Abstract
Background
Aedes albopictus, commonly known as the Asian tiger mosquito, has become one of the most invasive mosquito species. Over the last 5 decades, it has been introduced and established in various tropical and temperate regions worldwide. First reported in Europe in 1979 in Albania and later in Italy in 1990, the species is now established in 13 European Union (EU)/European Economic Area (EEA) countries and 337 regions (2023). In Portugal, Ae. albopictus was first detected in the Algarve and Penafiel regions in 2017, followed by Alentejo in 2022 and Lisbon in 2023. This mosquito species poses a significant public health risk as a vector for numerous pathogenic viruses, including dengue, Zika, and chikungunya.
Methods
Aedes albopictus collected in Lisbon in 2023 were analyzed using cytochrome c oxidase I (COX) gene sequencing to understand their genetic relationships.
Results
Our data indicate that the Ae. albopictus mosquito populations detected in three locations in Lisbon in 2023 correspond to recent but distinct introduction events.
Conclusions
Although there has been no local transmission of Aedes-transmitted viruses in mainland Portugal to date, the spread of the mosquito and increased international travel increase the risk of Aedes-borne disease outbreaks. The ongoing spread of Ae. albopictus in the country and the confirmed multiple introductions in new locations raise awareness of the need to monitor mosquito vectors to control and prevent autochthonous Aedes-borne disease outbreaks.
Graphical Abstract

Similar content being viewed by others
Background
First described by Skuse in India in 1894, Aedes (Stegomya) albopictus has been recognized as one of the most invasive mosquito species, successfully colonizing numerous tropical and temperate regions worldwide over the last 5 decades. Aedes albopictus was first reported in Europe in 1979 in Albania [1], followed by Italy in 1990 [2]. Italy is currently considered the most infested country in Europe, with Ae. albopictus established over large areas and thriving particularly in urban areas [3]. Since its introduction in Italy, Ae. albopictus has steadily spread throughout Europe, particularly to most Mediterranean countries. In 2023, Ae. albopictus was established in 13 European Union (EU)/European Economic Area (EEA) countries and 337 regions, while in 2013, it was established only in 8 countries and 114 European regions [4]. In Portugal, Ae. albopictus was first detected in 2017 through two different introduction events in the Algarve, the southernmost region [5], and Penafiel, in the Porto region [6]. In 2022, this vector was detected in the Alentejo region and, in late, September 2023 in Lisbon [7]. The National Vector Surveillance Network-Rede de Vigilância de Vectores (REVIVE) has been running since 2008 under the auspices of the Portuguese Ministry of Health [8]. REVIVE conducts nationwide surveillance of the most critical hematophagous arthropods for public health (mosquitoes, ticks, and sandflies). Regular surveillance of mosquito species and screening field-collected mosquitoes for arboviruses is conducted. Airports, ports, storage areas, and certain border regions with Spain are monitored throughout the year with the involvement of local and regional authorities.
In addition to the nuisance associated with establishing Aedes albopictus, its ability to act as a vector for a wide range of arboviruses remains a primary concern. This mosquito species has emerged as a significant global public health threat due to its ability to transmit several pathogenic flaviviruses (such as dengue, Zika, and yellow fever) and alphaviruses (especially chikungunya virus). The central tourist regions in the country are the Algarve, Lisbon, and Porto, where the presence of this vector species can be a significant concern due to the higher risk of incoming viremic travelers. Since 2007, cases of autochthonous transmission of chikungunya associated with Ae. albopictus have been documented in Europe [9]. Dengue has been reported in Europe since 2010, with autochthonous cases transmitted by Ae. albopictus in Croatia, France, Italy, and Spain [10].
To date, local transmission of Aedes-borne viruses has not yet been detected in mainland Portugal. However, the geographic expansion of Ae. albopictus across Portugal, combined with the increasing number of international travelers, often from regions with ongoing Aedes-borne outbreaks, highlights the importance of mosquito vector surveillance and control and raises public health concerns about the risk of increased introduction and autochthonous transmission of Aedes-borne viral infections. Here, we report a preliminary genetic analysis of the Ae. albopictus mosquitoes detected in Lisbon in 2023, using the primary barcode sequence for members of the animal kingdom, a partial sequence of the cytochrome c oxidase I (COX) gene, widely used to study the genetic relationships of Ae. albopictus [11,12,13,14] and previously used in Portuguese mosquito populations [15].
Methods
Mosquito samples and DNA extraction
Larvae samples were collected in natural and artificial water containers at three sites in the Lisbon region (Algés, Alvalade, and São Domingos de Benfica; Table 1). Mosquito samples from the Algarve and Porto populations, collected in 2022 and 2023, were also submitted for analysis. All mosquito samples were collected by the national REVIVE surveillance network [6] in public and private properties, with the knowledge and permission of the respective responsible/owners. The collected mosquitoes were reared to adults in the insectary (by collection site and date), and 10 adult mosquitoes were randomly selected from each collection site for analysis. Sampled mosquitoes collected in the Lisbon region were ground individually by grinding with a mortar and pestle with liquid nitrogen and further ground after adding 500 µL lysis buffer (NUCLISENS® easyMAG, Biomérieux). Nucleic acid extraction was performed with the prepared lysate suspensions in the automated platform NUCLISENS® easyMAG (Biomérieux), as previously described [6]. Larval and adult mosquitoes were morphologically confirmed as Ae. albopictus [16, 17].
Molecular analysis
Molecular identification was performed using the COX gene of mitochondrial DNA with primers LCO1490 and HCO 2198 [18], as previously described [6]. Aedes albopictus haplotype diversity for COX sequences was estimated using DnaSP v.6.10.01 [19] using default parameters.
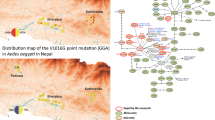
To integrate the mosquitoes circulating in Portugal and into the global Ae. albopictus genetic diversity, the consensus nucleotide partial sequences of the COX gene were aligned against several sequences available in GenBank (20 COX previously reported at the global level; Fig. 1) using BioEdit version 7.2.5 [20] and further used to construct a phylogenetic tree using the unweighted pair group method with arithmetic mean (UPGMA; 1000 bootstraps) in MEGA X [21]. For better visualization of the tree, the KX383935 sequence was used as an outgroup. Figure 1 was generated using BioRender.com.
A UPGMA phylogenetic tree constructed from 18 (9 novel) COX sequences obtained from mosquitoes circulating in Portugal (Table 1) and 20 sequences available in GenBank. Bootstrap values (1000 replicates) greater than 65 are shown above the branches, and branch lengths (in the same units as those of the evolutionary distances used to infer the phylogenetic tree) are shown below the branches. Evolutionary distances were calculated using maximum composite likelihood. Sequences are identified by GenBank accession numbers, country region, country, and year of collection (if available). Tree nodes are identified by the COX haplotype. Colored circles indicate the regions where mosquitoes from Portugal were collected. B Map showing the locations of mosquito sample collection and the most likely origin of introduction in Lisbon, based on available data. Composite figure created with https://www.biorender.com/
Results
The 10 partial COX sequences obtained for Ae. albopictus mosquitoes collected at each of the three sites in Lisbon (30 sequences in total) show low genetic diversity, with only one haplotype in Algés (GenBank accession number PP825984) and São Domingos de Benfica (GenBank accession number PP825985). In Alvalade, two haplotypes were identified, with one corresponding to nine collected mosquito specimens (GenBank accession number PP825983) and the other corresponding to only one (GenBank accession number PP825982). Sequences from mosquitoes collected in 2022 and 2023 in the Algarve and Porto regions are identical to previously detected haplotypes for these regions (Table 1; Fig. 1), which were identified as haplotype 1 (the Algarve and Porto) and haplotype 2 for the Algarve, as previously defined [15]. The haplotype diversity analysis for the sequences detected in the Lisbon region shows, in Alvalade, two haplotypes, haplotype 2 and haplotype 3, previously detected in the Algarve, and two new haplotypes for the other sites, haplotype 6 in Algés (with similarity to sequences from widespread sites) and haplotype 7 in São Domingos de Benfica, identical to sequences detected in Spain (Table 1 and Fig. 1).
Discussion
The observed low diversity of COX sequences in mosquitoes collected in Lisbon is consistent with recent introductions. Although the collection of immature mosquitoes may bias the population diversity, collections were made in heavily infested containers, corresponding to multiple female mosquito ovipositions. For most of these sites, the water containers containing immature mosquitoes were the only ones found with Ae. albopictus in the surveyed area. Some sites were analyzed following citizen science reports of mosquito presence, and others resulted from detailed inspections by the Lisbon and Tagus Valley Health Authority following the initial detection event.
The COX sequences from the three Lisbon sites differed, so these introductions can be considered separate events. Although preliminary, these data suggest that, at least in Alvalade, the introduction may have come from the Algarve. However, direct external introductions cannot be excluded, especially in São Domingos de Benfica and Algés. Nevertheless, more data are needed on the current mosquito populations circulating in Porto, the Algarve, and Alentejo. A finer genetic analysis, namely by mitogenome sequencing, with a broader sampling of mosquitoes in the coming season (2024) is underway to obtain more details. Nevertheless, the identification of three independent introduction events in one mosquito season highlights the potential of this species to invade new geographic areas in a short period. It also means that these events are likely underway and indicate naive regions that could be rapidly colonized.
Conclusion
In 2022, over 19 million travelers entered Europe from dengue-affected areas [22]. Between 2012 and 2022, our team at the National Reference Laboratory of the Portuguese National Institute of Health (INSA) detected 142 Aedes-borne infections in viremic travelers. However, the high rates of asymptomatic infection in humans and the relatively short viremic window of symptomatic patients suggest that many traveler infections may be under-recognized, and the number of viremic travelers is much higher. Europe is experiencing a warming trend, with more frequent and severe heat waves and floods and longer and warmer summers [10]. This creates more favorable conditions for invasive mosquito species such as Ae. albopictus and Ae. aegypti. The geographical spread of invasive mosquito species to previously unaffected areas in the EU/EEA is an ongoing reality. In most European countries, cold winters do not allow year-round transmission [10], but in the southernmost region of Portugal, the Algarve, adult Ae. albopictus mosquitoes are already present year-round, although at lower population levels in winter. Given climate change, with continued increases in temperature and subsequent milder winters, the conditions for virus transmission will undoubtedly increase.
Given the ongoing spread of Ae. albopictus in mainland Portugal, it is essential to raise awareness of mosquito-borne diseases among the general public, healthcare professionals, and travelers.
Availability of data and materials
The nucleotide sequence data reported in this paper have been deposited in the NBCI GenBank database under the accession numbers: PP825977–PP825985.
Abbreviations
- COX :
-
Cytochrome c oxidase I
- EU/EEA:
-
European Union/European Economic Area
- FCT:
-
Portuguese Foundation for Science and Technology
References
Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc. 1998;14:340–3.
Sabatini A, Raineri V, Trovato G, Coluzzi M. Aedes albopictus in Italia e possibile diffusione della specie nell’area mediterranea [Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area]. Parassitologia. 1990;32:301–4.
Valerio L, Marini F, Bongiorno G, Facchinelli L, Pombi M, Caputo B, et al. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis. 2010;10:291–4. https://doi.org/10.1089/vbz.2009.0007.
European Centre for Disease Prevention and Control, ECDC. Increasing risk of mosquito-borne diseases in EU/EEA following spread of Aedes species. 2023. https://www.ecdc.europa.eu/en/news-events/increasing-risk-mosquito-borne-diseases-eueea-following-spread-aedes-species. Accessed 13 Jun 2024.
Marabuto E, Rebelo MT. The Asian tiger mosquito, Aedes (Stegomyia) albopictus (Skuse), a vector of dengue, chikungunya and zika viruses, reaches Portugal (Diptera: Culicidae). Zootaxa. 2018;4413:197–200. https://doi.org/10.11646/zootaxa.4413.1.10.
Osório HC, Zé-Zé L, Neto M, Silva S, Marques F, Silva AS, et al. Detection of the invasive mosquito species Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Portugal. Int J Environ Res Public Health. 2018;15:820. https://doi.org/10.3390/ijerph15040820.
Osório HC, Zé-Zé L, Amaro F, Silva M, Freitas IC, Soares P, et al. REVIVE 2023 – Culicídeos. In: REVIVE 2023 - Culicídeos, Ixodídeos e Flebotómos: Rede de Vigilância de Vetores [REVIVE 2023 - Culicids, Ixodids and Sandflies: Vector Surveillance Network]. Instituto Nacional de Saúde Doutor Ricardo Jorge. 2024. http://hdl.handle.net/10400.18/9172. Accessed 25 May 2024. (In Portuguese).
Osório HC, Zé-Zé L, Amaro F, Alves MJ. Mosquito surveillance for prevention and control of emerging mosquito-borne diseases in Portugal 2008–2014. Int J Environ Res Public Health. 2014;11:11583–96. https://doi.org/10.3390/ijerph111111583.
Rezza R, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;30:1840–6. https://doi.org/10.1016/S0140-6736(07)61779-6.
World Health Organization, WHO. Disease outbreak news; Dengue—global situation. 2024. https://www.who.int/emergencies/disease-outbreak-news7item/2023-DON518. Accessed 01 Jun 2024.
Žitko T, Kovačić A, Desdevises Y, Puizina J. Genetic variation in East-Adriatic populations of the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), inferredfrom NADH5 and COI sequence variability. Eur J Entomol. 2011;108:501–8. https://doi.org/10.14411/eje.2011.065.
Beebe NW, Ambrose L, Hill LA, Davis JB, Hapgood G, Cooper RD, et al. Tracing the tiger: population genetics provides valuable insights into the Aedes (Stegomyia) albopictus invasion of the Australasian Region. PLoS Negl Trop Dis. 2013;7:e2361. https://doi.org/10.1371/journal.pntd.0002361.
Zhong D, Lo E, Hu R, Metzger ME, Cummings R, Bonizzoni M, et al. Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS ONE. 2013;8:e68586. https://doi.org/10.1371/journal.pone.0068586.
Eskildsen GA, Rovira JR, Smith O, Miller MJ, Bennett KL, McMillan WO, et al. Maternal invasion history of Aedes aegypti and Aedes albopictus into the Isthmus of Panama: Implications for the control of emergent viral disease agents. PLoS ONE. 2018;13:e0194874. https://doi.org/10.1371/journal.pone.0194874.
Zé-Zé L, Borges V, Osório HC, Machado J, Gomes JP, Alves MJ. Mitogenome diversity of Aedes (Stegomyia) albopictus: Detection of multiple introduction events in Portugal. PLoS Negl Trop Dis. 2020;14:e0008657. https://doi.org/10.1371/journal.pntd.0008657.
Ribeiro H, Ramos HC. Identification keys of the mosquitoes of Continental Portugal, Açores and Madeira. Eur Mosq Bull. 1999;3:1–11.
Schaffner F, Anges G, Geoffroy B, Hervy J-P, Rhaiem A, Brunhes J. The mosquitoes of Europe: an identification and training programme. Montepellier: IRD editions (CD-ROM); 2001.
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–9.
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol Biol Evol. 2017;34:3299–302. https://doi.org/10.1093/molbev/msx248.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–8.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
European Centre for Disease Prevention and Control, ECDC. Travel-associated dengue cases. 2024 https://www.ecdc.europa.eu/en/dengue/surveillance/dengue-virus-infections-travellers. Accessed 13 Jun 2024.
Acknowledgements
We are grateful to Marco Brustolin and Carles Aranda for providing evidence of the collection date and location in Spain of the sampled mosquitoes corresponding to sequences KU319443, KU319444, KU319446, and KU319447. We also thank the REVIVE team for collecting mosquitoes nationwide, especially Lisbon and Tagus Valley Health Authority. This work received support from the Portuguese Foundation for Science and Technology (FCT; reference: CEECINST/00049/2021/CP2817/CT0001, DOI: https://doi.org/10.54499/CEECINST/00049/2021/CP2817/CT0001; reference: FCT/MCTES UIB/00211/2020, https://doi.org/10.54499/UIDB/00211; reference: FCT/MCTES UIP/00211/2020, https://doi.org/10.54499/UIDP/00211).
Funding
This work was funded by the Institute of Environmental Health of the Faculty of Medicine of the University of Lisbon (ISAMB), with project references FCT UIDB/04295/2020 and UIDP/04295/2020. This work was partially funded by the MOBVEC—Mobile Bio-Lab to support first response in Arbovirus outbreaks (2023–2026) project, reference HORIZON-EIC-2022-PATHFINDEROPEN-01 under the Pathfinderopen program of the European Innovation Council (CEI), and Ph.D. fellowship reference 2022.13476.BDANA (FCT). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: LZZ and HCO. Performed the experiments: LZZ, ICF, MS, and HCO. Analyzed the data: LZZ, ICF, and PS. Drafting of the manuscript: LZZ and PS. Revised the manuscript: LZZ, ICF, MS, MJA, and HCO. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
No special permits were required for the field studies. Consent was obtained from the property owners for mosquito collection in private residential areas and with the knowledge and permission of the relevant authority in public areas. No sites were legally protected, and no endangered or protected species were involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zé-Zé, L., Freitas, I.C., Silva, M. et al. The spread of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in Portugal: a first genetic analysis. Parasites Vectors 17, 389 (2024). https://doi.org/10.1186/s13071-024-06460-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06460-w