD56dimCD57+ NK cells exhibited a selective reduction in SLE patients. A t-SNE map of CD45+ lymphocyte cells from HCs and active SLE patients. There were 8 subsets (CD3−CD19+CD27+CD20−CD38+ plasmablast, CD3−CD19+CD27+CD20+CD38− memory B, CD3−CD19+CD27− naive B, CD3+CD56+ NKT, CD3+T cell, CD3−CD56dimCD57− NK cell, CD3−CD56bright NK cell, and CD3−CD56dimCD57+ NK cell). t-SNE was performed simultaneously on four SLE patients with active disease activities and four HCs. B Heatmap representing scaled expression values of the 8 subsets between HC (n = 4) and SLE patients (n = 4). C Representative flow cytometric plot for NK cell subsets (including CD3−CD56dimCD57+ NK cells, CD3−CD56bright NK cells, and CD3−CD56dimCD57− NK cells) from a HC and a SLE patient (Left panel). The percentage of three NK cell subsets in total NK cells from patients (n = 37) and HCs (n = 30) (Middle panel). The proportion of NK cell subsets in CD45+ lymphocytes in SLE patients (n = 37) and HCs (n = 30) (Right panel). D The correlation of CD56dimCD57+ NK cell proportion with SLEDAI score. E The correlation of CD56dimCD57+ NK cell proportion with the level of C3. F The correlation of CD56dimCD57+ NK cell proportion with the level of C4. G The correlations of CD56dimCD57+ NK cell proportion with the levels of IgG, IgM and IgA. H The correlation of CD56dimCD57+ NK cell proportion with the level of PLT. I The correlation of CD56dimCD57+ NK cell proportion with the level of anti-dsDNA antibody. (* P < 0.05, **P < 0.01, ***P < 0.001). tSNE: t-distributed stochastic neighbour embedding; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index;C3: serum concentrations of complement C3; C4: serum concentrations of complement C4; IgG:immunoglobulin G; IgM:immunoglobulin M; IgA:immunoglobulin A;PLT: blood platelet; anti-dsDNA antibody: anti-double-stranded-DNA-specific antibody
Furthermore, correlation analysis showed that CD56dimCD57+ NK cell proportion was negatively correlated with SLEDAI score (r = − 0.425, P = 0.009) (Fig. 2D), positively correlated with levels of complement C3 (r = 0.403, P = 0.016) and C4 (r = 0.518, P = 0.001) (Fig. 2E, F). In addition, the levels of PLT and anti-dsDNA antibody were weakly associated with CD56dimCD57+ NK cell proportion (Fig. 2G-I). No significant correlation was found between CD56bright NK cell proportion and SLEDAI score (r = 0.107, P = 0.52) (Supplementary Fig. 3).
Together, these findings demonstrated that SLE patients had not only a global reduction in their peripheral NK cells but also an alteration in subpopulations of NK cells-a loss of mature CD56dimCD57+ NK cells which were negatively correlated with disease activities.
Peripheral CD56dimCD57+ NK cells underwent increased endogenous apoptosis in SLE patients
To delineate the underlying mechanisms for the apparent reduction of peripheral NK cells in SLE patients, Annexin-V/7-AAD double staining flow cytometry was applied to detect the proportion of apoptotic NK cells. Total apoptotic rate of peripheral CD56dimCD57+ NK cells (57.7 ± 16.0%, n = 19 versus 29.8 ± 6.2%, n = 10, P < 0.01) and CD56dimCD57− NK cells (52.0 ± 15.2%, n = 19 versus 27.9 ± 5.8%, n = 10, P < 0.01) in SLE patients were both increased compared with that in HCs (Fig. 3A). Total apoptotic rate of peripheral CD56dimCD57+ NK cells in SLE patients was increased compared with CD56dimCD57− NK cells in SLE patients (57.7 ± 16.0%, n = 19 versus 52.0 ± 15.2%, n = 19, P < 0.01) (Fig. 3A).
Peripheral CD56dimCD57+ NK cells underwent increased endogenous apoptosis in SLE patients. A Representative flow cytometric plot for apoptosis of NK cells (CD56dimCD57+ NK cell subset and CD56dimCD57− NK cell subset) in one HC and one SLE patient (Left panel). Comparison of total apoptotic rate of NK cell subsets between HCs (n = 10) and SLE patients (n = 19) (Right panel). B Comparison of Cleaved-caspase 3 level of CD56dimCD57+ NK cells in SLE patients (n = 6) and HCs (n = 4). C Comparison of Cleaved-caspase 9 level of CD56dimCD57+ NK cells in SLE patients (n = 8) and HCs (n = 8). (* P < 0.05, **P < 0.01, ***P < 0.001)
Cleaved-caspase 3 is an important regulator of apoptosis and Cleaved-caspase 9 mediates endogenous apoptosis. Compared with CD56dimCD57+ NK cells in HCs, CD56dimCD57+ NK cells in SLE patients showed increased expressions of Cleaved-caspase 3 (MFI: 129 ± 26.8, n = 4 versus 238 ± 75.3, n = 6, P < 0.05, Fig. 3B) and Cleaved-caspase 9 (MFI: 181 ± 20.3, n = 8 versus 262 ± 69.3, n = 8, P < 0.001, Fig. 3C).
Collectively, the selective reduction in CD56dimCD57+ NK cells was due to, at least in part, increased endogenous apoptosis level in lupus microenvironment.
Peripheral CD56dimCD57+ NK cells showed higher oxidative stress in SLE patients
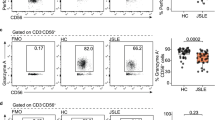
To identify cellular programs that may underlie low levels of NK cell activity in lupus, we analyzed a published dataset [19] (GSE63829) by GSEA for Hallmark gene sets, which are coherently expressed signatures derived by aggregating many MSigDB gene sets to represent well-defined biological states or processes. Since there was no appropriate data of human NK cells, we adopted GSE63829 from lupus prone mouse. Among 50 hallmark gene sets from MSigDB, ROS pathway was significantly enriched (NES = 1.528, p = 0.029, Fig. 4A) in NK cells from lupus, compared with controls. All enriched pathways were summarize in Supplementary Table 3. As mitochondria are key cellular sources of ROS, total and mitochondrial ROS levels were assessed in NK cells. Both ROS (67,357 ± 23,137, n = 14 versus 40,983 ± 9046, n = 8, P < 0.01, Fig. 4B) and mtROS ( 601 ± 157, n = 14 versus 353 ± 42.0, n = 11, P < 0.001, Fig. 4C) levels of CD56dimCD57+ NK cells were significantly increased in SLE patients.
Peripheral CD56dimCD57+ NK cells showed higher oxidative stress in SLE patients. A GSEA plots showing an increase in ROS signature (enrichment plot: HALLMARK_REACTIVE_OXYGEN_SPECIES_PATHWAY) in lupus NK cells compared with normal NK cells (GSE63829). B Production of ROS in CD56dimCD57+NK cells from SLE patients (n = 14) and HCs (n = 8). C Production of mtROS in CD56dimCD57+NK cells from SLE patients (n = 14) and HCs (n = 11). D GSEA plots showing an increase in ROS signature (enrichment plot: HALLMARK_REACTIVE_OXYGEN_SPECIES_PATHWAY) in CD57 + NK cells compared with CD57-NK cells (GSE23695). E Comparison of ROS level of CD56dimCD57+ NK cell subset and CD56dimCD57− NK cell subset in SLE patients (n = 14). F Comparison of mtROS level of CD56dimCD57+ NK cell subset and CD56dimCD57− NK cell subset in SLE patients (n = 14). G The correlation of ROS level and perforin expression in CD56dimCD57+ NK cell subset in SLE patients(n = 14). H The correlation of ROS level and total apoptosis in CD56dimCD57+ NK cell subset in SLE patients (n = 14). I Comparison of apoptosis level of CD56dimCD57+ NK cell subset and CD56dimCD57− NK cell subset from HC upon exposure to H2O2 for 24 h culture at concentrations of 20 µM, 40 µM and 80 µM, respectively (n = 5). GSEA: Gene set enrichment analysis; ROS: reactive oxygen species; mtROS: mitochondrial reactive oxygen species. (* P < 0.05, **P < 0.01, ***P < 0.001)
Interestingly, ROS pathway was highly activated (NES = 1.573, p = 0.018, Fig. 4D and Supplementary Table 4) in CD56dimCD57+ NK cells compared with the CD56dimCD57− NK cell subset when we analyzed another published dataset [6] (GSE23695). The two subsets in GSE23695 are CD3−CD56dimCD16+CD57+ and CD3−CD56dimCD16+CD57− NK cells. In peripheral blood of healthy people, CD56dim NK cells express high levels of CD16. So we use that data for our study comparing CD56dimCD57+ and CD56dimCD57− NK cells. Interestingly, ROS pathway was enriched in both GSE63829 and GSE23695. In addition, ROS level (67,357 ± 23,137, n = 14 versus 63,539 ± 16,767, n = 14, P = 0.1, Fig. 4E) was marginally higher and mtROS level (601 ± 157, n = 14 versus 464 ± 119, n = 14, P < 0.001, Fig. 4F) was much higher in CD56dimCD57+ NK cells in SLE patients compared with CD56dimCD57− NK cells. Of note, we observed a strong negative association between ROS level and perforin expression (r = − 0.649, P = 0.012, Fig. 4G), and a weak positive association between ROS level and total apoptosis (r = 0.443, P = 0.11, Fig. 4H) in CD56dimCD57+ NK cells from SLE patients. These indicators reflected a higher oxidative stress of CD56dimCD57+ NK cells in SLE patients, which may influence NK cell activities.
Therefore, we next performed a detailed analysis aiming to reveal the effect of ROS on the two NK subsets. Total PBMCs were treated with different concentrations of H2O2 to mimic physiological levels of ROS exposure [20]. With increased concentrations of H2O2, apoptotic cells were increased in both two NK cell subsets (Fig. 4I). At concentrations of 20 µM and above, apoptotic rates of CD56dimCD57+ NK cells were higher than CD56dimCD57− NK cells when they were exposed to the same H2O2 level, suggesting that CD56dimCD57+ NK cells were more prone to undergo apoptosis when exposed to ROS (Fig. 4I). To confirm this finding more clearly, we sorted NK cells from PBMCs. Consistent with the data from PBMCs, our results verified that apoptotic rates of CD57+ NK cells were higher than CD57− NK cells at the same H2O2 level (Supplementary Fig. 4A). What is more, ROS levels of CD57+ NK cells were higher than CD57− NK cells when they were exposed to the same H2O2 level (Supplementary Fig. 4B).
These studies suggested that CD56dimCD57+ NK cells were preferentially targeted for oxidative stress in SLE patients, leading to decreased activity of this NK subset.
Peripheral CD56dimCD57+ NK cells exhibited attenuated cytotoxicity and altered phenotypes in SLE patients
The distinctive characteristics of CD56dim NK cells and CD56bright NK cells have been well-characterized, and the former are predominant in the peripheral blood, with stronger cytotoxic function. To assess the functions of two CD56dim NK cell subsets (CD56dimCD57+ NK cells and CD56dimCD57− NK cells), we examined the markers associated with degranulation, activation and cytokine production.
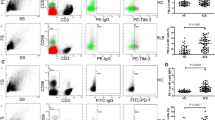
In our research, flow cytometry analysis confirmed the discrepancy of CD56dimCD57+ NK cells and CD56dimCD57− NK cells. The expression of CD107a on the cell membrane correlates with NK cell killing activity. Compared with CD56dimCD57− NK cells, CD56dimCD57+ NK cells exhibited higher expression of CD107a, perforin, granzyme B, CD16 and active receptor NKG2D in HCs (CD107a: 60.4 ± 18.6% versus 49.9 ± 13.7%, n = 4; perforin: 97.5 ± 3.01% versus 92.2 ± 6.50%, n = 10; granzyme B: 97.3 ± 4.47% versus 90.0 ± 4.69%, n = 6; CD16: 97.7 ± 3.01% versus 93.7 ± 4.30%, n = 12; NKG2D: 97.5 ± 2.65% versus 94.8 ± 3.49%, n = 10, Fig. 5A, B, all P < 0.05). With respect to inhibitory receptors specific for MHC class I, NKG2A expression was decreased on CD56dimCD57+ NK cells relative to that on CD56dimCD57− NK cells in HCs (8.54 ± 7.20% versus 42.9 ± 10.4%, n = 7, P < 0.001, Fig. 5A, B). The expression of IFN-γ (25.7 ± 19.4% versus 36.4 ± 16.1%, n = 5) and IL-10 (5.27 ± 3.17% versus 5.14 ± 2.51%, n = 5) did not differ between CD56dimCD57+ NK cells and CD56dimCD57− NK cells in HCs (Supplementary Fig. 5). The discrepancies between CD56dimCD57+ NK cells and CD56dimCD57− NK cells in SLE patients were similar (CD107a: 31.3 ± 5.46% versus 22.5 ± 4.30%, n = 4; perforin: 82.2 ± 15.1% versus 69.1 ± 20.1%, n = 24; granzyme B: 72.5 ± 23.6% versus 65.3 ± 22.6%, n = 9; CD16: 79.3 ± 17.5% versus 75.2 ± 16.8%, n = 17; NKG2D: 92.5 ± 5.90% versus 89.8 ± 8.87%, n = 10; NKG2A: 26.7 ± 17.3% versus 43.2 ± 23.8%, n = 11; IFN-γ: 16.3 ± 10.6% versus 24.1 ± 8.19%, n = 7; IL-10: 8.51 ± 3.64% versus 10.5 ± 3.82%, n = 6, Fig. 5A, B), but to a lesser extent than that in HCs. Our research demonstrated that CD56dimCD57+ NK cells exhibited enhanced cytotoxic function.
Peripheral CD56dimCD57+ NK cells exhibited attenuated cytotoxicity and altered phenotypes in SLE patients. A Representative flow cytometric plot for expression of CD107a, perforin, Granzyme B, CD16, NKG2D and NKG2A on NK cell subsets (CD56dimCD57+ NK cell subset and CD56dimCD57− NK cell subset) in HC and SLE patient. B Comparison of CD107a, perforin, Granzyme B, CD16, NKG2D and NKG2A expression on NK cell subsets. (* P < 0.05, **P < 0.01, ***P < 0.001)
We also observed a significant impairment in CD107a expression by CD56dimCD57+ NK cells from SLE patients relative to that from HCs (31.3 ± 5.46%, n = 4 versus 60.4 ± 18.6%, n = 4, P < 0.05, Fig. 5A, B). Compared with CD56dimCD57+ NK cells in HCs, CD56dimCD57+ NK cells in SLE patients showed decreased expressions of perforin (97.5 ± 3.01%, n = 10 versus 82.2 ± 15.1%, n = 24, P < 0.01), granzyme B (97.3 ± 4.47%, n = 6 versus 72.5 ± 23.6%, n = 9, P < 0.05), CD16 (97.7 ± 3.01%, n = 12 versus 79.3 ± 17.5%, n = 17, P < 0.01) and NKG2D (97.5 ± 2.65%, n = 10 versus 92.5 ± 5.90%, n = 10, P < 0.05), along with increased NKG2A expression (8.54 ± 7.20%, n = 7 versus 26.7 ± 17.3%, n = 11, P < 0.05) (Fig. 5A, B). There were no significant differences between the two groups in terms of IFN-γ (25.7 ± 19.4%, n = 5 versus 16.3 ± 10.6%, n = 7) and IL-10 (5.27 ± 3.17%, n = 5 versus 8.51 ± 3.64%, n = 6) expression (Supplementary Fig. 5). In CD56dimCD57− NK cells, there were differences in percentage of CD107a (49.9 ± 13.7%, n = 4 versus 22.5 ± 4.30%, n = 4, P < 0.05), perforin (92.2 ± 6.50%, n = 10 versus 69.1 ± 20.1%, n = 24, P < 0.05), granzyme B (90.0 ± 4.69%, n = 6 versus 65.3 ± 22.6%, n = 9, P < 0.05) and CD16 (93.7 ± 4.30%, n = 12 versus 75.2 ± 16.8%, n = 17, P < 0.05) between HCs and SLE patients, but no differences in expressions of NKG2D and NKG2A (Fig. 5A, B).
Together, CD56dimCD57+ NK cells in SLE patients exhibited not only lower proportion but also impaired in vitro function, especially the cytotoxic function.
Peripheral CD56dimCD57+ NK cells from SLE patients exhibited impaired cytotoxicity in response to activated CD4+ T cells
Activated CD4+ T cells are involved in multiple aspects of SLE pathogenesis [1]. NK cells can negatively regulate CD4+ T cell responses to protect from immunopathology, and most reports have found that this process is dependent on direct cytotoxicity [3, 4]. We observed specific lysis of autologous activated CD4+ T cells by NK cells, but no killing of resting CD4+ T cells (Fig. 6A, Supplementary Fig. 6), which is consistent with the findings of other investigators [4, 21]. In addition, sorted CD56dimCD57+ NK cells manifested significantly enlarged cytotoxicity relative to CD56dimCD57− NK cells in response to autologous activated CD4+ T cells from HCs (27.1 ± 1.19%, n = 3 versus 17.7 ± 2.24%, n = 3, P < 0.05, Fig. 6A). As there are differences in CD4+ T cells between HCs and SLE patients, we chose the same allogeneic CD4+ T cell as the target cells. CD56dimCD57+ NK cells from SLE patients showed significantly attenuated cytotoxicity compared with that from HCs when co-cultured with the same allogeneic activated CD4+ T cells (14.5 ± 0.75%, n = 3 versus 20.7 ± 2.38%, n = 3, P < 0.05, Fig. 6B).
Peripheral CD56dimCD57+ NK cells from SLE patients exhibited impaired cytotoxicity in response to activated CD4+ T cells. A Cytoxicity assay of CD4+ T cells in response to NK cell subsets (n = 3). B Cytoxicity assay of CD4+ T cells in response to CD56dimCD57+ NK cells from SLE patients and HCs (n = 3). C Comparison of ICOS expression on CD4+ T cells from SLE patients (n = 10) and HCs (n = 9). D Comparison of PD-1 expression on CD4+ T cells from SLE patients (n = 10) and HCs (n = 10). E Comparison of ICOS expression on CD4+ T cells after co-culture with NK subsets (n = 4). F The correlation of ICOS expression on CD4+ T cells and CD56dimCD57+ NK cell percentage in SLE patients (n = 10). G The correlation of PD-1 expression on CD4+ T cells and CD56dimCD57+ NK cell percentage in SLE patients (n = 10). ICOS: Inducible T cell costimulator; PD-1: programmed death 1. (* P < 0.05)
On CD4+ T cells, inducible T cell costimulator (ICOS) and programmed death 1 (PD-1) are activation markers which play critical roles in B cell activation and differentiation [1, 22]. Our study also confirmed that both the percentages of ICOS (5.24 ± 5.04%, n = 10 versus 1.36 ± 0.64%, n = 9, P < 0.05) and PD-1 (18.3 ± 8.13%, n = 10 versus 8.71 ± 4.65%, n = 10, P < 0.05) on CD4+ T cells were higher in SLE patients than in HCs (Fig. 6C, D). In addition, there was no statistical difference in PD-1 expression in NK cell subsets between the two groups (Supplementary Fig. 7). The expression of ICOS on CD4+ T cells decreased after co-culture with CD57+ NK cells from HCs (23.8 ± 1.49%, n = 4 versus 15.9 ± 4.64%, n = 4), and such an inhibitory effect was not significant for NK cell subsets from SLE patients (Fig. 6E). We further tested whether NK cell subset distribution was correlated with activation makers of CD4+ T cells in SLE. Interestingly, lower percentage of CD56dimCD57+ NK cells strongly correlated with higher expression of active maker ICOS (r = − 0.649, P = 0.042, Fig. 6F). We also observed a trend of, though not statistically significant, negative correlation between CD56dimCD57+ NK cell percentage and PD-1 expression (r = − 0.428, P = 0.217, Fig. 6G). The above results suggested a regulatory effect of NK cell subset distribution on activation of CD4+ T cells.
These data indicated that NK cells, especially CD56dimCD57+ NK cells, targeted activated CD4+ T cells. The selective reduction in CD56dimCD57+ NK cells and impaired function in SLE patients might impede their ability to eradicate activated pathogenic CD4+ T cells which are key cells that cause lupus disease.
Discussion
In this study, we demonstrated that the number and percentage of NK cells decreased substantially in the peripheral blood of treatment-naive SLE patients compared with those of HCs and treatment-naive RA patients. Beyond reduced numbers, NK cells in SLE patients had a selective loss of mature CD56dimCD57+ NK cell subset, which was conversely correlated with disease activity. CD56dimCD57+ NK cells exhibited increased cytotoxic effect on activated CD4+ T cells. Additionally, attenuated cytotoxicity function, up-regulated endogenous apoptosis and increased oxidative stress were observed in peripheral CD56dimCD57+ NK cells from SLE patients.
Previous studies have shown significantly down-regulated counts and abnormal phenotypes of peripheral NK cells in SLE patients [10,11,12]. To exclude the influence of glucocorticoids or immunosuppressive drugs, we enrolled treatment-naive patients. We identified an extremely reduced NK count (median 50 /µL) in SLE patients, which was less than a quarter of HCs (median 266 /µL). Our study confirmed that compared with both HCs and untreated RA patients, numbers and percentages of NK cells were remarkably down-regulated in SLE patients, suggesting that NK cell deficiency was mainly due to immune disorder underlying lupus itself regardless of treatment. Our study also indicated that SLE patients exhibited a selective reduction in CD56dimCD57+ NK cells which was negatively correlated with SLEDAI, positively correlated with both levels of complement C3 and C4, and weakly associated with anti-dsDNA antibody level. Our research suggests that CD56dimCD57+ NK cell deficiency might be an important intrinsic mechanism for the pathogenesis of SLE.
Beyond being important innate immune cells, NK cells may regulate adaptive immune responses in many aspects [3, 4, 23]. In vivo studies showed that, through a perforin-dependent pathway, NK cells can not only directly eliminate activated CD4+ T cells but also exhibit potent immunosuppressive capacity that repress antigen-specific B cell and immunoglobulin somatic mutations [3, 23]. Activated CD4+ T cells may express cell-surface NKG2D ligands and become susceptible to autologous NK-cell lysis [24, 25]. NK cell depletion can lead to the enhanced development of experimental autoimmune encephalomyelitis (EAE) due to increased numbers of auto-reactive CD4+ T cells [26]. Hemophagocytic lymphohistiocytosis (HLH), a disease linked to genetic defects in perforin mediated cytotoxicity, has been associated with impaired NK cells and increased proliferation of T cells [27]. The immunologic hallmark of SLE patients is the breakdown of tolerance, manifested as CD4+ T cell activation, B cell amplification and autoantibody production [1, 22]. ICOS is a crucial activation marker of CD4+ T cells and plays critical roles in T-B interaction for B cell activation and differentiation [22, 28]. Therefore, ICOS positive CD4+ T cells might be pathogenic in SLE. Our study confirmed that the expression of ICOS on CD4+ T cells was particularly low in healthy controls while significantly increased in SLE patients. In the co-culture experiments, the expression of ICOS on CD4+ T cells decreased after co-culture with NK cells, and such an inhibitory effect was more significant for CD57+ NK cells from healthy controls than those from SLE patients. Furthermore, we observed lysis of activated CD4+ T cells, but not resting CD4+ T cells, when cocultured with NK cells. In addition, CD56dimCD57+ NK cells exhibited stronger cytotoxicity in response to activated CD4+ T cells relative to CD56dimCD57− NK cells. Our research demonstrated that two CD56dim NK cell subsets exhibited lower expression of CD107a, perforin and granzyme B in SLE patients. Whlie the single-cell RNA-seq from children with SLE (cSLE) has demonstrated that NK cells, with up-regulated cytotoxicity-encoding genes, were expanded in cSLE patients [29]. Discrepancy in the results may be explained by differences in ages, levels of disease activities and treatment regimen, as SLE is a heterogeneous autoimmune disease. Our research suggests that altered NK cell subsets and impaired function might impede their ability to eradicate activated pathogenic CD4+ T cells.
On the other hand, SLE patients are susceptible to infections which have also been shown to play a role in the development and exacerbation of SLE [2, 30, 31]. NK cell deficiency and its blunted cytotoxicity function may be an important risk factor for infections in SLE patients, especially the opportunistic infections.
Novel drugs that target NK cells are currently being explored in cancers and autoimmune diseases [32]. Atopic dermatitis (AD) patients show deficiency in circulating NK cells and administration of NK cell-boosting IL-15 superagonist leads to clinical amelioration in AD mice [33]. Daclizumab (targeting IL-2Ralpha chain) therapy may relieve the symptoms of multiple sclerosis patients, which is associated with expansion of CD56bright NK cells and a gradual decline in circulating CD4+ T cells [34]. Restoring NK cell activity may contribute to the elimination of plasma cells from SLE patients, and elotuzumab (targeting SLAMF7) and daratumumab (targeting CD38) may contribute to the elimination of antibody producing cells in vitro [35]. Recent studies have verified that low-dose IL-2 treatment was effective in SLE patients and could increase NKG2D+ NK cells [36]. Taken together, it is justifiable to suppose that restoration of NK cell activity (e.g. through low-dose IL-2) could potentially help regain immune balance and minimize both systemic autoimmunity and the risk of infection in SLE patients.
Oxidative stress characterizes the lupus immune system and promotes its pathogenesis at multiple levels [37, 38]. Treatment with MitoTempo, an inhibitor of mtROS production, delays disease progression in lupus mice [37]. And treatment with the ROS scavenger N-acetyl-cysteine (NAC) relieves disease activity of SLE patients in a randomized, double-blind, placebo-controlled trial study [38]. ROS facilitates metastasis of melanoma cells by down-regulating functions of NK cells, and ROS inhibition may restore NK cell-mediated clearance of these malignant cells [39]. Our study confirmed that the elevated levels of ROS and mtROS in CD56dimCD57+NK cells from lupus patients were associated with increased apoptosis and decreased function of NK cells. A previous research has demonstrated that CD56dim NK cells, but not CD56bright NK cells, are likely to succumb to apoptosis when undergoing oxidative stress [40]. Consistent with this view, CD56bright NK cells did not change significantly in our study. We further indicated that, in the CD56dim NK cell population, CD57+ cells suffered from higher oxidative stress and more cell apoptosis, which may explain the selective reduction of CD56dimCD57+ NK cells seen in lupus patients.
We recognized that there were several limitations in our study. The total number of patients was somewhat small and larger prospective cohorts are required to perform further analyses. We described the contribution of ROS to the function of NK cell subsets using in vitro experiments, further investigations conducted in vivo will be more valuable. And the molecular pathways involved in the cross-talk between CD4+ T cells and NK cells need further research.
Conclusion
In summary, we demonstrated decreased number and impaired in vitro function of CD56dimCD57+ NK cells in SLE patients. This NK cell subset was negatively correlated with the severity of SLE patients. The selective reduction may due to preferential apoptosis of CD56dimCD57+ NK cells under increased oxidative stress in SLE patients. More importantly, CD56dimCD57+ NK cells exhibited stronger cytotoxicity in response to activated CD4+ T cells. Altered NK cell subsets and impaired function might impede their ability to eradicate activated pathogenic CD4+ T cells which are key cells that cause lupus disease.
Availability of data and materials
The data used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- SLE:
-
Systemic lupus erythematosus
- RA:
-
Rheumatoid arthritis
- ACR:
-
American College of Rheumatology
- HC:
-
Healthy control
- PLT:
-
Blood platelet
- C3:
-
Serum concentrations of complement C3
- C4:
-
Serum concentrations of complement C4
- anti-dsDNA antibodies:
-
Anti-double-stranded-DNA antibodies
- ANA:
-
Antinuclear antibodies
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IgA:
-
Immunoglobulin A
- SLEDAI:
-
Systemic Lupus Erythematosus Disease Activity Index
- DAS28:
-
28-Joint disease activity score
- LN:
-
Lupus nephritis
- PBMCs:
-
Peripheral blood mononuclear cells
- PMA:
-
Phorbol 12-myristate 13-acetate
- 7-AAD:
-
7-Amino-actinomycin D
- ROS:
-
Reactive oxygen species
- mtROS:
-
Mitochondrial reactive oxygen species
- DCFH-DA:
-
2',7'-Dichlorodihydrofluorescein diacetate
- MFI:
-
Mean fluorescence intensity
- MACS:
-
Magnet associated cell sorting
- FACS:
-
Fluorescence activated cell sorting
- FBS:
-
Foetal Bovine Serum
- CFSE:
-
Carboxyfluorescein diacetate succinimidyl ester
- FVD:
-
Fixable Viability Dyes
- H2O2 :
-
Hydrogen peroxide
- t-SNE:
-
T-distributed stochastic neighbour embedding
- GSEA:
-
Gene Set Enrichment Analysis
- GEO:
-
Gene Expression Omnibus
- NES:
-
Normalized enrichment score
- MSigDB:
-
Molecular Signature v7.2 Database
- IQR:
-
Interquartile range
- ICOS:
-
Inducible T cell costimulator
- PD-1:
-
Programmed death 1
References
Chen PM, Tsokos GC. T Cell Abnormalities in the Pathogenesis of Systemic Lupus Erythematosus: an Update. Curr Rheumatol Rep. 2021;23(2):12. https://doi.org/10.1007/s11926-020-00978-5 PMID: 33512577.
Pego-Reigosa JM, Nicholson L, Pooley N, Langham S, Embleton N, Marjenberg Z, Barut V, Desta B, Wang X, Langham J, Hammond ER. The risk of infections in adult patients with systemic lupus erythematosus: systematic review and meta-analysis. Rheumatology (Oxford). 2021;60(1):60–72. https://doi.org/10.1093/rheumatology/keaa478.PMID:33099651;PMCID:PMC7785308.
Rydyznski CE, Cranert SA, Zhou JQ, Xu H, Kleinstein SH, Singh H, Waggoner SN. Affinity Maturation Is Impaired by Natural Killer Cell Suppression of Germinal Centers. Cell Rep. 2018;24(13):3367-3373.e4. https://doi.org/10.1016/j.celrep.2018.08.075.PMID:30257198;PMCID:PMC6192537.
Crouse J, Xu HC, Lang PA, Oxenius A. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol. 2015;36(1):49–58. https://doi.org/10.1016/j.it.2014.11.001 PMID: 25432489.
Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, Kubota M, Matsumoto R, Thapa P, Szabo PA, Li Poon MM, Li J, Arakawa-Hoyt J, Shen Y, Fong L, Lanier LL, Farber DL. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell. 2020;180(4):749-763.e13. https://doi.org/10.1016/j.cell.2020.01.022 Epub 2020 Feb 13. PMID: 32059780; PMCID: PMC7194029.
Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–74. https://doi.org/10.1182/blood-2010-04-282301 Epub 2010 Aug 23. PMID: 20733159; PMCID: PMC2981540.
Collins PL, Cella M, Porter SI, Li S, Gurewitz GL, Hong HS, Johnson RP, Oltz EM, Colonna M. Gene Regulatory Programs Conferring Phenotypic Identities to Human NK Cells. Cell. 2019;176(1–2):348-360.e12. https://doi.org/10.1016/j.cell.2018.11.045 Epub 2018 Dec 27. PMID: 30595449; PMCID: PMC6329660.
Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177(1):155–64. https://doi.org/10.1084/jem.177.1.155.PMID:8418197;PMCID:PMC2190856.
Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, Cepika AM, Acs P, Turner J, Anguiano E, Vinod P, Kahn S, Obermoser G, Blankenship D, Wakeland E, Nassi L, Gotte A, Punaro M, Liu YJ, Banchereau J, Rossello-Urgell J, Wright T, Pascual V. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell. 2016;165(3):551–65. https://doi.org/10.1016/j.cell.2016.03.008 Epub 2016 Mar 31.
Spada R, Rojas JM, Barber DF. Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J Leukoc Biol. 2015;98(4):479–87.
Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P, et al. Phenotype and Function of Natural Killer Cells in Systemic Lupus Erythematosus. Arthritis Rheum. 2011;63(6):1698–706.
Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, et al. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol. 2011;186(6):3421–31.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. https://doi.org/10.1002/art.1780400928 PMID: 9324032.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91 PMID: 11838846.
Linderman GC, Steinerberger S. Clustering with t-SNE, provably. SIAM J Math Data Sci. 2019;1(2):313–32. https://doi.org/10.1137/18m1216134 Epub 2019 May 28. PMID: 33073204; PMCID: PMC7561036.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7. https://doi.org/10.1089/omi.2011.0118 Epub 2012 Mar 28. PMID: 22455463; PMCID: PMC3339379.
Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12(3):191–200. https://doi.org/10.1038/nri3158.Erratum.In:NatRevImmunol.2012Jun;12(6):471.PMID:22343568;PMCID:PMC3409649.
Voynova EN, Skinner J, Bolland S. Expansion of an atypical NK cell subset in mouse models of systemic lupus erythematosus. J Immunol. 2015;194(4):1503–13. https://doi.org/10.4049/jimmunol.1402673 Epub 2015 Jan 16. PMID: 25595787; PMCID: PMC4323852.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–83. https://doi.org/10.1038/s41580-020-0230-3 Epub 2020 Mar 30 PMID: 32231263.
Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170(7):3572–6. https://doi.org/10.4049/jimmunol.170.7.3572 PMID: 12646619.
Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205(12):2873–86. https://doi.org/10.1084/jem.20080840 Epub 2008 Nov 3. PMID: 18981236; PMCID: PMC2585848.
Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481(7381):394–8. https://doi.org/10.1038/nature10624.PMID:22101430;PMCID:PMC3539796.
Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther. 2013;15(4):216. https://doi.org/10.1186/ar4232.PMID:23856014;PMCID:PMC3979027.
Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 2007;110(2):606–15. https://doi.org/10.1182/blood-2006-10-052720.
Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186(10):1677–87. https://doi.org/10.1084/jem.186.10.1677.PMID:9362528;PMCID:PMC2199138.
Kogawa K, Lee SM, Villanueva J, Marmer D, Sumegi J, Filipovich AH. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002;99(1):61–6. https://doi.org/10.1182/blood.v99.1.61 PMID: 11756153.
Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, Qi H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517(7533):214–8. https://doi.org/10.1038/nature13803 Epub 2014 Oct 15 PMID: 25317561.
Nehar-Belaid D, Hong S, Marches R, Chen G, Bolisetty M, Baisch J, Walters L, Punaro M, Rossi RJ, Chung CH, Huynh RP, Singh P, Flynn WF, Tabanor-Gayle JA, Kuchipudi N, Mejias A, Collet MA, Lucido AL, Palucka K, Robson P, Lakshminarayanan S, Ramilo O, Wright T, Pascual V, Banchereau JF. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat Immunol. 2020;21(9):1094–106. https://doi.org/10.1038/s41590-020-0743-0.
Yuan Q, Xing X, Lu Z, Li X. Clinical characteristics and risk factors of infection in patients with systemic lupus erythematosus: A systematic review and meta-analysis of observational studies. Semin Arthritis Rheum. 2020;50(5):1022–39. https://doi.org/10.1016/j.semarthrit.2020.06.004 Epub 2020 Jun 17 PMID: 32911280.
Doaty S, Agrawal H, Bauer E, Furst DE. Infection and Lupus: Which Causes Which? Curr Rheumatol Rep. 2016;18(3):13. https://doi.org/10.1007/s11926-016-0561-4 PMID: 26951251.
Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17(9):1025–36. https://doi.org/10.1038/ni.3518 PMID: 27540992.
Mack MR, Brestoff JR, Berrien-Elliott MM, Trier AM, Yang TB, McCullen M, Collins PL, Niu H, Bodet ND, Wagner JA, Park E, Xu AZ, Wang F, Chibnall R, Council ML, Heffington C, Kreisel F, Margolis DJ, Sheinbein D, Lovato P, Vivier E, Cella M, Colonna M, Yokoyama WM, Oltz EM, Fehniger TA, Kim BS. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci Transl Med. 2020;12(532):eaay1005. https://doi.org/10.1126/scitranslmed.aay1005 PMID: 32102931; PMCID: PMC7433875.
Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103(15):5941–6. https://doi.org/10.1073/pnas.0601335103 Epub 2006 Apr 3. PMID: 16585503; PMCID: PMC1458677.
Humbel M, Bellanger F, Fluder N, Horisberger A, Suffiotti M, Fenwick C, Ribi C, Comte D. Restoration of NK Cell Cytotoxic Function With Elotuzumab and Daratumumab Promotes Elimination of Circulating Plasma Cells in Patients With SLE. Front Immunol. 2021;22(12): 645478. https://doi.org/10.3389/fimmu.2021.645478.PMID:33828555;PMCID:PMC8019934.
He J, Zhang R, Shao M, Zhao X, Miao M, Chen J, Liu J, Zhang X, Zhang X, Jin Y, Wang Y, Zhang S, Zhu L, Jacob A, Jia R, You X, Li X, Li C, Zhou Y, Yang Y, Ye H, Liu Y, Su Y, Shen N, Alexander J, Guo J, Ambrus J, Lin X, Yu D, Sun X, Li Z. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79(1):141–9. https://doi.org/10.1136/annrheumdis-2019-215396 Epub 2019 Sep 19. PMID: 31537547; PMCID: PMC6937406.
Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;2:146–53. https://doi.org/10.1038/nm.4027 Epub 2016 Jan 18. PMID: 26779811; PMCID: PMC4742415.
Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, Perl A. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–46. https://doi.org/10.1002/art.34502.PMID:22549432;PMCID:PMC3411859.
He H, Wang C, Liu G, Ma H, Jiang M, Li P, Lu Q, Li L, Qi H. Isobavachalcone inhibits acute myeloid leukemia: Potential role for ROS-dependent mitochondrial apoptosis and differentiation. Phytother Res. 2021;35(6):3337–50. https://doi.org/10.1002/ptr.7054.
Harlin H, Hanson M, Johansson CC, Sakurai D, Poschke I, Norell H, Malmberg KJ, Kiessling R. The CD16- CD56(bright) NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16+ CD56(dim) subset. J Immunol. 2007;179(7):4513–9. https://doi.org/10.4049/jimmunol.179.7.4513 PMID: 17878347.
Acknowledgements
We thank all the patients and healthy donors involved in the study.
Funding
This work was supported by the National Natural Science Foundation of China [grant number. 81601410, 81671606, 82071834], Dalian Science and Technology Innovation Fund (2021JJ13SN48), Dalian key laboratory of human homeostasis microbiology and disease immunology, Natural Science Foundation of Liaoning Provincial Department Education (LZ2020051), Natural Science Foundation of Liaoning Province (2020-BS-191).
Author information
Authors and Affiliations
Contributions
XL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. XL, JZ, YZ and ZL conceived of and designed the study. ZL, YT, ZB, JL, YZ, JQ, MJ and JZ acquired the data. ZL, YT, ZB, JL, JQ and MJ analyzed and interpreted the data. All authors were involved in drafting the article or making critical revisions for important intellectual content, and the authors read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki, and the design of the work was approved by the ethics committee of Affiliated Hospital of Nantong University and the ethics committee of the Dalian Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Antibody list. Supplementary Table 2. Baseline characteristics of patients and healthy controls in this study. Supplementary Table 3. Hallmark gene sets((Lupus vs Control)*. Supplementary Table 4. Hallmark gene sets((CD57+ vs CD57-)*. Supplementary Figure 1. The NK cell subset gating strategies. Supplementary Figure 2. Supplementary analysis of clinical data and peripheral NK count in SLE and RA patients. Supplementary Figure 3. The correlation of CD56dimCD57+ NK cell percentage with SLEDAI (n=37). Supplementary Figure 4. Apoptosis and ROS levels of sorted NK cells upon exposure to H2O2. Supplementary Figure 5. Cytokine expression of CD56dimCD57+ NK cells in SLE patients and HCs. Supplementary Figure 6. No cytotoxicity of resting CD4+ T cells by NK cells(n=3). Supplementary Figure 7. PD-1 expression of NK cell subsets in SLE patients (n=8) and HCs (n=8).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, Z., Tian, Y., Bai, Z. et al. Increased oxidative stress contributes to impaired peripheral CD56dimCD57+ NK cells from patients with systemic lupus erythematosus. Arthritis Res Ther 24, 48 (2022). https://doi.org/10.1186/s13075-022-02731-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02731-y