Abstract
Background
Infrapatellar fat pad (IFP) has recently emerged as a potential source of inflammation in knee arthropathies. It has been proposed to be one source of adipocytokines, fatty acids (FA), and FA-derived lipid mediators that could contribute to the pathophysiological processes in the knee joint. Alterations in synovial fluid (SF) lipid composition have been linked to both osteoarthritis (OA) and rheumatoid arthritis (RA). The aim of the present study was to compare the FA signatures in the IFP and SF of RA and OA patients.
Methods
Pairs of IFP and SF samples were collected from the same knees of RA (n = 10) and OA patients (n = 10) undergoing total joint replacement surgery. Control SF samples (n = 6) were harvested during diagnostic or therapeutic arthroscopic knee surgery unrelated to RA or OA. The FA composition in the total lipids of IFP and SF was determined by gas chromatography with flame ionization and mass spectrometric detection.
Results
Arthropathies resulted in a significant reduction in the SF proportions of n-6 polyunsaturated FA (PUFA), more pronouncedly in OA than in RA. OA was also characterized with reduced percentages of 22:6n-3 and lower product/precursor ratios of n-3 PUFA. The proportions of total monounsaturated FA increased in both RA and OA SF. Regarding IFP, RA patients had lower proportions of 20:4n-6, total n-6 PUFA, and 22:6n-3, as well as lower product/precursor ratios of n-3 PUFA compared to OA patients. The average chain length of SF FA decreased in both diagnoses and the double bond index in OA.
Conclusions
The observed complex alterations in the FA signatures could have both contributed to but also limited the inflammatory processes and cartilage destruction in the RA and OA knees.
Similar content being viewed by others
Background
The potential inflammatory role of the infrapatellar fat pad (IFP) has recently become a topic of interest for osteoarthritis (OA) [1] and rheumatoid arthritis (RA) research [2]. IFP is an intracapsular but extrasynovial organ that does not directly interact with the articular cartilage [3]. It has, however, been proposed to be a source of fatty acids (FA), FA-derived lipid mediators (LM), and adipocytokines that could contribute to the pathophysiological processes in knee arthropathies [1, 2, 4]. IFP-conditioned medium is able to stimulate OA fibroblast-like synoviocytes to produce prostaglandin E2 (PGE2), cytokines, and cartilage-degrading enzymes [5], which suggests a role as an anatomical site in the complex process of knee joint inflammation. RA synovial tissues typically have a more inflammatory phenotype with more infiltrating immune cells and a higher expression of cytokines than OA joints [6]. The IFP of RA patients is also infiltrated by a higher number of immune cells, while the types and levels of secreted adipocytokines are similar to OA [2].
Articular synovial fluid (SF) is the synovial membrane-produced ultrafiltrate of blood plasma that contains lubricating compounds, such as hyaluronan and phospholipids (PL). The concentrations of cholesterol, lipoproteins, and apolipoproteins increase in RA SF [7]. In addition, the levels of various glycero-PL and sphingolipids are elevated in RA and OA SF [8, 9]. FA and their derivatives have been proposed to play roles in the pathophysiology of joint diseases [10]. Changes in the desaturation and chain length of FA could affect the anti-friction and lubricating properties of surface-active PL that cover the articular cartilage [11, 12]. Local FA concentrations could also contribute to inflammatory processes and cartilage degradation [10]. Generally, n-6 polyunsaturated FA (PUFA), which are overrepresented in the Western diet [13, 14], are precursors to pro-inflammatory LM, while n-3 PUFA are converted to less inflammatory or resolving ones [10, 15]. In fact, n-3 PUFA, especially 20:5n-3 (eicosapentaenoic acid), have anti-inflammatory and anti-destructive effects on cartilage [16, 17]. In agreement with this, dietary fish oil supplements containing high proportions of 20:5n-3 and 22:6n-3 (docosahexaenoic acid) can reduce the tender joint count and morning stiffness in RA patients [18]. Increased consumption of long-chain n-3 PUFA may also have beneficial effects on pain alleviation and function of OA joints, although the evidence is less convincing [19].
The major dietary n-6 PUFA, 18:2n-6 (linoleic acid), when first converted to 20:4n-6 (arachidonic acid), can have pro-inflammatory effects on cartilage via increased PGE2 production [20]. OA joints have been documented to accumulate n-6 PUFA, especially 20:4n-6, the immediate precursor of PGE2 [21, 22]. The major monounsaturated FA (MUFA), 18:1n-9 (oleic acid), inhibits cartilage destruction, while the role for the most common saturated FA (SFA), 16:0 (palmitic acid), remains controversial [20, 23]. According to Lu et al. [24], a high dietary intake of SFA can be associated with the progression of knee OA, while dietary PUFA and MUFA have potentially protective effects. PGE2 levels can be elevated in SF/plasma of RA and OA patients [10]. However, pro-resolving LM can be also detected in both RA and OA joints, where they could potentially contribute to the resolution pathways [25,26,27].
Previous studies focusing on the detailed assessment of FA signatures in RA or OA joints are scarce. To assess the contribution of IFP to knee arthropathies, our aim was to examine the proportions of a comprehensive array of FA in the IFP and SF of RA and OA patients. To the best of our knowledge, this is the first time the FA profiles of IFP and SF sample pairs from the same knees are compared between RA and OA. It was hypothesized that RA would be characterized with a more pro-inflammatory FA signature compared to OA in both IFP and SF.
Methods
Subjects, ethics, and sampling
Patients with knee joint disorders (seropositive RA: men n = 3, women n = 7; primary OA: men n = 2, women n = 8; Table 1) were recruited at the Oulu University Hospital with the permission of the Ethical Committee of the Hospital (decision #29/2011, amendment 2/24/2014) in compliance with the Helsinki Declaration. As the selection criteria, we only sampled patients who had reached the age of majority (18 years) and were undergoing knee surgery for pre-existing medical indications (Table 2). Prior to the surgery, all patients signed consent forms to donate their IFP and SF samples. General data were recorded as follows: sex, age, body mass, height, body mass index (BMI), operation, operative diagnosis, and medication. No data that would enable the identification of the patients were registered.
SF samples were collected with sterile needles and syringes during joint replacement surgery performed at the Oulu University Hospital in 2011–2017 and stored at − 70 °C. IFP samples were harvested from the same patients during the removal of periarticular tissues according to the demands of the joint replacement procedure. All samples were obtained during surgery for pre-existing indications, and they represented tissues that would have been removed during surgery regardless of the study. The total duration of the surgery increased only by a minimal amount of time due to sampling. For comparison, control SF samples (men n = 2, women n = 4) were collected during arthroscopic knee surgery performed as diagnostic arthroscopy or due to trauma without underlying RA/OA at the Kuopio University Hospital in 2014 with the permission of the Ethical Committee of the Hospital (decision #79//2013) in compliance with the Helsinki Declaration. The samples were stored at − 80 °C. The selection criteria for control patients and the collection of general data were similar to patients with RA and OA.
FA analyses
Subsamples of IFP and SF were transmethylated in methanolic H2SO4 under nitrogen atmosphere [28], and the formed FA methyl esters (FAME) were extracted with hexane and analyzed by a Shimadzu GC-2010 Plus gas chromatograph (Shimadzu, Kyoto, Japan) employing flame ionization detector (FID). The FAME structures were confirmed by using electron impact mass spectra recorded by a Shimadzu GCMS-QP2010 Ultra with a mass selective detector. The resulting chromatographic peaks from FID were manually integrated with the GCsolution software (v2.41.00) by Shimadzu. The results represent the FA composition (mol-%) of IFP and SF total lipids, and when calculating indices, as defined below, the mol-% values of the FA were always used. The n-6 and n-3 PUFA product/precursor ratios were calculated as follows: (20:3n-6 + 20:4n-6)/18:2n-6 and (20:5n-3 + 22:6n-3)/18:3n-3. The double bond index (DBI) was calculated by using the formula: [(1 × (Σ MUFA)) + (2 × (Σ dienoic FA)) + (3 × (Σ trienoic FA)) + (4 × (Σ tetraenoic FA)) + (5 × (Σ pentaenoic FA)) + (6 × (Σ hexaenoic FA))]/100. The total average chain length (TACL) was calculated as follows: [(14 × (Σ 14C FA)) + (15 × (Σ 15C FA)) + (16 × (Σ 16C FA)) + (17 × (Σ 17C FA)) + (18 × (Σ 18C FA)) + (19 × (Σ 19C FA)) + (20 × (Σ 20C FA)) + (22 × (Σ 22C FA)) + (24 × (Σ 24C FA))]/100. The ∆9-desaturation index was calculated with the formula: [(14:1n-5 + 16:1n-9 + 16:1n-7 + 16:1n-5 + 17:1n-8 + 18:1n-9 + 18:1n-7 + 18:1n-5 + 20:1n-11 + 20:1n-9 + 20:1n-7 + 22:1n-11 + 22:1n-9 + 22:1n-7 + 24:1n-9)/(14:0 + 16:0 + 17:0 + 18:0 + 20:0 + 22:0 + 24:0)], which included all the MUFA irrespective of whether their production also required chain-elongation or chain-shortening steps after the desaturation of the SFA precursor. The ∆6-desaturation index was calculated as 18:3n-6/18:2n-6 (since 18:4n-3 did not accumulate in the studied tissues, 18:4n-3/18:3n-3 ratio was not used) and the ∆5-desaturation index as 20:4n-6/20:3n-6 and 20:5n-3/20:4n-3.
Statistical analyses
Comparisons between the study groups were performed with the generalized linear model executed with the normal probability distribution (IBM SPSS v25 software, IBM, Armonk, NY, USA). The examined parameter was the dependent variable and the study group the model factor. Relative differences in the FA proportions between IFP and SF were tested with the independent samples t test. Sex ratios in the study groups were tested with Fisherʼs exact test. Correlations were calculated with the Spearman correlation coefficient (rs). The p value < 0.05 was considered statistically significant. The results are presented as the mean ± standard error of the mean (SEM). To carry out a general assessment of the FA profiles, we also performed the discriminant analysis by classifying FA data by discriminant functions to determine how the samples in the study groups differed from one another, which variables separated them most clearly, and how well the analysis was able to classify the samples into their respective study groups: control, RA, or OA.
Results
General demographic data of the patients are presented in Table 1. The RA and OA patients were significantly older than those in the control group. The body masses or BMI were not affected by the diagnoses. Neither did sex have an effect on the general characteristics of the patients, but there was a significant group × sex interaction regarding age and BMI.
In the discriminant analysis, the five study groups were clearly separated from each other based on the FA signatures (Fig. 1). The FA having the largest separation power included 18:2n-6, 22:4n-6, 18:3n-3, 22:6n-3, 18:1n-9, and 20:4n-6. The analysis classified 100% of the samples correctly into their respective study groups. The general differences in the FA profiles between IFP and SF can be examined in Table 3.
Discriminant analysis depicting the classification of fatty acid signatures in the studied tissues based on discriminant functions 1 and 2. With these first two functions, 98.4% of the variance was explained. IFP infrapatellar fat pad, SF synovial fluid, OA osteoarthritis, RA rheumatoid arthritis. Note that the scaling is different in the x- and y-axes
According to the generalized linear model, RA SF had higher proportions of 16:1n-5, 17:0ai, DMA 18:1n-7 (dimethyl acetal), and total MUFA compared to control SF, while 18:2n-6, 24:0, and TACL were lower (Table 3). Regarding OA SF, the proportions of 14:1n-5, 15:0, DMA 18:1n-7, 18:1n-9, and total MUFA were elevated and those of 18:2n-6, 20:4n-6, 24:0, 22:6n-3, total PUFA, and total n-6 PUFA reduced, and the product/precursor ratios of n-3 PUFA, TACL, and DBI decreased compared to control SF. RA SF had higher percentages of 16:1n-5, 20:4n-6, and 22:6n-3 than OA SF. Regarding IFP, RA lipids had higher proportions of 14:0 and 20:1n-9 but lower percentages of 20:4n-6, 22:4n-6, 22:6n-3, total PUFA, and n-6 PUFA, and decreased product/precursor ratios of n-3 PUFA.
The n-3/n-6 PUFA ratios of SF correlated positively with the n-3/n-6 PUFA ratios of IFP in RA patients (rs = 0.903, p < 0.0004) but not in OA patients (rs = 0.200, p = 0.580). Similarly, the n-3 PUFA sums correlated positively between SF and IFP in RA patients (rs = 0.709, p = 0.022) but not in OA patients (rs = 0.321, p = 0.365), and the 18:1n-9 proportions between SF and IFP in OA patients (rs = 0.648, p = 0.043) but not in RA patients (rs = − 0.321, p = 0.365). In addition, the BMI correlated inversely with the SF proportions of 22:6n-3 in RA patients (rs = − 0.723, p = 0.018) but not in OA patients (rs = − 0.406, p = 0.244). There were no significant correlations between the BMI and n-3 or n-6 PUFA sums, nor the n-3/n-6 PUFA ratios in SF or IFP (rs = − 0.479–0.200, p = 0.162–0.947) except for the n-6 PUFA sums in the IFP of RA group (rs = − 0.729, p = 0.017). In the generalized linear model with BMI as a covariate, the effects of the BMI on the SF and IFP n-3 PUFA sums, n-6 PUFA sums, and n-3/n-6 PUFA ratios were nonsignificant (p = 0.266–0.566).
Discussion
The significance of age, sex, and obesity in joint disorders is well-documented, which was also confirmed in the discriminant analysis of the present study. However, the novelty of our results is in the FA part of the study and in the potential dialogue between the FA profiles and inflammatory phenomena in the two major joint diseases, RA and OA. The main findings of the present study were as follows: (i) n-6 PUFA reduced in proportion in RA SF and especially in OA SF compared to control knees, (ii) 22:6n-3 and product/precursor ratios of n-3 PUFA decreased in OA SF, (iii) TACL and DBI reduced especially in OA SF, and (iv) total n-6 PUFA, 20:4n-6, 22:6n-3, and product/precursor ratios of n-3 PUFA were lower in RA IFP than in OA IFP.
It was initially hypothesized that the RA knee would exhibit a more pro-inflammatory FA signature compared to the OA knee. However, we observed a loss of n-6 PUFA in the SF of both RA and OA patients, as well as lower 20:4n-6 and total n-6 PUFA proportions in RA IFP compared to OA IFP. There is a growing consensus for a detrimental role of n-6 PUFA and their derivatives in joint health. It has been established that n-6 PUFA, especially 20:4n-6, can accumulate in OA joints [21, 22] and that they increase cyclooxygenase-2 protein levels [29] and PGE2 production in chondrocytes [20]. In plasma PL, 20:4n-6 levels were positively associated with the degree of knee synovitis [30], and low dietary 20:4n-6 intake reduced inflammation in RA patients [31]. In addition, the secretion of 20:4n-6 was higher from the IFP of OA patients compared to post-mortem donors without OA [4]. However, the situation is more complex, as n-6 PUFA also have potential roles in the pathways of resolution of inflammation [32]. Previously, 18:3n-6 (gamma-linolenic acid) was noted to improve signs and symptoms of RA disease activity [33], and in a recent study, 18:2n-6 levels in erythrocytes were inversely associated with the risk of RA [34]. According to Van de Vyver et al. [35], 20:4n-6 concentrations decreased in the SF free FA of patients with end-stage knee OA, supporting the present results.
To the best of our knowledge, the observed reduction in the proportions of n-6 PUFA has not been previously documented for RA SF or IFP, but some similarities have been reported for the circulating levels of n-6 PUFA in RA patients [36,37,38]. It is tempting to hypothesize that the decreased relative proportions of n-6 PUFA in SF could reduce inflammation and, in this way, provide a potentially protective mechanism in knee arthropathies. Alternatively, the reduced SF proportions of 18:2n-6 and 20:4n-6 could have been due to their intensified conversion to short-lived LM. This view is supported by the SF activity of phospholipase A2 [39], an enzyme that cleaves PUFA from PL for LM synthesis. In addition, based on the few liquid chromatography–tandem mass spectrometry studies conducted, SF contains several pro- and anti-inflammatory LM originating from n-6 and n-3 PUFA [25, 40].
In addition to the loss of n-6 PUFA, we documented reduced 22:6n-3 proportions in OA SF, also reflected in the decreased n-3 PUFA product/precursor ratios. Previous data have shown that n-3 PUFA can have protective effects on joint tissues, for instance, by reducing the production of cyclooxygenase-2, inflammatory cytokines, and cartilage-degrading enzymes [16, 17, 29] and, for these reasons, the relative decrease of 22:6n-3 in the OA SF samples was expected. Earlier, the mobilization of 22:6n-3 from IFP was higher in OA patients than in post-mortem donors without OA [4]. As 22:6n-3 is a precursor of pro-resolving LM, it could contribute to the resolution pathways in diseased joints [25,26,27]. The n-3 PUFA levels in the SF of RA patients of the present study were not different from controls, and their 22:6n-3 percentages were somewhat higher than in OA SF. This is opposite to the findings of Navarro et al. [37], who documented decreased n-3 PUFA levels in the SF of RA patients compared to those with OA. These data agree with the literature in which a higher dietary intake of long-chain n-3 PUFA has been associated with a lower risk of developing RA [41] and in which dietary fish oil supplements have reduced tender joint count and stiffness of RA joints [18]. In contrast to our SF results, the lower 22:6n-3 proportions in RA IFP compared to OA IFP would be in line with earlier literature.
A high n-6/n-3 PUFA ratio in the diet and in the body is associated with obesity and several other pathological conditions [13, 14]. Our research group observed an increase in the IFP n-6/n-3 PUFA ratio due to experimentally induced early OA in a rabbit model [42] and, in human plasma, a high n-6/n-3 PUFA ratio was associated with greater pain and functional limitations of the OA knee [43], suggesting implications in joint diseases. However, there was no relationship between SF n-3/n-6 PUFA ratios and OA severity or synovitis in an obese mouse model of OA [44]. In the present study, the n-3/n-6 PUFA ratios in SF and IFP were unaffected by RA or OA, which could mean that both types of PUFA are used for LM production, also supported by the previous liquid chromatography–tandem mass spectrometry profiling studies of SF LM [25, 27, 40]. We did not find increased product/precursor ratios of n-6 PUFA, different from previous literature on serum PL [36, 38]. Neither were there statistically significant differences in the ∆6- or ∆5-desaturation indices according to the diagnoses. These results are in line with the study by de Pablo et al. [34], who found no significant association between these indicators of PUFA metabolism and the risk of RA.
The present results also revealed an increase in the SF proportions of total MUFA in both RA and OA. Previously, Navarro et al. [37] documented elevated percentages of long-chain MUFA in RA SF, possibly in association with increased sphingomyelin levels. In the present study, 18:1n-9 showed increased percentages in OA SF and, previously, it was identified as a critical metabolite for discriminating between early- and late-stage OA with increased levels in SF during disease progression [45]. The possible role for MUFA in joint diseases is not clear, but 18:1n-9 has exerted anti-destructive effects on chondrocytes and cartilage in vitro [20]. In this way, the elevated MUFA proportions could counteract pathologic processes in RA and OA.
A high dietary intake of SFA was previously connected to the disease progression of knee OA [24] and the major SFA, 16:0, induced inflammation, articular cartilage breakdown, and chondrocyte apoptosis in vitro [23]. However, 16:0 was also documented to inhibit cartilage destruction [20]. A recent study using an obese mouse model of OA reported negative association of SF SFA with OA severity [44]. We did not find significant changes in the proportions of major SFA between RA and OA SF or IFP, while Navarro et al. [37] observed increased 16:0 and total SFA levels in the SF of RA compared to OA patients. To sum up the present results, the major differences between the control knees and the RA and OA joints were an increase in MUFA and a simultaneous decrease in n-6 PUFA. It has been previously proposed that IFP could inhibit catabolic processes in the end-stage OA [46]. Together, these findings suggest that in addition to destructive cascades in the diseased joint, protective biochemical phenomena may also arise, when a share of n-6 PUFA is replaced with MUFA, perhaps following intense consumption of n-6 PUFA precursors for LM production. These observations have potential translational applications, as altering the FA profile could help alleviate the pathological processes in both RA and OA.
Even though PL levels in SF have been documented to be elevated in RA and OA [8, 9], articular surfaces have been proposed to become deficient in surface-active PL in OA [47]. Changes in the desaturation and length of FA chains could influence the anti-friction and lubricating properties of these PL covering the articular cartilage [11, 12]. Kosinska et al. [12] suggested that there could be an increase in the PL species with longer FA chains in RA and OA, which could hypothetically reduce friction and protect articular surfaces. We found decreased TACL in both RA and OA SF and reduced DBI in OA SF. Provided that PL with longer-chain and more unsaturated FA would reduce the coefficient of friction in joints [48], the decreased TACL and DBI could be examples of harmful FA alterations in RA and OA, in contrast to the observed decrease in n-6 PUFA and the increase in MUFA.
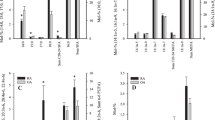
It has been proposed that most SF FA would derive from plasma [37], but IFP has also been suggested to be one source of FA and LM affecting the knee joint [4, 5, 49]. IFP has been assumed to induce both protective and disease-aggravating activities in the OA knee [49]. The interrelations between the IFP and SF FA profiles were assessed here by the simultaneous analysis of these tissues from the same knees, and the relative differences in the proportions of FA between IFP and SF are represented in Fig. 2. The proportions of several long-chain SFA and MUFA were higher in SF, and their source could possibly be plasma sphingomyelin known to harbor these FA [50]. We only found a few significant correlations in the FA proportions between IFP and SF. This could indicate that the release of FA from IFP may not be as prominent as could be assumed or that it is mostly the FA-derived LM that are released from IFP to SF via the synovial membrane. SF lipids may also derive directly from tissue destruction in diseased joints causing the IFP and SF FA profiles to diverge.
The relative differences (%) in the proportions of selected fatty acids (FA) between tissues. IFP infrapatellar fat pad, SF synovial fluid, OA osteoarthritis, RA rheumatoid arthritis. The bars with negative values indicate that a FA had higher percentages in IFP than in SF, and the bars with positive values indicate higher percentages in SF than in IFP [(mol-% in SF–mol-% in IFP)/mol-% in IFP; mean + SEM]. Asterisk denotes significant difference between OA and RA (t test, p < 0.05)
When investigating the reasons behind the tissue- and diagnosis-specific differences in the FA composition, the relative importance of the changes due to systemic PUFA distribution in the body vs. locally different PUFA metabolism is difficult to address. However, no tissues of any wild type mammals are able to convert n-6 PUFA to n-3 PUFA or vice versa, a trait only possible through introduced genes from invertebrates [51]. Still, these structurally different PUFA families affect each other’s metabolism by competing for the same enzymatic conversions, especially for the further ∆6- and ∆5-desaturations of PUFA precursors that already have the n-3 or n-6 double bond in place [52]. However, this competition that can be found in the structural modifications of PUFA does not seem to occur between n-3 and n-6 PUFA in the cycles of incorporation and hydrolysis that remodel the lipid acyl chains [53]. In addition, different phospholipases have preferred substrates, and the hydrolysis rates depend on the lipid molecule head group and acyl chains [54]. Thus, specific PUFA may be hydrolyzed and consumed at different rates when cyclooxygenase, lipoxygenase, and cytochrome P450 monooxygenase enzymes convert n-6 and n-3 PUFA to various LM [55, 56].
Some potential limitations regarding the present study should be acknowledged. The control group for SF was relatively small and, in addition, the IFP samples unfortunately lacked a control group. This was due to ethical reasons, as IFP is not routinely removed during arthroscopic surgery unlike in total joint replacement. Furthermore, the control SF samples do not represent healthy but RA- and OA-free joints, and knee trauma can influence the SF lipid composition [57]. In addition, the control patients were significantly younger than the RA and OA groups, which is quite unavoidable considering the demographics of degenerative joint diseases vs. knee trauma. The effects of age on body FA profiles cannot be excluded [58]. While this variable may be a confounding factor regarding the study population, it would be logistically difficult to obtain an age-standardized sample population regarding knee diseases. By the time of the knee replacement surgery, the patients had a long disease history that had been treated with systemic and local medications, and we cannot exclude the possibility that some of these could have influenced the FA profiles, especially regarding the usually more intensively medicated RA patients.
Conclusions
The FA signatures in IFP and SF were clearly different between the studied diagnoses. These changes could contribute to disease progression by influencing inflammatory processes, cartilage destruction, and friction in the joint. The FA manifestations of RA and OA could not be regarded as strictly pro-inflammatory or ameliorating but likely reflected both inflammation and protective biochemical phenomena. The major changes from the control knees to the RA and OA joints were an increase in MUFA and a simultaneous decrease in n-6 PUFA. There is accumulating evidence for a protective role of n-3 PUFA in joint health, but the potential roles of the n-6 PUFA loss and the increase in MUFA should be investigated in future studies on RA and OA.
Abbreviations
- 16:0:
-
Palmitic acid
- 18:1n-9:
-
Oleic acid
- 18:2n-6:
-
Linoleic acid
- 18:3n-6:
-
Gamma-linolenic acid
- 20:4n-6:
-
Arachidonic acid
- 20:5n-3:
-
Eicosapentaenoic acid
- 22:6n-3:
-
Docosahexaenoic acid
- BMI:
-
Body mass index
- DBI:
-
Double bond index
- DMA:
-
Dimethyl acetal
- FA:
-
Fatty acid
- FAME:
-
Fatty acid methyl ester
- FID:
-
Flame ionization detector
- IFP:
-
Infrapatellar fat pad
- MUFA:
-
Monounsaturated fatty acid
- OA:
-
Osteoarthritis
- PGE2 :
-
Prostaglandin E2
- PL:
-
Phospholipid
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
- TACL:
-
Total average chain length
- TAG:
-
Triacylglycerol
- UFA:
-
Unsaturated fatty acid
References
Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RGHH, Zuurmond A, Stojanovic-Susulic V, Van Osch GJVM, Toes REM, Ioan-Facsinay A. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–7.
de Jong AJ, Klein-Wieringa IR, Kwekkeboom JC, Toes REM, Kloppenburg M, Ioan-Facsinay A. Inflammatory features of infrapatellar fat pad in rheumatoid arthritis versus osteoarthritis reveal mostly qualitative differences. Ann Rheum Dis. 2018;77:1088–90.
Macchi V, Stocco E, Stecco C, Belluzzi E, Favero M, Porzionato A, De Caro R. The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J Anat. 2018;233:146–54.
Gierman LM, Wopereis S, van El B, Verheij ER, Werff-van der Vat BJC, Bastiaansen-Jenniskens YM, van Osch GJVM, Kloppenburg M, Stojanovic-Susulic V, Huizinga TWJ, Zuurmond A-M. Metabolic profiling reveals differences in concentrations of oxylipins and fatty acids secreted by the infrapatellar fat pad of donors with end-stage osteoarthritis and normal donors. Arthritis Rheum. 2013;65:2606–14.
Eymard F, Pigenet A, Citadelle D, Flouzat-Lachaniette C-H, Poignard A, Benelli C, Berenbaum F, Chevalier X, Houard X. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol. 2014;66:2165–74.
de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, Zuurmond A-M, Schoones J, Toes REM, Huizinga TWJ, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthr Cartil. 2012;20:1484–99.
Prete PE, Gurakar-Osborne A, Kashyap ML. Synovial fluid lipids and apolipoproteins: a contemporary perspective. Biorheology. 1995;32:1–16.
Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, Lasczkowski G, Rickert M, Schmitz G, Steinmeyer J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013;65:2323–33.
Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, Lasczkowski G, Rickert M, Schmitz G, Steinmeyer J. Sphingolipids in human synovial fluid - a lipidomic study. PLoS One. 2014;9:e91769.
Brouwers H, von Hegedus J, Toes R, Kloppenburg M, Ioan-Facsinay A. Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract Res Clin Rheumatol. 2015;29:741–55.
Chen Y, Crawford RW, Oloyede A. Unsaturated phosphatidylcholines lining on the surface of cartilage and its possible physiological roles. J Orthop Surg Res. 2007;2:14.
Kosinska MK, Ludwig TE, Liebisch G, Zhang R, Siebert H-C, Wilhelm J, Kaesser U, Dettmeyer RB, Klein H, Ishaque B, Rickert M, Schmitz G, Schmidt TA, Steinmeyer J. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One. 2015;10:e0125192.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79.
Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128.
Russo GL. Dietary n – 6 and n – 3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–46.
Zainal Z, Longman AJ, Hurst S, Duggan K, Caterson B, Hughes CE, Harwood JL. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr Cartil. 2009;17:896–905.
Knott L, Avery NC, Hollander AP, Tarlton JF. Regulation of osteoarthritis by omega-3 (n-3) polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthr Cartil. 2011;19:1150–7.
Fortin PR, Lew RA, Liang MH, Wright EA, Beckett LA, Chalmers TC, Sperling RI. Validation of a meta-analysis: the effects of fish oil in rheumatoid arthritis. J Clin Epidemiol. 1995;48:1379–90.
Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology. 2018;57:iv61–74.
Bastiaansen-Jenniskens YM, Siawash M, van de Lest CHA, Verhaar JAN, Kloppenburg M, Zuurmond A-M, Stojanovic-Susulic V, Van Osch GJVM, Clockaerts S. Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions: a preliminary study. Cartilage. 2013;4:321–8.
Lippiello L, Walsh T, Fienhold M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism. 1991;40:571–6.
Plumb MS, Aspden RM. High levels of fat and (n-6) fatty acids in cancellous bone in osteoarthritis. Lipids Health Dis. 2004;3:12.
Alvarez-Garcia O, Rogers NH, Smith RG, Lotz MK. Palmitate has proapoptotic and proinflammatory effects on articular cartilage and synergizes with interleukin-1. Arthritis Rheumatol. 2014;66:1779–88.
Lu B, Driban JB, Xu C, Lapane KL, McAlindon TE, Eaton CB. Dietary fat intake and radiographic progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res. 2017;69:368–75.
Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, Serhan CN, Mayboroda OA. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC–MS/MS. Biochim Biophys Acta. 2012;1821:1415–24.
Serhan CN. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature. 2014;510:92–101.
Jónasdóttir HS, Brouwers H, Kwekkeboom JC, van der Linden HMJ, Huizinga T, Kloppenburg M, Toes REM, Giera M, Ioan-Facsinay A. Targeted lipidomics reveals activation of resolution pathways in knee osteoarthritis in humans. Osteoarthr Cartil. 2017;25:1150–60.
Christie WW. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Christie WW, editor. Advances in lipid methodology – two. Dundee: Oily Press; 1993. p. 69–111.
Hurst S, Rees SG, Randerson PF, Caterson B, Harwood JL. Contrasting effects of n-3 and n-6 fatty acids on cyclooxygenase-2 in model systems for arthritis. Lipids. 2009;44:889–96.
Baker KR, Matthan NR, Lichtenstein AH, Niu J, Guermazi A, Roemer F, Grainger A, Nevitt MC, Clancy M, Lewis CE, Torner JC, Felson DT. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthr Cartil. 2012;20:382–7.
Adam O, Beringer C, Kless T, Lemmen C, Adam A, Wiseman M, Adam P, Klimmek R, Forth W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol Int. 2003;23:27–36.
Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–8.
Zurier RB, Rossetti RG, Jacobson EW, DeMarco DM, Liu NY, Temming JE, White BM, Laposata M. Gamma-linolenic acid treatment of rheumatoid arthritis. Arthritis Rheum. 1996;39:1808–17.
de Pablo P, Romaguera D, Fisk HL, Calder PC, Quirke A-M, Cartwright AJ, Panico S, Mattiello A, Gavrila D, Navarro C, Sacerdote C, Vineis P, Tumino R, Ollier WE, Michaud DS, Riboli E, Venables PJ, Fisher BA. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case–control study. Ann Rheum Dis. 2018;77:981–7.
Van de Vyver A, Clockaerts S, van de Lest CHA, Wei W, Verhaar J, Van Osch GJVM, Bastiaansen-Jenniskens YM. Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage. 2018. https://doi.org/10.1177/1947603518798891.
Bruderlein H, Daniel R, Boismenu D, Julien N, Couture F. Fatty acid profiles of serum phospholipids in patients suffering rheumatoid arthritis. Prog Lipid Res. 1981;20:625–31.
Navarro E, Esteve M, Olivé A, Klaassen J, Cabré E, Tena X, Fernández-Bañares F, Pastor C, Gassull MA. Abnormal fatty acid pattern in rheumatoid arthritis. A rationale for treatment with marine and botanical lipids. J Rheumatol. 2000;27:298–303.
Jacobsson L, Lindgärde F, Manthorpe R, Åkesson B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann Rheum Dis. 1990;49:901–5.
Pruzanski W, Vadas P, Stefanski E, Urowitz MB. Phospholipase A2 activity in sera and synovial fluids in rheumatoid arthritis and osteoarthritis. Its possible role as a proinflammatory enzyme. J Rheumatol. 1985;12:211–6.
de Grauw JC, van de Lest CHA, van Weeren PR. A targeted lipidomics approach to the study of eicosanoid release in synovial joints. Arthritis Res Ther. 2011;13:R123.
Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis. 2014;73:1949–53.
Mustonen A-M, Käkelä R, Finnilä MAJ, Sawatsky A, Korhonen RK, Saarakkala S, Herzog W, Paakkonen T, Nieminen P. Anterior cruciate ligament transection alters the n-3/n-6 fatty acid balance in the lapine infrapatellar fat pad. Lipids Health Dis. 2019;18:67.
Sibille KT, King C, Garrett TJ, Glover TL, Zhang H, Chen H, Reddy D, Goodin BR, Sotolongo A, Petrov ME, Cruz-Almeida Y, Herbert M, Bartley EJ, Edberg JC, Staud R, Redden DT, Bradley LA, Fillingim RB. Omega-6:omega-3 PUFA ratio, pain, functioning, and distress in adults with knee pain. Clin J Pain. 2018;34:182–9.
Wu C-L, Kimmerling KA, Little D, Guilak F. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci Rep. 2017;7:44315.
Kim S, Hwang J, Kim J, Ahn JK, Cha H-S, Kim KH. Metabolite profiles of synovial fluid change with the radiographic severity of knee osteoarthritis. Joint Bone Spine. 2017;84:605–10.
Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, Zuurmond A-M, Stojanovic-Susulic V, Bridts C, de Clerck L, DeGroot J, Verhaar JAN, Kloppenburg M, van Osch GJVM. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71:288–94.
Hills BA, Monds MK. Deficiency of lubricating surfactant lining the articular surfaces of replaced hips and knees. Br J Rheumatol. 1998;37:143–7.
Sarma AV, Powell GL, LaBerge M. Phospholipid composition of articular cartilage boundary lubricant. J Orthop Res. 2001;19:671–6.
Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15:225.
Phillips GB, Dodge JT. Composition of phospholipids and of phospholipid fatty acids of human plasma. J Lipid Res. 1967;8:676–81.
Kang JX, Wang J, Wu L, Kang ZB. Fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504.
Blanchard H, Pédrono F, Boulier-Monthéan N, Catheline D, Rioux V, Legrand P. Comparative effects of well-balanced diets enriched in α-linolenic or linoleic acids on LC-PUFA metabolism in rat tissues. Prostaglandins Leukot Essent Fatty Acids. 2013;88:383–9.
Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13:267–72.
Batchu KC, Hänninen S, Jha SK, Jeltsch M, Somerharju P. Factors regulating the substrate specificity of cytosolic phospholipase A2-alpha in vitro. Biochim Biophys Acta. 2016;1861:1597–604.
Astudillo AM, Balboa MA, Balsinde J. Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:772–83.
Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–69.
Rabinowitz JL, Gregg JR, Nixon JE. Lipid composition of the tissues of human knee joints II. Synovial fluid in trauma. Clin Orthop Relat Res. 1984;190:292–8.
Holman RT, Smythe L, Johnson S. Effect of sex and age on fatty acid composition of human serum lipids. Am J Clin Nutr. 1979;32:2390–9.
Acknowledgements
The technical assistance of Sanna Sihvo, Tarja Huhta, and Minna Savilampi is greatly acknowledged.
Funding
The work was financially supported by the Jane and Aatos Erkko Foundation (to A-MM). The funding source had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Availability of data and materials
All relevant data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
PN and A-MM designed and coordinated the study. PL, JH, ST, AJ, TK, and HK conducted the sampling of the patients and contributed to the preliminary sample and patient analysis. RK and TP performed the laboratory analyses. PN integrated the chromatograms and performed the statistical analyses. A-MM wrote the first draft of the manuscript. All authors commented on the manuscript and accepted its final content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Ethical Committees of the Oulu University Hospital (decision #29/2011, amendment 2/24/2014) and the Kuopio University Hospital (decision #79//2013). Written informed consent was obtained from each patient.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mustonen, AM., Käkelä, R., Lehenkari, P. et al. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res Ther 21, 124 (2019). https://doi.org/10.1186/s13075-019-1914-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-019-1914-y