Abstract
Background
Rheumatoid arthritis (RA) knowledge has been constructed with studies performed in Caucasians patients; Latin American patients present unique characteristics. Empowerment is a social multidimensional construct that has been associated to better health-related quality of life in RA. There is no validated instrument for use with Spanish-speaking patients. The objective of the study was to adapt the Spanish version of the Health Empowerment Scale (S-HES), which was selected for its psychometric properties and suitability for low-literacy populations, for RA Hispanic patients (RAEH), and to perform its psychometric validation.
Methods
RAEH adaptation, pilot testing, and psychometric validation were performed. Three convenience samples of RA outpatients from a national tertiary care level center were used. For RAEH adaptation, the word “health” was substituted with “RA” in the original S-HES, integrated by 8 items. Pilot testing (in 50 patients) assessed feasibility. Psychometric validation included content validity (nine experts rated item convenience, clarity, and cultural semantic accuracy), internal consistency (in 200 patients, Cronbach’s alpha) and test–retest (in a subsample of 50 patients, ICC and 95% CI), construct validity (factor analysis), and face validity (in 20 patients, % of agreement). Patients gave written informed consent.
Results
Patients were primarily middle-aged females and had typical long-standing disease, although early disease was represented. In the psychometric validation sample, the majority of the outpatients had autoantibodies; meanwhile, half of them had no evidence of disease activity, with acute reactants phase determinations within normal range. Patients with comorbidities and joint replacement were also included.
Experts agreed upon the attributes of content validity: 83–100% considered the item was essential, 100% agreed on the item’s clarity and 80–100% on the cultural semantic accuracy. In the pilot sample, ≥ 80% of the patients agreed with the item’s clarity and format.
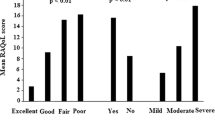
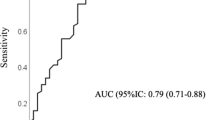
In the psychometric validation sample, mean RAEH was 34 (maximum possible score: 40 = highest score). RAEH had a good internal consistency, Cronbach’s α = 0.86, and moderately good reliability (ICC [95% CI] test–retest: 0.79 [0.62–0.88]). Factor analysis for construct validity showed a single factor explaining 52% of the variance.
Patients agreed with each item content validity (85–100%) and clarity (75–100%).
Conclusions
RAEH was valid and reliable to evaluate empowerment in Spanish-speaking RA patients.
Similar content being viewed by others
Background
Patient empowerment is a social multidimensional construct [1], largely accomplished by individuals themselves. Most empowerment definitions focus on the individual’s capacity to make decisions about their health-related behavior and to have or take control over aspects of their lives that relate to health [2, 3]. Empowerment has been conceived as a health-enhancing process [3, 4], as a measurable health-related psychosocial outcome [2, 5] and even as an aspect of health-related quality of life (HRQoL) [6]. Currently, there is not a universal definition of empowerment and it has been argued that the construct may be context- and population-specific [7]; culture, age and socioeconomic resources influence empowerment level and the degree to which the different social groups can be and wish to be empowered differ [2]. A study performed in 33 developing countries, 21 of them African, demonstrated considerable variation between countries with regard to the relationship between women’s empowerment and their use of maternal health services; the authors highlighted the need to develop locally sensitive and meaningful measures of (women’s) empowerment [8]. In the field of chronic (non-rheumatic) diseases, empowerment facilitates patients’ involvement in their care and has been related to treatment safety and effectiveness [9, 10], to lower dependence on health services [9, 11], to better treatment adherence, and to improvement in specific health outcomes [9, 10].
Rheumatoid arthritis (RA) is a worldwide chronic inflammatory disease that impacts patients’ HRQoL and may result in increased mortality. Early and aggressive treatment with disease modifying anti-rheumatic drugs (DMARDs) improves outcomes [12]. The 2013 European League against Rheumatism (EULAR) treatment-recommendations for RA emphasizes that treatment must be based on a shared decision between the patient and the rheumatologist [12]. In order to have patients form a partnership with their primary physician in optimizing their health-related disease, patients require knowledge about their disease, skills to self-manage their disease and participate in medical decision making, and power [13]; all of them reflect empowerment attributes.
RA patients from Latin American countries present distinctive characteristics; the literature highlights a lower prevalence [14], a younger age at presentation [14, 15], and a higher female:male ratio [14, 15] in this population, compared with Caucasians. In addition, patients from Latin America present individual and situational sources of vulnerability [14,15,16] that affect compliance with medications and negatively impact outcomes [15, 17]. Two studies assessed empowerment in RA patients and its association with patient-related outcomes. In the first one, performed in the USA, RA patients found an association between enhancing patients’ empowerment (no validated scale was used) and improvement in some 36-item Short Form (SF-36) categories [18]. The second one was performed in Swedish patients with rheumatic diseases (including RA) and showed an association between patients’ empowerment, as assessed according to a scale that was validated in the same study, and better self-reported health [19]. Few additional studies described empowerment-enhancing approaches in RA patients, although no validated scales were used [20,21,22,23]. None of these studies had been performed in Latin American populations, which limits the comprehensiveness of the topic.
Assessment of empowerment requires the use of validated tools with appropriate psychometric properties. A recent systematic review detailed 19 measures with varying quality, 6 of them generic and 13 developed for a specific condition [24]. In addition, a Spanish questionnaire to assess empowerment for self-care has been described, although limited to climacteric women [25]. The Swedish Rheumatic Disease Empowerment Scale (SWE-RES-23) is the only questionnaire adapted for rheumatic diseases and was developed in Swedish [19]. The scale, adapted from the Swedish version of a Diabetes Empowerment Scale, showed adequate psychometric properties in Swedish patients with rheumatic diseases; there is no Spanish version. The Spanish version of the Elders Health Empowerment Scale (S-HES) is a generic scale that reflects the attributes of empowerment construct; its psychometric properties were tested in urban senior citizens (men and women) from Rosario, Argentina [26]. The literature highlights important differences between Hispanic and non-Hispanic Whites, particularly in regard to health-related decision making [27], which is conceptually close to empowerment construct. In addition, the S-HES has shown good validity and reliability (in Spanish-speaking people), is easy to apply as the instrument includes 8 items, and is considered simple to understand according to an elementary literacy level [26, 28], which is relevant for Latin American countries. Based on those considerations, we selected the S-HES, adapted the scale as an RA Empowerment Scale for Hispanic patients (RAEH), and perform its psychometric validation. We present herein the results.
Methods
The study was conducted in three steps: (1) RAEH adaptation, (2) pilot testing, and (3) psychometric validation.
Description of samples
Three different convenience samples of consecutive RA patients were considered; patients included in each sample belonged to the outpatient clinic of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador-Zubirán (INCMyN-SZ), a tertiary care level and national referral center for rheumatic diseases. All the patients had RA diagnosis, which was established according to their primary physician criteria (rheumatologist or trainee in rheumatology, no validated classification criteria were used), who additionally classified each patient as in remission or with active disease (no standard definition was used). In addition, all the patients had major comorbidity assessed according to the Charlson score [29]. In the three samples, the patients’ selection was directed to have age, gender (female preponderance), disease duration, and disease activity quotes represented.
The first sample included 50 patients and was used to evaluate feasibility; the second one included 200 patients and was used for the psychometric validation; the last sample included 20 patients and was used for face validity.
Sample size calculation for psychometric validation
Considering the number of RAEH items (8 items), a sample size of at least 80 RA outpatients was required as the exploratory factor analysis recommends 5 to 10 respondents per item [30]. However, taking into account additional published recommendations (at least 150 to 200 patients), we decided to include 200 RA patients (fair sample size) in the final sample [31].
Step (1) RAEH adaptation (see Additional file 1)
The S-HES author was contacted in order to have him involved in the process. The RAEH was adapted from the S-HES, that retained 8 items (one per subscale), with 8 subscales: satisfaction and dissatisfaction related to health, identification and achievement of personally meaningful goals, application of a systematic problem-solving process, coping with the emotional aspects of living with health, stress management, appropriate social support, self-motivation and making cost/benefit decisions about making behavior changes. Each item is scored on a 5-point Likert scale, ranging from 5 (strongly agree) to 1 (strongly disagree). In the scale, higher scores indicate stronger level of health-related empowerment, with scale scores ranging from 1 to 5.
First, in each item of the S-HES, the word “health” was substituted by “rheumatoid arthritis”. Then, each of three researchers (one rheumatologist, one trainee in rheumatology and one social worker, a PhD candidate in Health Sciences) suggested one sentence per item, in order to have different perspectives represented; researchers were blinded to each other proposals. After a consensus was obtained, the three researchers selected one sentence per item and integrated a preliminary version of the RAEH. RAEH was scored as in the original scale, but the sum of individual item-scores was provided; accordingly, RAEH score ranged from 8 to 40.
Step (2) Pilot testing
Feasibility was tested (in 50 patients) according to the following criteria: time required to fill the scale, patients’ perceived item’s clarity, and patients’ format acceptance.
Step (3) Psychometric validation
Content validity
Content validity was tested by a Validation Expert Committee (VEC) that was integrated by six rheumatologists and two psychiatrists who received relevant literature related to empowerment construct, and the author of the original S-HES (a geriatrist and psychiatrist). Content validity was examined by asking members of the VEC to rate each of the eight sentences (one per item) according to three categories: unnecessary, important but not necessary or essential. In addition, the VEC rated the item’s clarity and cultural semantic accuracy.
Reliability
Internal consistency (assessed in 200 patients) and test–retest (assessed in a subsample of 50 patients, by the same researcher, with an interval of 3 ± 1 weeks) were evaluated to determine the reliability of the scale.
Construct validity
Construct validity was determined with factor analysis.
Face validity
Previously, a brief explanation of the empowerment concept was offered to 20 patients, who were, in a second step, directed to rate each item as a valid measure of the respective empowerment dimension (Yes/No) along with each sentence’s clarity (Yes/No).
Statistical analysis
Descriptive statistics was performed to estimate the frequencies and percentages (for categorical variables), means and standard deviation (SD) or medians and 25th–75th interquartile (IQ) ranges (for continuous variables) of sociodemographic- and disease characteristics-related variables.
RAEH construct validity was evaluated by confirmatory factor analysis (maximum likelihood) with Varimax rotation. Sampling adequacy was confirmed by the Kaiser-Mayer-Olkin (KMO) measure (appropriate value ≥ 0.5); use of factor analysis was supported by the Bartlett’s test of sphericity (significant value p < 0.05), eigenvalue > 1 and correlations coefficients > 0.30 [32]. Floor and ceiling effects were determined as the percentage of patients who achieved the lowest and highest score of the scale, respectively.
Cronbach’s α and inter-item correlation for the complete scale and for each dimension was used to assess RAEH internal consistency of the questionnaire. Cronbach’s alpha interpretation was as follows: < 0.70 indicates that individual items provide an inadequate contribution to the overall scale and values of > 0.90 suggest redundancy, [33].
RAEH test–retest reliability was evaluated by the t test comparison between total test scores and by between partial test dimension score. In addition, intra-class correlation coefficients (ICC) and their 95% confident intervals (CI) were calculated based on a single measurement, absolute-agreement, two-way mixed-effects model. According to the ICC, values < 0.5 indicate poor reliability, between 0.5–0.75 moderate reliability, between 0.75 and 0.9 good reliability and values > 0.9 indicate excellent reliability. Finally, 95% CI estimates between 0.83 and 0.94 were considered as good reliability level and those between 0.95 and 0.99 estimates, as excellent reliability level [34].
All statistical analyses were performed with Statistical Package for the Social Sciences version 21.0 (IBM Corp., Armonk, NY, USA). A value of p ≤ 0.05 (two tails) was considered to be statistically significant.
Ethical considerations
The study received ethical approval from the Comité de Ética en Investigación of the INCMyN-SZ (reference number: 2226-17/ 18-1. Written informed consent was obtained from all the patients who agreed to participate.
Results
Description of the population characteristics of the three samples
During the study, there were 270 RA outpatients recruited; they were divided in three samples. Table 1 summarizes their characteristics. Patients were primarily middle-aged females, married, had basic education and medium-low socioeconomic status. The population had long-standing disease although patients with ≤ 5 years of disease duration were also represented.
In the sample for the psychometric validation, more than half of the patients were in remission and had acute reactants phase determinations (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) within normal range; also, the majority of the patients had disease-specific autoantibodies, particularly rheumatoid factor (RF) and antibodies to cyclic citrullinated peptides (ACCP).
Finally, in the three samples, patients with a major comorbid condition and patients with a surgical joint replacement were also represented.
Patients and disease characteristics were similar in the three samples, but patients from the face validation sample were more frequently married and had more disease duration ≤ 5 years (p = 0.02 and p = 0.03, respectively).
Feasibility
Early during pilot testing (five patients enrolled), patients manifested a preference for a different order in the item’s disposition; the suggestion was adopted and maintained in the final version of the RAEH used during the validation process (see Additional file 2).
(Mean) time required to fill the scale was 7 min and all the patients agreed the time was convenient. Eighty-five percent of the patients agreed on the item’s clarity and 95% of them agreed on the scale format.
Content validity
The preliminary version of the RAEH was integrated by 8 items, one per subscale. This version was submitted the VEC who agreed that each sentence/item was essential (83–100%), and about each item clarity (100%) and cultural semantic accuracy (100%), as summarized in Table 2.
Reliability
The median RAEH score for the sample was 34 (31–37), with a minimum possible score of 8 and a maximum possible score of 40. Coefficient of Kurtosis (4.5) and skewness (1.29) showed non-normal data distribution. RAEH exhibited good internal consistency, with Cronbach’s α = 0.86 for the full scale. Floor and ceiling effects were of 0.5% and 7.5%, respectively; for individual items, the percentage of patients scoring at the floor was 0.5–2% and the percentage of patients scoring at the ceiling was 26. 5–52.5%. Details of statistics, Cronbach’s α, inter-item correlation and floor and ceiling effects for each particular item are summarized in Table 3.
Mean (±SD) time between the two measurements in the test–retest analysis was of 19.3 (±6.9) days; ICC was of 0.79 (95% CI: 0.62–0.88, p ≤ 0.001). Finally, comparison of means of the total score and each dimension-score between the two measurements did not significantly differed (data not shown).
Construct validity
Construct validity was demonstrated by KMO = 0.884 and Barlett’s test of sphericity (X2 = 610.93, p ≤ 0.001) which confirmed the adequacy of the sample size for conducting factor analysis. A single factor structure was extracted, accounting for 52% of the variance. Table 3 summarizes results for items construct validity.
Patients’ RAEH face validity
Table 4 summarizes results from this process; the majority of the patients agreed that the items were a valid measure of the corresponding dimension (85–100%) and that items were clear (75–100%).
Handling of incomplete questionnaires
Only 10 scales (5% of the psychometric validation sample) had ≤ 1 missing response/item. There was no consistency in the items with omitted response. In all cases, patients stated that they skipped it by mistake. No item was considered objectionable or did not apply to the patient. Incomplete scales were returned back to the patients, who filled them in.
Discussion
In the present study, we performed a psychometric validation of the RAEH, which was adapted from a generic scale that assessed health-related empowerment. RAEH psychometric properties were adequate in terms of construct, content and face validity, and reliability, which were evaluated with internal consistency and test–retest, as recommended [30]. In addition, RAEH was also feasible based on users’ evaluation. Importantly, different samples of consecutive RA outpatients were used to perform analysis. We suggest that RAEH can be used to evaluate empowerment in Hispanic RA patients; the scale is simple and suitable for low-literacy patients and its use could be generalized to Spanish-speaking RA patients from other countries. Although ethnic heterogeneity is characteristic of the Latin American population, RA patients from this geographic area present distinctive characteristics when compared to white populations from the United States and Europe [35].
The RAEH showed adequate internal consistency reliability; Cronbach’s α coefficient for the total scale and the eight empowerment dimensions were good, with α value of 0.86 [33]. The test–retest reliability assessed in 50 patients by the same researcher showed an ICC of 0.79, indicating good reliability [34], with 95% CI of 0.62–0.88. The construct validity was demonstrated by KMO sampling and Barlett’s test of sphericity, both confirming the adequacy of the sample size for conducting factor analysis [33], and a single factor structure was extracted, accounting for 52% of the variance. Face and content validity were examined by patients, and a multidisciplinary committee of health care providers involved in RA management. Patients (RAEH face validity) agreed that items were a valid measure of the corresponding dimension and that items were clear; additional patients confirmed RAEH feasibility. Patients involvement may be particularly relevant as one would expect the indicators of empowerment to be defined by the patients themselves, rather than (or in addition to) by health care professionals. Finally, the RAEH (total score) did not show neither floor nor ceiling effect, which had been defined when more than 15% of the patients achieved the lowest or highest score, respectively [30]. Both, floor and ceiling effects can reduce the possibility of detecting change over time.
This is the first scale validated in the Hispanic population that measures empowerment in patients with RA. There is only one additional scale, the SWE-RES-23, which assessed empowerment in patients with rheumatic diseases [19]. The scale was developed in Swedish and psychometric properties were evaluated in a population primarily represented by middle-aged females; in addition, although patients with different rheumatic diagnosis were included, 74% of them were classified with inflammatory joint diseases, which include RA diagnosis. The RAEH and the SWE-RES-23 showed similar psychometric properties in terms of construct validity, internal consistency and reliability although stability reliability was not tested in the SWE-RES-23, meanwhile the RAEH showed good ICC. Regarding RAEH ceiling effects, it should be mentioned there were ceiling effects in all the individual’s dimensions, which indicates that patients start with higher empowerment skills than the average and may lack room for improvement [30]. Arvidsson et al. also found ceiling effects in two subscales of the SWE-RES-23 and the total score [19], as did Serrani in six out of eight subscales of the S-HES [25] (see Additional file 3).
The median RAEH score for our target population was 34, which confirms that our patients showed an empowerment level above medium values, as has been found in other studies [19, 25]. The average of the empowerment measurement cannot be compared between the RAEH and the SEW-RES-23, because they had different number of reagents and dimensions. Nonetheless, total RAEH mean value (4.2 ± 0.57) was superior to that from either the SWE-RES-23 (vs. 3.53 ± 0.57, p = 0.001) or the S-HES (vs. 3.51 ± 0.73, p = 0.001). Our higher scale mean value may be considered unexpectedly high for a population with limited literacy, but could be explained by the long-term RA duration present in our patients (12 years of disease duration).
Additional studies have tried to measure the level of empowerment in patients with RA; however, empowerment has been measured in a subjective manner. Geller et al. [18] used an empowerment program for 6 months in patients with chronic pain (six of them had RA) and assessed the quality of life, but did not quantitatively measure the level of empowerment. Allam et al. [36] tested the effects of online social support and gamification on health outcomes in 157 RA patients; empowerment levels changed over time, more so in the groups having access to online support or a gamified experience of the website. van der Vaart et al. [37] applied questionnaires and measured use, satisfaction and impact of a web portal, which provided patients with home access to their electronic medical records, in 360 RA patients; only 54% of the respondents (to the questionnaires) had viewed their electronic medical records; among those who had logged in, 44% reported feeling more involved in their treatment, 37% felt they had more knowledge about their treatment, but differences over time were not found on the empowerment-related instruments. Different empowerment-enhancing approaches had been described in RA patients in order to support the patients [20] and promote patient independence [21]. Clinical trials may be considered complex systems, and patients enrolled explained their participation as an experience that substantially improved their control and adaptation by a better understanding of their disease, which may ultimately enhance empowerment [22]. Similarly, alternative models of RA patients’ follow-up, whereby patients initiate appointments themselves, may offer greater self-management of the underlying rheumatic disease [23].
The study has some limitations. First, we did not assess RAEH response to change, neither had indication of minimal important change nor minimal important difference, and without such information, it is impossible to understand if changes in levels of empowerment matters to patients [24]. Second, the RAEH was adapted and validated in a particular population of Hispanic RA outpatients from México and, given cultural differences in Latin America, results present here need to be reproduced in other Spanish-speaking countries. Nonetheless, the RAEH is simple and easy to apply particularly in populations with poor levels of literacy. Third, the RAEH questionnaire assessed empowerment skills at the individual level, but did not account for organizational and community levels that may additionally impact empowerment, particularly in patients with chronic conditions [7]. Finally, the RAEH evaluates empowerment limited to RA; nonetheless, empowerment is a complex construct and it may be necessary to set up a disease-specific context to understand empowerment implications, in terms of outcomes relevant to patients.
Conclusions
In conclusion, RAEH is valid, reliable, and feasible to evaluate empowerment in Spanish-speaking RA patients. Patients from Latin America present unique characteristics and research needs to focus on such populations in order to complete the picture of RA, knowledge of which has been constructed based on studies performed primarily in Caucasians patients. There is (qualitative) evidence in non-rheumatic diseases suggesting that enhancing patient empowerment over their health is highly valued and enables patients to better manage their lives. These, if reproduced in patients with rheumatic diseases, in particular RA, should be conceived as a valuable outcome. The RAEH could be applied in a practical way in studies that use empowerment-enhancing programs in Spanish-speaking RA patients.
Abbreviations
- ACCP:
-
Antibodies to cyclic citrullinated peptide
- C-RP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- HRQoL:
-
Health-related quality of life
- ICC:
-
Intra-class correlation coefficient
- INCMyN-SZ:
-
Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
- KMO:
-
Kaiser-Meyer-Olkin sampling
- RA:
-
Rheumatoid arthritis
- RAEH:
-
Rheumatoid Arthritis Empowerment Scale for Hispanic patients
- RF:
-
Rheumatoid factor
- SD:
-
Standard deviation
- S-HES:
-
Spanish-Health Empowerment Scale
- SWE-RES-23:
-
Swedish Rheumatic Disease Empowerment Scale
- VEC:
-
Validation Expert Committee
References
Tengland P-A. Empowerment: A conceptual discussion. Health Care Anal. 2008;16:77–96.
McAllister M, Dunn G, Payne K, Davies L, Todd C. Patient empowerment: The need to consider it as a measurable patient-reported outcome for chronic conditions. BMC Health Serv Res. 2012;12:157.
Aujoulat I, d’Hoore W, Deccache A. Patient empowerment in theory and practice: Polysemy or cacophony? Patient Educ Counseling. 2007;66:13–20.
Bergsma LJ. Empowerment education: the link between media literacy and health promotion. Am Behav Sci. 2004;48:152–64.
Anderson RM, Funnell MM. Patient empowerment: Myths and misconceptions. Patient Educ Couns. 2010;79:277–82.
Tengland P-A. Empowerment: A goal or a means of health promotion? Med Health Care and Philosophy. 2007;10:197–207.
Zimmerman MA. Empowerment theory: psychological, organizational and community levels of analysis. In: Rappaport J, Seldman E, editors. Handbook of Community Psychology. New York: Plenum; 2000.
Ashmed S, Creanga AA, Gillespie DG, Tsui AO. Economic status, education and empowerment: implications for maternal health service utilization in developing countries. PLoS One. 2010;6:e11190.
Voshaar MJH, Nota I, van der Laar MAFJ, van der Bemt BJF. Patient-centred care in established rheumatoid arthritis. Best Pract and Res Clin Rheumatol. 2015;29:643–63.
Elwyn G, Laitner S, Coulter A, Walker E, Watson P, Thomson R. Implementing share decision-making in the NHS. British Med J. 2010;341:c5146.
Counter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. Br Med J. 2007;335:24–7.
Smolen JS, Landerwe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biologic disease modifying anti-rheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Hibbard JH. Moving toward a more patient-centred health care delivery system. Health Aff (Millwood) 2004 Suppl Variation: var 133–5.
Mody GM, Cardiel MH. Challenges in the management of rheumatoid arthritis in developing countries. Best Pract Clin Rheumatol. 2008;22:621–41.
Contreras-Yáñez I, Pascual-Ramos V. Window of opportunity to achieve major outcomes in early rheumatoid arthritis patients: How persistence with therapy matters. Arthritis Res Ther. 2015;17:177. https://doi.org/10.1186/s13075-015-0697-z.
Pérez-Román DI, Ortiz-Haro AB, Ruiz-Medrano E, Contreras-Yáñez I, Pascual-Ramos V. Outcomes after rheumatoid arthritis patients complete their participation in a long-term observational study with tofacitinib combined with methotrexate: Practical and ethical implications in vulnerable populations after tofacitinib discontinuation. Rheumatol Int. 2018;38(4):599–606.
Van Den Bemt BJF, Zwikker HE, Van Den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8:337–51.
Geller JS, Kulla J, Shoemaker A. Group medical visits using empowerment based model as treatment for women with chronic pain in an underserved community. Global Adv Health Med. 2015;4:27–31.
Arvidsson S, Bergman S, Arvidsson B, Fridlund B, Tingström P. Psychometric properties of the Swedish rheumatic disease empowerment scale, SWE-RES-23. Musculoskeletal Care. 2012;10:101–9.
Arvidsson SB, Petersson A, Nilsson I, Andersson B, Arvidsson B, Petersson IF, et al. A nurse led rheumatology clinic’s impact on empowering patients with rheumatoid arthritis: A qualitative study. Nurs Health Sci. 2006;8:133–9.
De la Torre-Aboki J. Aportación de la consulta de enfermería en el manejo del paciente con artritis reumatoide. Reumatol Clin. 2011;6(S3):S16–9.
De Jorge M, Parra S, De la Torre-Aboki J, Herrero-Beaumont G. Randomized clinical trials as reflexive-interpretative process in patients with rheumatoid arthritis: a qualitative study. Rheumatol Int. 2015;35(8):1423–30. https://doi.org/10.1007//S00296-015-3218-0.
Child S, Goodwin VA, Perry MG, Gericke C, Byng R. Implementing a patient-initiative review system in rheumatoid arthritis: a qualitative evaluation. BMC Health Serv Res. 2015;15:157.
Barr PJ, Scholl I, Bravo P, Faber MJ, Elwyn G, McAllister M. Assessment of patient empowerment: A systematic review of measures. PLoS One. 2015;10(5):e0126553. https://doi.org/10.1371/journal.pone.0126553.
Doubova SV, Espinosa-Alarcón P, Infante C, Aguirre-Hernández R, Rodríguez-Aguilar R, Olivares-Santos R, et al. Adaptation and validation of scales to measure self-efficacy and empowerment for self-care in Mexican climateric stage women. Salud Pública Mex. 2013;55(3):257–66.
Serrani Azcurra DJL. Elders health empowerment scale. Spanish adaptation and psychometric analysis. Columbia Médica. 2014;45:179–85.
Katz JN, Lyons N, Wolff LS, Silverman J, Emrani P, Holt HL, et al. Medical-decision making among Hispanics and non-Hispanics with chronic back and knee pain: A qualitative study. BMC Musculoskelet Disord. 2011;12:78.
Barrio-Cantalejo IM, Simón-Lorda P, Melguizo M, Escalona I, Marijuán MI, Hernando P. Validación de la escala INFLESZ para evaluar la legibilidad de los textos dirigidos a pacientes. Anales Sis San Navarra. 2008;31(2):135–52.
Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83.
Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42.
Fleiss J. The design and analysis of clinical experiments. New York: Wiley; 1986.
Pett MA, Lackey NR, Sullivan JJ. Making sense of factor analysis: The use of factor analysis for instrument development in health care research. Thousand Oaks: Sage Publications Inc; 2003.
Bland J, Altman D. Statistics notes: Cronbach’s alpha. BMJ. 1997;314:275.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63.
Cardiel MH, Latin American Rheumatology Associations of the Pan American League of Associations for Rheumatology (PANLAR); Grupo Latino Americano de Estudio de Artritis Reumatoide GLADAR). First Latin-American position paper on the pharmacological treatment of rheumatoid arthritis. Rheumatology (Oxford). 2006;45(Suppl 2):ii7–ii22.
Allam A, Kostova z NK, Schulz PJ. The effect of social support features and gamification on a web-based intervention for rheumatoid arthritis patients: Randomized controlled trial. J Med Intern Res. 2015;17(1):e14.
van der Vaart R, Drossaert CHC, Taal E, Drossaers-Bakker KW, Vonkeman HE, van der Laar MAFJ. Impact on patient-accessible electronic medical records in rheumatology: use, satisfaction and effects on empowerment among patients. BMC Musculoskeletal Dis. 2014;15:102.
Acknowledgements
None.
Funding
This work was carried out with a grant from UCB Mexico Biopharmaceutical Company.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author (VPR). The data are not publicly available due to them containing information that could compromise research participant consent.
Author information
Authors and Affiliations
Contributions
ICY participated in the conception and design of the study and performed the statistical analysis. ERM participated in the conception of the study and reviewed the manuscript. LCHR participated in the conception of the study and reviewed the manuscript. VPR participated in the conception and design of the study; performed the statistical analysis, and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
All authors read and approved this manuscript.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán with the reference number IRE-2226-17/ 18-1. All necessary written consent was obtained from any patients involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial interests that could create a potential conflict of interest with regard to the work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Items head-to-head comparison between the S-HES and the RAEH. Table shows the items in Spanish and English of the S-HES and RAEH scales. (PDF 304 kb)
Additional file 2:
Figure S1. Rheumatoid Arthritis Empowerment Scale for Hispanic patients (RAEH). Final version of the Spanish version of the Health Empowerment Scale for RA Hispanic patients used during the validation process. (PDF 568 kb)
Additional file 3:
Table S2. Comparison of SWE-RES-23, S-HES and RAEH psychometric validation properties. Table shows the statistics of the tests carried out in the validation of the SWE-RES-23, S-HES, and RAEH scales. (PDF 301 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Contreras-Yáñez, I., Ruiz-Medrano, E., Hernández, L.d.R. et al. Psychometric validation of an empowerment scale for Spanish-speaking patients with rheumatoid arthritis. Arthritis Res Ther 20, 244 (2018). https://doi.org/10.1186/s13075-018-1741-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-018-1741-6