Abstract
Background
The eutherian IGF2R imprinted domain is regulated by an antisense long non-coding RNA, Airn, which is expressed from a differentially methylated region (DMR) in mice. Airn silences two neighbouring genes, Solute carrier family 22 member 2 (Slc22a2) and Slc22a3, to establish the Igf2r imprinted domain in the mouse placenta. Marsupials also have an antisense non-coding RNA, ALID, expressed from a DMR, although the exact function of ALID is currently unknown. The eutherian IGF2R DMR is located in intron 2, while the marsupial IGF2R DMR is located in intron 12, but it is not yet known whether the adjacent genes SLC22A2 and/or SLC22A3 are also imprinted in the marsupial lineage. In this study, the imprinting status of marsupial SLC22A2 and SLC22A3 in the IGF2R imprinted domain in the chorio-vitelline placenta was examined in a marsupial, the tammar wallaby.
Results
In the tammar placenta, SLC22A3 but not SLC22A2 was imprinted. Tammar SLC22A3 imprinting was evident in placental tissues but not in the other tissues examined in this study. A putative promoter of SLC22A3 lacked DNA methylation, suggesting that this gene is not directly silenced by a DMR on its promoter as seen in the mouse. Based on immunofluorescence, we confirmed that the tammar SLC22A3 is localised in the endodermal cell layer of the tammar placenta where nutrient trafficking occurs.
Conclusions
Since SLC22A3 is imprinted in the tammar placenta, we conclude that this placental imprinting of SLC22A3 has been positively selected after the marsupial and eutherian split because of the differences in the DMR location. Since SLC22A3 is known to act as a transporter molecule for nutrient transfer in the eutherian placenta, we suggest it was strongly selected to control the balance between supply and demand of nutrients in marsupial as it does in eutherian placentas.
Similar content being viewed by others
Background
Genomic imprinting is a complex epigenetic process that leads to expression of a subset of genes in a parent-of-origin specific way [1, 2]. Amongst vertebrates, this phenomenon has been found in therian mammals (eutherians and marsupials), but there is no evidence for imprinting so far in monotreme mammals or non-mammalian vertebrates [3,4,5,6]. Since many imprinted genes function in the mammalian placenta, genomic imprinting is thought to have evolved in concert with mammalian placentation [7,8,9,10,11,12]. In this context, the ‘supply and demand’ theory suggests that imprinted genes in the placenta evolved to regulate the balance of nutritional interactions between mother and the fetus carrying father’s genes by controlling the supply of nutrients [13]. However, postnatal imprinting has been characterised in both marsupials and eutherians [14,15,16,17], so imprinting is involved not only in placentation, but also in postnatal growth and development. The question remains as to how and why genomic imprinting evolved only in therian mammals amongst vertebrates. As imprinting must have evolved in the common ancestor of therian mammals, by comparing the characteristics of imprinting between marsupials and eutherians, we may be able to identify ancestral features of imprinting and understand how it evolved.
Imprinted genes of eutherians tend to be clustered in the genome [18, 19]. The grouping of imprinted genes within clusters allows sharing of common regulatory elements such as non-coding RNAs and differentially methylated regions (DMRs). When these regulatory elements control the imprinting status of clustered genes, they are known as imprinting control regions (ICRs). In eutherians, one of the best characterised imprinting clusters is the Insulin-like growth factor 2 receptor (IGF2R) gene locus [20,21,22,23,24,25,26]. In mice, the Igf2r gene is a paternally imprinted (maternally expressed) gene, and its imprinting is regulated by an intrinsic CpG island, which is a DMR, and the antisense long non-coding (lnc) RNA, Airn [23, 25,26,27,28]. The DMR is established during gametogenesis and has differential epigenetic modifications between gametes [29, 30]. It acts as a promoter of the lncRNA, Airn [27]. Airn is a maternally imprinted (paternally expressed) gene and silences Igf2r expression on the paternal genome by transcriptional overlap [23]. This lncRNA also regulates the imprinting of a 10 + Mb region that includes two neighbouring genes in the placenta, the solute carrier family 22 members 2 and 3 (Slc22a2 and Slc22a3) [22, 28, 31] as well as seven distal genes more than 2 Mb away from the Igf2r locus [32]. Airn establishes epigenetic silencing of Slc22a3 by recruiting a histone modification enzyme, euchromatin histone methyltransferase 2 (EHMT2, also known as G9a) [31]. Although the exact mechanism by which Airn inactivates the paternal Slc22a2 is currently unknown, Airn may recruit histone 3 lysine 27 trimethylation (H3K27me3) and the polycomb repressive complex 2 (PRC2) to the gene locus [22]. Therefore, the DMR at the intrinsic CpG Island which controls Airn expression is the ICR of the Igf2r imprinted domain in mice [20, 25, 26]. Although the lncRNA-based mechanisms for establishing Igf2r imprinted domain in mice are well documented, imprinting of IGF2R and its neighbouring genes in other eutherian mammals is different from that of mice. In cows, IGF2R, AIRN, SLC22A2 and SLC22A3 are imprinted in their placentas [33]. Although there is no information about imprinting of SLC22A2 and SLC22A3, IGF2R is imprinted in sheep and dogs [34, 35]. In humans, genes of the IGF2R imprinted domain show an indicator of imprinting, but it is polymorphically imprinted in a subset of placentas [36]. In contrast, in pigs, the evidence is confused since in one study there is paternal IGF2R expression [37], in a second study there is maternal IGF2R expression [38] and in the third study there is bi-allelic expression [39]. SLC22A3 is not imprinted in their placentas [40]. Therefore, while the IGF2R imprinted domain is likely to have been present in the common ancestor of eutherian mammals it may not have been strongly selected in some species such as pigs. Whether the IGF2R imprinted domain evolved in a marsupial ancestor or developed by convergent evolution in marsupial lineages is currently unknown. Comparing the IGF2R gene locus between marsupials and eutherians would clarify its evolution.
The marsupial IGF2R gene is also imprinted [41,42,43]. However, until recently, it was assumed that IGF2R in marsupials lacked key regulatory features such as Airn and a DMR [41, 43] as there was no DMR at intron 2, the location of the mouse Igf2r DMR/ICR. However, we described a novel DMR in intron 12 which has a 687 bp antisense lncRNA, Antisense LncRNA in IGF2RDMR (ALID) [43]. The DMR location is totally different between eutherians (intron 2) [26, 30] and marsupials (intron 12) [43]. ALID is much shorter than mouse Airn [43], so the imprinting mechanism of IGF2R in marsupials may be different from that of mouse Igf2r. However, it is still possible that ALID may have an analogous mode of action in silencing the flanking genes SLC22A2/SLC22A3 in the placenta. If SLC22A2 and/or SLC22A3 are imprinted in the marsupial lineage, it would provide strong evidence that placenta-specific imprinting has been strongly selected for therian mammals, despite the differences in the DMR location.
In this study, the imprinting status of SLC22A2 and SLC22A3 in marsupial placentas was investigated using a marsupial, the tammar wallaby (Macropus eugenii). The tammar has an epithelio-chorial placenta which consists of two regions, the avascular bilaminar omphalopleure (BOM) and the vascular trilaminar omphalopleure (TOM) separated by the terminal blood vessel, the sinus terminalis [44]. Marsupial orthologues of SLC22A2 and SLC22A3 were investigated by combining molecular experiments and analysis of tammar transcriptome data sets. Imprinting analysis was thereafter performed in the tammar placentas and other tissues to confirm its imprinting. We further evaluated protein localisation to determine conserved transporter function. Here, we report that SLC22A3 but not SLC22A2 is imprinted in the tammar placenta. By confirming SLC22A3 imprinting, this study revealed that the IGF2R imprinted domain has been strongly selected in marsupial mammals after either transposition or independent acquisition of the marsupial IGF2R DMR.
Results
Identification of marsupial SLC22A2
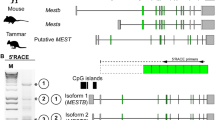
To characterise the imprinting cluster of the marsupial IGF2R gene locus, the marsupial orthologue of SLC22A2 was searched for using the wallaby genome database (Wallabase: https://wallabase.science.unimelb.edu.au/) comparing with mouse SLC22A2 (Accession number: NM_013667.3). 1693 bp of putative tammar SLC22A2 were identified. The putative tammar SLC22A2 was found in the vicinity of the tammar IGF2R gene. Furthermore, another SLC22A family gene, the SLC22A1 gene candidate, was found between the putative SLC22A2 and IGF2R, as seen in eutherians. Based on this conserved synteny, we considered the putative SLC22A2 as a candidate gene for our downstream analysis. While mouse and human SLC22A2 have 11 exons including 5ʹ and 3ʹ UTRs, the putative tammar SLC22A2 had 9 exons without a 3ʹ UTR (Fig. 1A). To characterise potential isoforms of SLC22A2 in placentas, full-length of tammar SLC22A2 transcripts were examined by 5' and 3'. Rapid amplification of cDNA ends (RACE) reactions using BOM cDNA (Fig. 1B). While the 5ʹ RACE reaction produced a distinct single band, the 3' RACE reactions produced multiple bands depending on primers used (Fig. 1B). By comparing three different 3' RACE reactions, we confirmed that only the largest RACE product contained partial SLC22A2 with a poly-A tail and upstream polyadenylation signals (Fig. 1B). The identified full-length tammar SLC22A2 contained 11 exons and encoded 554 a.a (Fig. 1B). The identified tammar SLC22A2 had a high consensus with mouse Slc22a2 (similarity: 90%, identity: 79%) and human SLC22A2 (similarity: 88%, identity: 78%). The tammar SLC22A2 shared the major facilitator superfamily (MFS) domain with mouse Slc22a2 and human SLC22A2 (Fig. 1C).
Identification of marsupial orthologue of SLC22A2 in the tammar. A Exon structure of mouse Slc22a2, human SLC22A2 and tammar putative SLC22A2. Black boxes and white boxes represent protein-coding exon and UTRs, respectively. B 5ʹ and 3ʹ RACE primers and RACE results. Full-length of tammar SLC22A2 encoding 554 a.a. was determined by RACE experiments. Asterisks indicate RACE product containing partial SLC22A2 sequences. 3ʹ RACE product contained poly-A signal (red-letters) and poly-A tail (green letters). C Protein alignment. Red-coloured highlight represents the major facilitator superfamily (MFS) domain of the tammar SLC22A2
The tammar SLC22A2 is not imprinted in placenta tissues
In order to identify potential single nucleotide polymorphism (SNP) sites, publically available tammar transcriptome data sets were analysed (SRA accession number: DRP001145). After analysing several adult tissues (testis, liver, lung, heart, spleen and brain), transcriptome data derived from liver were confirmed to have mapped-reads to the SLC22A2 exons which were confirmed by the RACE experiments. Fortunately, the liver transcriptome data had an informative SNP at the last exon of SLC22A2 (Fig. 2A). The C/G SNP was further confirmed by PCR using fetal gDNA followed by direct sequencing of the PCR products. After confirming the heterozygous C/G SNP in fetal gDNA, allelic expression analysis of SLC22A2 transcript was performed. In both BOM and TOM tissues, SLC22A2 clearly showed bi-allelic expression (Fig. 2B).
Tammar SLC22A2 is not imprinted in placenta tissues. A Detecting a single nucleotide polymorphism (SNP) in tammar SLC22A2 using liver transcriptome data. A SNP candidate (C/G) was detected at the 3'UTR of the last exon of tammar SLC22A2. The grey-coloured graph represents mapped RNA-seq reads. Black box with a line represents exon structure of the gene. B Allelic expression analysis of tammar SLC22A2 in BOM and TOM tissues by direct sequencing followed by PCR amplification
Identification of marsupial SLC22A3
To characterise the imprinting cluster of marsupial IGF2R gene locus, marsupial orthologue of SLC22A3 was searched for using the wallaby genome database (Wallabase: https://wallabase.science.unimelb.edu.au/) with mouse SLC22A3 (Accession number: NM_011395.2). 1692 bp of putative tammar SLC22A3 were identified. The putative tammar SLC22A3 was found next to the tammar SLC22A2 gene, as seen in eutherians. Based on this conserved synteny, we considered the putative SLC22A3 as a candidate gene for our downstream analysis. While mouse and human SLC22A3 have 11 exons including 5ʹ and 3’ʹ UTRs, the putative tammar SLC22A3 had 11 exons without the 3ʹ UTR (Fig. 3A). To characterise potential isoforms of SLC22A3 in placentas, full-length of tammar SLC22A3 transcripts were examined by 5' and 3' RACE reactions using BOM cDNA (Fig. 3B). While the 5’RACE reaction produced a distinct single band, the 3' RACE reactions produced two different RACE products (Fig. 3B). After cloning and sequencing each RACE product, two different isoforms were confirmed. Both isoforms had poly-A tail and upstream polyadenylation signals (Fig. 3B). Although there were at least two isoforms based on the differences in the exon structure, all isoforms encoded the same 563 amino acids. The identified tammar SLC22A3 had a high homology with mouse Slc22a3 (similarity: 89%, identity: 81%) and human SLC22A3 (similarity: 91%, identity: 84%). The tammar SLC22A3 shared the MFS domain with mouse and human SLC22A3 (Fig. 3B and C).
Identification of marsupial orthologue of SLC22A3 in the tammar. A Exon structure of mouse Slc22a3, human SLC22A3 and tammar putative SLC22A3. Black boxes and white boxes represent protein-coding exon and UTRs, respectively. B 5’ and 3’ RACE primers and RACE results. Two isoforms of tammar SLC22A3 was determined by RACE experiments, and both encoded 563 a.a. Asterisks indicate RACE product containing partial SLC22A3 sequences. 3ʹ RACE products contained poly-A signal (red-letters) and poly-A tail (green letters). C Protein alignment. Red-coloured highlight represents the major facilitator superfamily (MFS) domain of the tammar SLC22A3
The tammar SLC22A3 is imprinted in bilaminar placenta tissues
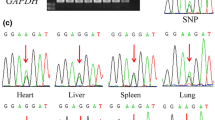
In order to identify potential SNP sites, published tammar transcriptome data sets were analysed. After analysing several adult tissues (testis, liver, lung, heart, spleen and brain), transcriptome data sets derived from heart and spleen were confirmed to have mapped-reads matched the SLC22A3 exons based on the RACE experiments. Fortunately, both heart and spleen transcriptome data had an informative SNP at the first exon of SLC22A3 (Fig. 4A). After confirming the T/G SNP by PCR reaction, allelic expression analysis of SLC22A3 transcripts was performed using the SNP information. Of the 20 biological replicates examined in this study, 3 samples had a clear heterozygous SNP. In BOM tissues (n = 3), SLC22A3 showed strongly skewed allelic expression with the signal intensities that differed between the two alleles more than fivefold and 2 out of the 3 animals showed clear maternal expression as the mothers had homozygous SNP (Fig. 4B). The other animal also showed a strongly skewed allelic expression (Fig. 4B). However, since the mother of the animal was heterozygous, we could not conclude its parental origin-specific expression. The trilaminar placenta tissues of the same animals did not show the same strongly biased allelic expression as seen in the BOM tissue (Fig. 4C).
Tammar SLC22A3 is imprinted in BOM tissues. A Detecting a single nucleotide polymorphism (SNP) in tammar SLC22A3 using heart and spleen transcriptome data. A SNP candidate (T/G) was detected at the first exon of tammar SLC22A3. The grey-coloured graph represents mapped RNA-seq reads. Black box with a line represents exon structure of the gene. B Allelic expression analysis of tammar SLC22A3 by direct sequencing followed by PCR amplification. Animal#1 and Animal#2 showed clear maternal expression in BOM tissues
SLC22A3 has variable expression in pouch young heart and spleen
Since mouse Slc22a3 imprinting is only observed in the placenta [20], the tammar SLC22A3 may have a similar tissue-specific imprinting pattern. To confirm tissue-specific imprinting of SLC22A3 in the tammar, allelic expression of SLC22A3 was investigated using the tammar pouch young (PYs). Heart and spleen tissues derived from PYs were used for this analysis as the transcriptome data showed SLC22A3 expression in those tissues. After checking 12 animals, 6 animals had the heterozygous T/G SNP. In all of the 6 animals, SLC22A3 showed bi-allelic expression in heart (Fig. 5). In spleen, while two males had skewed expression with signal intensities that differed between the two alleles more than twofold, the other males and the three females showed bi-allelic expression (Fig. 5).
Biallelic expression of SLC22A3 in the tammar heart and spleen. Allelic expression analysis of tammar SLC22A3 in heart and spleen was examined in 6 PYs (3 males and 3 females) by direct sequencing followed by PCR amplification. In heart, all animals showed bi-allelic expression of SLC22A3. However, in spleen, two males had skewed expression while the other male and females showed bi-allelic expression of SLC22A3
SLC22A3 imprinting is not promoter DNA methylation dependent
Given the imprinting of tammar SLC22A3 in the tammar placenta, we next analysed DNA methylation at its promoter to ask whether tammar SLC22A3 imprinting is DMR dependent or not. Based on the MethPrimer programme, we identified two CpG islands located over the putative promoter region of the tammar SLC22A3 (Fig. 6). Bisulphite sequencing of the CpG islands demonstrated that the majority of CpG sites were unmethylated in the entire region of the putative SLC22A3 promoter in both BOM and TOM tissues (Fig. 6).
Tammar SLC22A3 lacked promoter DNA methylation. DNA methylation of the putative promoter of SLC22A3 in the tammar BOM and TOM placenta. Aqua-coloured region represent CpG islands determined by MethPrimer programme. DNA methylation of the two CpG islands were examined by Bisulphite sequencing. Filled and opened circle represent methylated and unmethylated cytosine residue, respectively
SLC22A3 is present in endodermal cells of the BOM and TOM
To determine its conserved role in placental tissues, protein localisation of SLC22A3 in the BOM and TOM was examined (Fig. 7). SLC22A3 was localised at the luminal surface of endometrial tissues (Fig. 7B). SLC22A3 protein was detected in the endodermal cells of both BOM and TOM placental tissues (Fig. 7C).
Tammar SLC22A3 localised at the placental endodermal cells. A An example of the early tammar fetus and placenta. Schematic diagram below the photo represents tammar placental structure and placental cell types. While the bilaminar omphalopleure (BOM) is the non-vascularised part of placenta, the trilaminar omphalopleure (TOM) is the vascularised part of placenta. The sinus terminalis (ST) is the blood vessel that marks the boundary between the vascular and non-vascular regions, BOM and TOM contains trophoblast cells (Tr) and endodermal cells (En). The mesodermal (Me) layer of TOM has red blood cells (RBC) in the developing blood vessels. B Immunofluorescence with IgG negative control. C Immunofluorescence of SLC22A3 in BOM placenta tissue. D Immunofluorescence of SLC22A3 in TOM placenta tissue. Red and blue colours represent SLC22A3 and DAPI staining, respectively. Right-hand side panels show high power photos. Scale bars: 100 μm and 40 μm (high power and low power) panels. Endo: endometrium
Discussion
In the tammar placentas, SLC22A3 but not SLC22A2 was imprinted. As in the mouse, tammar SLC22A3 imprinting was evident in BOM placenta tissues but not in the other tissues examined in this study. We conclude that the SLC22A3 imprinting in mammalian placentas evolved within the IGF2R imprinted domain in both marsupial and eutherian mammals.
SLC22A3 is a poly-specific organic cation transporter that transfers a wide variety of substrates and toxins across the cell membrane [45,46,47]. In Slc22a3 knockout mice, although there are no obvious placental and fetal growth defects, there are effects on placental transfer functions [48]. In concert with its transporter activity, mouse Slc22a3 is localised in the labyrinth layer of its chorio-allantoic placenta [49,50,51] where nutrient transfer from maternal blood occurs [52]. Mouse Slc22a3 is also present in the visceral endoderm of the yolk sac [51], where nutrient uptake into the embryo occurs [53]. In the tammar chorio-vitelline placenta, BOM appears to be responsible for a greater uptake of nutrients from uterine secretions than TOM [54,55,56,57]. SLC22A3 imprinting was evident in BOM tissues, suggesting that SLC22A3 imprinting is associated with uptake of nutrients from uterine secretions by the avascular BOM. Based on immunofluorescence, we confirmed that the tammar SLC22A3 is localised in the endodermal cell layer of both BOM and TOM side of the tammar yolk sac (chorio-vitelline) placenta. The tammar placental endodermal cells are also derived from trophoblast, and in this and the mouse study [51] SLC22A3 expression in the endoderm cells of the yolk sac is conserved between mouse and the tammar. Furthermore, since a labyrinth layer marker, GCM1, is also present in the endodermal cell layer of the tammar placenta [56, 58], our data suggest that SLC22A3 is a conserved nutrient transporter in therian placentas and supports the previous suggestions [56, 59] that the endodermal cell layer functions as a centre of nutrient trafficking in the tammar placenta.
In the tammar placenta, the SLC22A3 imprinting was evident only in BOM tissues. However, it is currently unknown why only BOM tissues but not TOM tissues show imprinted SLC22A3 expression in the tammar placenta. In concert with their functional role in either nutrient transport (BOM) or respiration (TOM) [44, 57], BOM and TOM have different transcriptional profiles [56]. This suggests that BOM tissues are differentially transcriptionally regulated, resulting in imprinted expression of SLC22A3. Alternatively, other cells such as mesodermal cells and nucleated red blood cells in TOM tissues may express the SLC22A3 gene. In mice, Slc22a3 is highly expressed in the visceral endoderm and is present, albeit at low expression, in the mesoderm cells of the visceral yolk sac [51], so, it is possible that mesoderm cells masks SLC22A3 imprinted expression in the tammar TOM.
The mouse Igf2r imprinted domain is regulated by the lncRNA, Airn [20, 28, 31]. The Airn transcript recruits a histone modification enzyme, EHMT2, and adds an inactive histone-3 lysine-9 di-methylation at the Slc22a3 gene locus [31]. In addition, Airn recruits H3K27me3 to Slc22a2 and Slc22a3 to establish the broad imprinted domain in mice [22], and there is no promoter DNA methylation at either Slc22a2 or Slc22a3 in mice [20, 51], suggesting that both are regulated by histone modification-based imprinting. Similarly, human SLC22A2 and SLC22A3 also lack a promoter DMR in placenta [36]. Like the tammar, the cow has a chorio-epithelial placenta, and has SLC22A3 but not SLC22A2 with a DMR at the promoter region in its placenta [33]. Since AIRN is conserved across eutherians [33, 60], the bovine study indicates that SLC22A3 imprinting mechanism can be varied even within eutherian mammals. In our analysis, CpG islands at the putative promoter of the tammar SLC22A3 lacked DNA methylation. This suggests that tammar SLC22A3 imprinting is not directly silenced by a DMR on its promoter as in mice and humans. In this context, the tammar lncRNA, ALID, is likely to have a similar function to Airn as this lncRNA is also expressed from a DMR which may be an ICR [43]. Further analysis of the interactions between EHMT2, PRC2 and ALID would be interesting in subsequent studies to determine potential function of ALID in silencing SLC22A3 in the tammar placenta.
In marsupials, the IGF2R DMR is located in intron 12 while the eutherian IGF2R DMR is located in intron 2 [26, 43, 61] (Fig. 8A). Based on this difference in genomic position, marsupial and eutherian IGF2R DMRs may have been translocated after the divergence of marsupials and eutherians (Fig. 8B and C), or they may have been acquired independently in each mammalian lineage after the marsupial–eutherian split (Fig. 8D) [43]. In either case, our study demonstrates that the SLC22A3 imprinting in placentas has been strongly selected in therian mammals during mammalian evolution (Fig. 8). Since SLC22A3 is a poly-specific transporter in placental tissues that regulates placental transfer, this selection may have occurred to control the balance between supply and demand of nutrients.
The evolution of the IGF2R imprinted domain and the intragenic DMR. A Schematic diagram of the tammar IGF2R imprinted domain and mouse Igf2r imprinted domain. The IGF2R DMR is located in intron 12 in the tammar whereas the eutherian IGF2R DMR is located in the promoter and intron 2. Red-coloured boxes and blue-coloured boxes represent maternally expressed genes and paternally expressed genes, respectively. White and grey-coloured boxes represent non-imprinted and silenced genes, respectively. Arrows indicate the direction of transcription. There are three scenarios for the evolution of IGF2R DMR and SLC22A3 imprinting in therian mammals. A Transposition of the IGF2R DMR in marsupials. In this case, the IGF2R DMR evolved in the common ancestor of therian mammals and the DMR acts as an ICR to establish epigenetic silencing of SLC22A2 and SLC22A3 as in mice. Even after the transposition, the SLC22A3 imprinting but not SLC22A2 was positively maintained in marsupial lineage. B Transposition of the IGF2R DMR in eutherians. In this case, the IGF2R DMR evolved in the common ancestor of therian mammals and the DMR acts as an ICR to establish epigenetic silencing of SLC22A3 as in the tammar. After the transposition, the SLC22A2 imprinting evolved in eutherian lineage. C Independent acquisition of the IGF2R DMR in each mammalian lineage. In this case, the evolution of SLC22A3 imprinting would have accompanied the de novo acquisition of IGF2R DMR in each mammalian lineage. Yellow and red lines indicate that SLC22A2 imprinting and SLC22A3 imprinting, respectively
Conclusions
By confirming imprinting of the SLC22A3 gene in a marsupial placenta, our data suggest the SLC22A3 imprinting has been strongly selected in both marsupials and eutherians the differences in the DMR locations. Our data further suggest that the control of nutrient transport in the placenta is a critical evolutionary function of genomic imprinting in marsupial, as well as in eutherian placentas.
Material and methods
Animals
Tammar wallabies (Macropus eugenii), of Kangaroo Island origin, were held in open grassy yards in our breeding colony at the University of Melbourne. Adults and pouch young (PY) were killed humanely as previously described [14, 55, 57]. Placentas from fetuses in the final third of gestation [day 19–25 days of the 26.5 day pregnancy (n = 20)] were collected post mortem from adult females and either snap frozen or fixed in 4% (w/v) paraformaldehyde (PFA) in phosphate buffered saline (PBS) (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH7.4), washed and stored in 100% methanol before histological analysis. PY aged between day 33 and day 81 after birth (n = 12) were dissected post mortem and heart and spleen snap frozen immediately. All animal experiments were approved by the University of Melbourne Animal Experimental Ethics Committees and followed the Australian National Health and Medical Research Council (2013) guidelines.
RNA extraction and cDNA synthesis
Snap-frozen placenta tissues consisting of the separated avascular bilaminar omphalopleure (BOM) and the vascular trilaminar omphalopleure (TOM) (see Fig. 7A), PY spleen and PY heart were used for RNA extraction using the GenElute Mammalian total RNA Miniprep Kit (Sigma-Aldrich, Missouri, USA) following the manufacturer’s instructions. The extracted RNA was treated with the DNA-free DNase treatment and removal kit (Thermo Fisher Scientific, Massachusetts, USA) to remove residual genomic DNA (gDNA). After confirming removal of gDNA by a Nanodrop (Thermo Fisher Scientific, Massachusetts, USA) and PCR using RNA as a template, either 200 ng of placenta RNA or up to 800 ng of PY spleen RNA or up to 400 ng of PY heart RNA were used as templates for cDNA synthesis using SuperScript IV First strand Synthesis System (Invitrogen, Carlsbad, USA).
5' and 3' rapid amplification of cDNA ends (RACE)
To determine the complete full sequence of marsupial SLC22A2 and SLC22A3, 5' and 3', rapid amplification of cDNA ends (RACE) experiments were performed using a SMARTer RACE 5'/3' kit (Clontech, California, USA). The first RACE reactions were performed with BOM cDNA using SeqAmp DNA Polymerase (Clontech, California, USA) with gene specific primers (Additional file 1). The nested 5' and 3' RACE reactions were performed by GoTaq DNA polymerase (Promega, Wisconsin, USA) and the RACE products were cloned using pGEM-T Easy Vector (Promega, Wisconsin, USA) and Escherichia coli JM109 competent cells (Promega, Wisconsin, USA). Plasmids were extracted using Wizard Plus SV Minipreps DNA Purification System (Promega, Wisconsin, USA) and directly sequenced with M13 primers (Additional file 1).
Comparative analysis of therian SLC22A2 and SLC22A3
DNA sequences of human SLC22A2, human SLC22A3, mouse Slc22a2 and mouse Slc22a3 were obtained from NCBI (https://www.ncbi.nlm.nih.gov). Amino acid sequences retrieved from DDBJ/EMBL/GenBank/RefSeq database were used for generating alignment using CLC sequence viewer 8 (https://resources.qiagenbioinformatics.com/manuals/clcsequenceviewer/current/index.php?manual=CLC_Sequence_Viewer_vs_Workbenches.html). Accession numbers: Homo sapiens SLC22A2, NM_003058.4; Mus musculus Slc22a2, NM_013667.3; H. sapiens SLC22A3, NM_021977.4; M. musculus Slc22a3, NM_011395.2. Functional domains of deduced amino acid sequences were examined with the Prosite server (http://prosite.expasy.org/).
Transcriptome analysis
To characterise the IGF2R imprinted domain as well as identifying informative single nucleotide polymorphisms (SNPs), tammar transcriptome data sets derived from various tissues (testis, liver, lung, heart, spleen and brain) were analysed. Publicly available tammar raw RNA-seq data sets (DRP001145) were downloaded from NCBI SRA (https://www.ncbi.nlm.nih.gov/sra). All RNA-seq reads were trimmed using TrimGalore! (v0.6.5) (https://github.com/FelixKrueger/TrimGalore) with default settings. The trimmed reads were aligned to the wallaby genome.v3 (https://wallabase.science.unimelb.edu.au) using HISAT2 (v2.1.0) [62] with a parameter—rna-strandness FR to reflect the strandedness of sequenced RNA. The mapped reads were assigned to each strand by Samtools (v1.9) [63]. SNP sites were called using BCFtools (v1.9) and the output file was compared with the mapped reads on Integrative genome viewer (IGV) [64, 65].
Genomic DNA extraction
Snap-frozen BOM, TOM, PY tails and endometrial tissues were used for genomic DNA (gDNA) extraction. BOM and TOM were the fetal gDNA source and endometrium was the maternal gDNA source. PY tails were used for genotyping. DNA extraction was performed with Wizard Genomic DNA purification kit (Promega, Wisconsin, USA) following the manufacturer’s instructions.
Allelic expression analysis
Extracted gDNA was used as a template for PCR amplification. PCR reaction was performed using gene specific primers (Additional file 1) with Go-Taq polymerase (Promega, Wisconsin, USA) under the following cycle conditions: 95 °C 30 s, 65 °C 30 s, and 72 °C 1 min. To analyse sequences of the tammar SLC22A2 and SLC22A3 transcripts, strand-specific cDNA synthesis was performed using each gene specific reverse primer (Additional file 1). The synthesised cDNA was used as a template for PCR amplification with Go-Taq polymerase (Promega, Wisconsin, USA) under the following cycle conditions: 95 °C 30 s, 65 °C 30 s, and 72 °C 1 min. After performing gel electrophoresis, confirmed PCR products from gDNA and cDNA were extracted and directly sequenced by Sanger sequencing to confirm SNP sites and allele specific expression. We consider a biased expression as a strongly skewed expression when the signal intensities between the two alleles differed more than fivefold.
Bisulphite sequencing
Purified genomic DNA derived from the fetus used for allelic expression analysis was treated with sodium bisulphite solution using EpiMark Bisulfite Conversion kit (New England Biolabs, Massachusetts, USA). After the bisulphite treatment of the genomic DNA, 40 cycles of PCR were carried out using EpiTaq polymerase (Takara Bio, Shiga, Japan) with the primers (Additional file 1) designed by MethPrimer (https://www.urogene.org/methprimer/) [66]. The PCR products were cloned using a pGEM T-easy vector (Promega, Wisconsin, USA) and E. coli JM109 competent cells (Promega, Wisconsin, USA). Plasmids were purified using Wizard Plus SV Minipreps DNA Purification System (Promega, Wisconsin, USA) and directly sequenced using M13 primers (Additional file 1). The sequence data were analysed by quantification tool for methylation analysis (QUMA) programme (http://quma.cdb.riken.jp) [67].
Immunofluorescence (IF) staining
PFA-fixed placenta samples (BOM and TOM at day 22 of gestation) were washed in 1 × PBS and re-hydrated through an ethanol series before being embedded in paraffin. Embedded samples were serially sectioned at 5 μm and mounted on poly-lysine coated slides (ThermoFisher Scientific, Massachusetts, USA). The sections were de-waxed, rehydrated through decreasing concentrations of ethanol, and then incubated in 0.1% (v/v) Triton X-100 in 1 × PBS (PBST) for 15 min at room temperature to permeabilise the tissue. Slides were boiled in Tris–EDTA (pH 8.0) for 20 min. The sections were treated with 0.3% (w/v) Sudan Black in 70% (v/v) EtOH solution for 15 min to reduce auto-fluorescent background. The Sudan Black treated sections were washed by 70% (v/v) EtOH and 1 × PBS before blocking. Thereafter, the sections were incubated for 1 h with 10% (w/v) goat serum diluted in 1 × PBS. After blocking, sections were incubated with primary antibody solution (Additional file 2) at 4 °C for 16 h. The following day, sections were washed three times with PBS and then incubated with fluorescent secondary antibodies (Additional file 2) for 1 h. The sections were washed three times with 1 × PBS again and then incubated for 10 min with 4', 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, Missouri, USA). DAPI-treated sections were mounted with fluorescence mounting solution. The controls for all treatments were no primary antibody and IgG isotype control antibodies. Images were collected on a Nikon A1R Confocal Laser Microscope System (Nikon, Tokyo, Japan).
Availability of data and materials
The datasets analysed during the current study are available in the NCBI SRA (https://www.ncbi.nlm.nih.gov/sra) as DRP001145.
Abbreviations
- DMR:
-
Differentially methylated region
- SLC22A2:
-
Solute carrier family 22 member 2
- SLC22A3:
-
Solute carrier family 22 member 3
- ICR:
-
Imprinting control region
- IGF2R:
-
Insulin-like growth factor 2 receptor
- lnc:
-
Long non-coding
- H3K27me3:
-
Histone 3 lysine 27 trimethylation
- PRC2:
-
Polycomb repressive complex 2
- ALID:
-
Antisense lncRNA in IGF2R DMR
- BOM:
-
Bilaminar omphalopleure
- TOM:
-
Trilaminar omphalopleure
- RACE:
-
Rapid amplification of cDNA ends
- a.a:
-
Amino acids
References
Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32.
Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–75.
Renfree MB, Hore TA, Shaw G, Marshall Graves JA, Pask AJ. Evolution of genomic imprinting: insights from marsupials and monotremes. Annu Rev Genomics Hum Genet. 2009;10:241–62.
Griffith OW, Brandley MC, Belov K, Thompson MB. Allelic expression of mammalian imprinted genes in a matrotrophic lizard Pseudemoia entrecasteauxii. Dev Genes Evol. 2016;226:79–85.
Pask AJ, Papenfuss AT, Ager EI, McColl KA, Speed TP, Renfree MB. Analysis of the platypus genome suggests a transposon origin for mammalian imprinting. Genome Biol. 2009;10:R1.
Frésard L, Leroux S, Servin B, Gourichon D, Dehais P, Cristobal MS, et al. Transcriptome-wide investigation of genomic imprinting in chicken. Nucleic Acids Res. 2014;42:3768–82.
Renfree MB, Ager EI, Shaw G, Pask AJ. Genomic imprinting in marsupial placentation. Reproduction. 2008;136:523–31.
Ono R, Nakamura K, Inoue K, Naruse M, Usami T, Wakisaka-Saito N, et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat Genet. 2006;38:101–6.
Kaneko-Ishino T, Ishino F. Retrotransposon silencing by DNA methylation contributed to the evolution of placentation and genomic imprinting in mammals. Dev Growth Differ. 2010;52:533–43.
Renfree MB, Suzuki S, Kaneko-Ishino T. The origin and evolution of genomic imprinting and viviparity in mammals. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120151.
Kaneko-Ishino T. The regulation and biological significance of genomic imprinting in mammals. J Biochem. 2003;133:699–711.
Hall JG. Genomic imprinting: review and relevance to human diseases. Am J Hum Genet. 1990;46:857–73.
Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, et al. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003;547:35–44.
Stringer JM, Suzuki S, Pask AJ, Shaw G, Renfree MB. Selected imprinting of INS in the marsupial. Epigenetics Chromatin. 2012;5:14.
Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, et al. The imprinted signaling protein XLαs is required for postnatal adaptation to feeding. Nat Genet. 2004;36:818–26.
Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Azim SM. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–9.
Keverne EB, Curley JP. Epigenetics, brain evolution and behaviour. Front Neuroendocrinol. 2008;29:398–412.
Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat Rev Genet. 2014;15:517–30.
Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet. 2005;21:457–65.
Zwart R, Sleutels F, Wutz A, Schinkel AH, Barlow DP. Bidirectional action of the Igf2r imprint control element on upstream and downstream imprinted genes. Genes Dev. 2001;15:2361–6.
Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014. https://doi.org/10.1101/cshperspect.a018382.
Andergassen D, Muckenhuber M, Bammer PC, Kulinski TM, Theussl HC, Shimizu T, et al. The Airn lncRNA does not require any DNA elements within its locus to silence distant imprinted genes. PLoS Genet. 2019. https://doi.org/10.1371/journal.pgen.1008268.
Latos PA, Pauler FM, Koerner M, v., Şenergin HB, Hudson QJ, Stocsits RR, et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–72.
Sleutels F, Tjon G, Ludwig T, Barlow DP. Imprinted silencing of Slc22a2 and Slc22a3 does not need transcriptional overlap between Igf2r and Air. EMBO J. 2003;22:3696–704.
Santoro F, Mayer D, Klement RM, Warczok KE, Stukalov A, Barlow DP, et al. Imprinted Igf2r silencing depends on continuous Airn lncRNA expression and is not restricted to a developmental window. Development. 2013;140:1184–95.
Wutz A, Smrzka OW, Schweifer N, Schellander K, Wagner EF, Barlow DP. Imprinted expression of the Igf2r gene depends on an intronic CpG island. Nature. 1997;389:745–9.
Braidotti G, Baubec T, Pauler F, Seidl C, Smrzka O, Stricker S, et al. The Air noncoding RNA: an imprinted cis-silencing transcript. Cold Spring Harb Symp Quant Biol. 2004;69:55–66.
Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–3.
Ishihara T, Griffith OW, Suzuki S, Renfree MB. Presence of H3K4me3 on paternally expressed genes of the paternal genome from sperm to implantation. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.838684.
Hiura H, Obata Y, Komiyama J, Shirai M, Kono T. Oocyte growth-dependent progression of maternal imprinting in mice. Genes Cells. 2006;11:353–61.
Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–20.
Andergassen D, Dotter CP, Wenzel D, Sigl V, Bammer PC, Muckenhuber M, et al. Mapping the mouse allelome reveals tissue-specific regulation of allelic expression. Elife. 2017. https://doi.org/10.7554/eLife.25125.
Liu X, Huo H, Jin L, Dong Y, Li D, Zhang C, et al. Genomic imprinting of the IGF2R/AIR locus is conserved between bovines and mice. Theriogenology. 2022;180:121–9.
Young LE, Schnieke AE, McCreath KJ, Wieckowski S, Konfortova G, Fernandes K, et al. Conservation of IGF2-H19 and IGF2R imprinting in sheep: effects of somatic cell nuclear transfer. Mech Dev. 2003;120:1433–42.
O’Sullivan FM, Murphy SK, Simel LR, McCann A, Callanan JJ, Nolan CM. Imprinted expression of the canine IGF2R, in the absence of an anti-sense transcript or promoter methylation. Evol Dev. 2007;9:579–89.
Monk D, Arnaud P, Apostolidou S, Hills FA, Kelsey G, Stanier P, et al. Limited evolutionary conservation of imprinting in the human placenta. Proc Natl Acad Sci. 2006;103:6623–8.
Wu YQ, Zhao H, Li YJ, Khederzadeh S, Wei HJ, Zhou ZY, et al. Genome-wide identification of imprinted genes in pigs and their different imprinting status compared with other mammals. Zool Res. 2020;41:721–5.
Bischoff SR, Tsai S, Hardison N, Motsinger-Reif AA, Freking BA, Nonneman D, et al. Characterization of conserved and nonconserved imprinted genes in swine. Biol Reprod. 2009;81:906–20.
Braunschweig MH. Biallelic transcription of the porcine IGF2R gene. Gene. 2012;500:181–5.
Yin Z, Zhang X, Li J, Jiao Y, Kong Q, Mu Y. Identification of imprinted genes and their differentially methylated regions in porcine. Russ J Genet. 2019;55:1488–98.
Weidman JR, Dolinoy DC, Maloney KA, Cheng JF, Jirtle RL. Imprinting of opossum Igf2r in the absence of differential methylation and Air. Epigenetics. 2006;1:49–54.
Killian JK, Byrd JC, Jirtle JV, Munday BL, Stoskopf MK, MacDonald RG, et al. M6P/IGF2R imprinting evolution in mammals. Mol Cell. 2000;5:707–16.
Suzuki S, Shaw G, Renfree MB. Identification of a novel antisense noncoding RNA, ALID, transcribed from the putative imprinting control region of marsupial IGF2R. Epigenetics Chromatin. 2018;11:55.
Renfree MB. Review: marsupials: placental mammals with a difference. Placenta. 2010;31:S21–6.
Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, et al. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem. 1998;273:15971–9.
Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, et al. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am J Physiol Renal Physiol. 2000;279:F449–58.
Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, et al. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–86.
Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3- deficient mice. Mol Cell Biol. 2001;21:4188–96.
Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J, et al. Sex- and diet-specific changes of imprinted gene expression and dna methylation in mouse placenta under a high-fat diet. PLoS ONE. 2010. https://doi.org/10.1371/journal.pone.0014398.
Verhaagh S, Barlow DP, Zwart R. The extraneuronal monoamine transporter Slc22a3/Orct3 co-localizes with the Maoa metabolizing enzyme in mouse placenta. Mech Dev. 2001;100:127–30.
Hudson QJ, Seidl CIM, Kulinski TM, Huang R, Warczok KE, Bittner R, et al. Extra-embryonic-specific imprinted expression is restricted to defined lineages in the post-implantation embryo. Dev Biol. 2011;353:420–31.
Woods L, Perez-Garcia V, Hemberger M. Regulation of placental development and its impact on fetal growth—new insights from mouse models. Front Endocrinol. 2018. https://doi.org/10.3389/fendo.2018.00570.
Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43:183–205.
Renfree MB, Tyndale-Biscoe CH. Intrauterine development after diapause in the marsupial Macropus eugenii. Dev Biol. 1973;32:28–40.
Renfree MB. Proteins in the uterine secretions of the marsupial Macropus eugenii. Dev Biol. 1973;32:41–9.
Guernsey MW, Chuong EB, Cornelis G, Renfree MB, Baker JC. Molecular conservation of marsupial and eutherian placentation and lactation. Elife. 2017. https://doi.org/10.7554/eLife.27450.
Renfree MB. The composition of fetal fluids of the marsupial Macropus eugenii. Dev Biol. 1973;33:62–79.
Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25:311–4.
Ager E, Suzuki S, Pask A, Shaw G, Ishino F, Renfree MB. Insulin is imprinted in the placenta of the marsupial Macropus eugenii. Dev Biol. 2007;309:317–28.
Yotova IY, Vlatkovic IM, Pauler FM, Warczok KE, Ambros PF, Oshimura M, et al. Identification of the human homolog of the imprinted mouse air non-coding RNA. Genomics. 2008;92:464–73.
Stöger R, Kubicka P, Liu CG, Kafri T, Razin A, Cedar H, et al. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71.
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–15.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9.
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6.
Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–92.
Li L-C, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31.
Kumaki Y, Oda M, Okano M. QUMA: quantification tool for methylation analysis. Nucleic Acids Res. 2008;36:W170–5.
Acknowledgements
We thank all members of the wallaby research group for help with the animals and the dissections. The authors thank the Melbourne University Biological Optical Microscopy Platform (BOMP) for assistance with the confocal microscopy.
Funding
This study was supported by a Melbourne Research Scholarship to TI and grants from the Australian Research Council to MBR and Prof. Geoff Shaw.
Author information
Authors and Affiliations
Contributions
TI, OWG, SS and MBR designed the research; TI performed experiments; TI and MBR collected the samples; TI, OWG, SS and MBR discussed the data, and TI and MBR wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Experimental procedures conformed to Australian National Health and Medical Research Council (2013) guidelines and were approved by the Animal Experimentation Ethics Committees of the University of Melbourne.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Primers used for this study.
Additional file 2.
Antibodies used for this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ishihara, T., Griffith, O.W., Suzuki, S. et al. Placental imprinting of SLC22A3 in the IGF2R imprinted domain is conserved in therian mammals. Epigenetics & Chromatin 15, 32 (2022). https://doi.org/10.1186/s13072-022-00465-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13072-022-00465-4