Abstract
Starting in October 2021, quarterly malacological surveys have been undertaken in Malawi, with the sampling of 12 specified freshwater habitats throughout a calendar year. Each survey monitors the presence of aquatic intermediate snail hosts of medical and veterinary importance. In March 2023, the alien lymnaeid species Pseudosuccinea columella was encountered for the first time in the surveys, in Nsanje District. This species identity was later confirmed upon DNA analysis of mitochondrial ribosomal 16S sequences. In July 2023, P. columella was also noted at single sites within Mangochi and Chikwawa Districts, and again in Nsanje District, with an additional location observed. Of particular importance, our sampled location in Mangochi District was directly connected to Lake Malawi, which expands the species list of invasive molluscs in this lake. While P. columella is a well-known intermediate snail host for human and animal fascioliasis, screening collected snails for trematode cercariae, alongside molecular xenomonitoring, did not yield equivocal evidence of active fluke infection. However, the newly recognized presence of this alien intermediate snail host within Lake Malawi, and along the Shire River Valley, flags a new concern in altered local transmission potential for human and animal fascioliasis.
Graphical Abstract

Similar content being viewed by others
Pseudosuccinea columella (Say, 1817), an invasive snail species originating from the Americas, has successfully colonized many freshwater habitats in Africa [1]. In southern Africa, its dispersion is recorded in Namibia, South Africa and Zimbabwe [2,3,4,5]. As P. columella is an alien intermediate host of liver fluke, its distribution is of medical and veterinary interest.
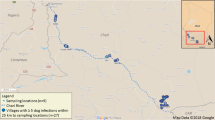
Our unexpected encounter with P. columella arose from broader malacological surveys for intermediate snail hosts of schistosomiasis, as part of activities performed within the framework of the “Hybridisation in UroGenital Schistosomiasis (HUGS)” project. Commencing in October 2021, then at quarterly intervals, HUGS has been inspecting 12 locations using standard freshwater snail collection protocols in Mangochi District (n = 7 sampling locations), Chikwawa District (n = 2) and Nsanje District (n = 3) (Fig. 1). The chosen locations are exemplars of high-risk water contact sites for human and animal schistosomiasis, and each location was inspected by a team of three, with snails collected by hand or by metal scoop depending on the habitat type [6].
Sketch maps of the distribution of Pseudosuccinea columella in Mangochi (a), Chikwawa (b) and Nsanje (c) Districts, southern Malawi. Red circles indicate HUGS survey sites where P. columella was found; grey circles are surveyed sites where this snail was not found. The locations are: Mangochi 1 (− 14.31373°, 35.14174°); Chikwawa 1 (− 16.03759°, 34.84091°); Nsanje 4 (− 16.88780°, 35.27475°); Nsanje 5 (− 16.92985°, 35.26552°) with corresponding location photograph. Note that the panorama image of Mangochi 1 clearly shows the stream, flowing left to right, directly connected to Lake Malawi. HUGS, Hybridisation in UroGenital Schistosomiasis (project)
Owing to its distinctive shell micro-sculpture [7], which permits quick in-field differentiation from Radix natalensis (Krauss, 1848), P. columella was first noticed during the March 2023 survey. Thereafter, a more purposeful search for living snails was made during the July 2023 survey. In so doing, sufficient specimens (n = 6) were obtained for an anatomical inspection, molecular snail taxonomy investigation and molecular liver fluke xenomonitoring investigation, with additional specimens (n = 29) checked for shedding liver fluke cercariae. Several specimens of R. natalensis were collected concurrently for later comparison.
For the anatomical investigation, each snail was placed in water for 2 min at 80 °C. Soft tissues were then carefully removed from the shell with forceps, and the empty shell viewed under a dissecting microscope and photographed (Fig. 2a, b, e, f), alongside more detailed inspection of the shell’s periostracum (Fig. 2c, g). To view the radula, head tissue was first separated and incubated in lactic acid for 3 days; the radula was then mounted onto a glass slide, with glass coverslip overlaid, and photographed under a light microscope (magnification: ×1000). Particular attention was given to the morphology of the central and first lateral teeth (Fig. 2d, h).
Conchological and anatomical comparison of Pseudosuccinea columella (top row) and Radix natalensis (bottom row). a–d P. columella conchology (a, b), shell microsculpture of the black square hatched area (c) and radular teeth (d) e–h R. natalensis conchology (e, f), shell microsculpture of the black square hatched area (g) and radular teeth (h). Although there is minor variation in the shape of the inner cusp of the first lateral teeth, the discriminatory feature is the periostracum’s spiral ridges
For the molecular taxonomy study, snail genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method, as adapted from [8]. Prior to tissue lysis, Phocine Herpes Virus (PhHV) was added to snail tissues as an internal extraction and later PCR amplification control for molecular xenomonitoring. Extracted genomic DNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) then normalized to 10 ng/ul using ddH2O. In this study, we targeted a partial region of the mitochondrial ribosomal 16S gene using the universal primers 16brm (5′-CCGGTCTGAACTCTGATCAT-3′) and 16arm (5′-CGCCTGTTTATCAAAAACAT-3′) for PCR amplification, as described by Remigio [9]. After amplification, the purity of the amplicons was determined by agarose gel (2%) electrophoresis with SYBR™ Safe DNA Gel Stain (Invitrogen, Thermo Fisher Scientific) before Sanger sequencing with forward and reverse primers at Source BioScience (Source BioScience, Cambridge, UK). Upon analysis of the forward and reverse sequences with MEGA11 software [10], a single ribosomal 16S consensus sequence of 402 nucleotides in length was obtained and then submitted to a BLAST search [11]. Newly obtained sequences were then deposited in GenBank (accession number OR801605).
To perform molecular xenomonitoring of liver fluke infection, we used the TaqMan real-time PCR-based assay of Alasaad et al. [12], using the genus-specific primers SSCPFaF (5′-TTGGTACTCAGTTGTCAGTGTG-3′) and SSCPFaR (5′-AGCATCAGACACATGACCAAG-3′) with species-specific TaqMan probes to detect Fasciola hepatica (Linnaeus, 1758) (ProFh: 5′-[6FAM]ACCAGGCACGTTCCGTCACTGTCACTTT[BHQ1]-3′) and Fasciola gigantica (Cobbold, 1856) (ProFg: 5′-[HEX]ACCAGGCACGTTCCGTTACTGTTACTTTGTC[BHQ1]-3′). Real-time PCR reactions were performed using a MIC thermocycler (Bio Molecular Systems, Upper Coomera, Queensland, Australia) with genomic DNA from adult worms of F. gigantica originating from cattle in Uganda as the positive control.
Our BLAST search identified a sequence with 100% similarity: P. columella isolate LS3 mitochondrion genome (accession number NC_042905.1) from North America. Twenty additional identical 16S matches were noted, including P. columella from Brazil [13] and South Africa [14]. While we did not observe any snails shedding fluke cercariae, we did note, from molecular xenomonitoring, very weak amplification DNA signatures, with cycle threshold (Ct) values of 35. We consider these to most likely arise from spurious amplification of other trematode larvae [15]. In Africa, human and animal liver fluke is typically transmitted by freshwater snails of the genus Galba or Radix [16], giving rise to an often allopatric transmission of F. hepatica and F. gigantica, respectively [17]. Given the ability of P. columella to transmit both species of liver fluke [18], this alien intermediate host snail potentially adds a new dimension to this snail-parasite relationship in Malawi, although our current conclusion is that there was no evidence for active liver fluke infection within our sampled snails.
To provide an insight into the ecology of P. columella, we review here, in brief, the aquatic habitats where it was found. As shown in Fig. 1, sampling site Mangochi 1 is predominantly a stream habitat, immediately marginal and directly connected to Lake Malawi itself. Since Lake Malawi is well-known internationally as a global hotspot of biological diversity, the addition of P. columella to its species list is not trivial in the least. Indeed, its presence likely adds to the expanding list of ecological change within the lake and is pertinent to other snail-borne diseases locally [6, 19]. This stream’s natural water supply is augmented by a pisciculture facility some 2–3 km inland, at − 14.32813°, 35.128351°. Here, water is directly taken from the lake and pumped underground, returning aboveground following this stream’s natural path. Before pisciculture, this stream was seasonal but is now a conducive habitat throughout the year, and it is reasonable to speculate that the presence of P. columella here was fully or partially attributable to local development(s) in pisciculture along the Lake Malawi shoreline. Another contributing factor would be the introduction and subsequent dispersion of invasive aquatic plants, such as water hyacinth (Pontederia crassipes Mart., 1823), now common across all collecting sites, on which P. columella was often found.
Following the Upper then Lower Shire River some 200 km southward, P. columella was found at Chikwawa 1, a natural and permanent oxbow lake of the Lower Shire River (Fig. 1). Upon more extreme seasonal flooding, this oxbow is directly connected to the river, which recently occurred in March 2023 by cyclone Freddy. Moving a further 100 km southward, both Nsanje 4 and Nansje 5 are seasonally flooded areas for informal pisciculture and small holder rice farming. Each site is temporarily connected to the Lower Shire Valley upon natural inundation(s) and by managed sluice gates (Fig. 1).
Water chemistry data were collected for each snail sampling site at each visit, including data for temperature (°C), pH, conductivity (µS) and total dissolved solids (TDS; ppm). Average values for these four water chemistry parameters at the sampling sites were: (i) Mangochi 1: 27.8 °C, pH 8.1, 517.6 µS and 264.6 ppm, respectively; (ii) Chikwawa 1: 32.7 °C, pH 8.4, 627.2 µS and 308.7 ppm, respectively; (iii) Nsanje 4: 32.5 °C, pH 8.3, 443 µS and 221.8 ppm, respectively; and (iv) Nsanje 5: 30.1 °C, pH: 8.0, 436.3 µS and 202.7 ppm, respectively. Across all 12 sites surveyed and across all time points, the average water chemistry data were 29.3 °C, pH 8.2, 487.9 µS (conductivity) and 244.9 (TDS). These environmental parameters are broadly consistent to those where P. columella has been found elsewhere [18] and are typical of predominantly permanent water bodies with a muddy substrate and various vegetation present.
Current information on the epidemiology of liver fluke infection of livestock in Malawi is scant as formal reporting of infection in slaughtered animals has ceased. In the past, however, northern parts of the country carried the greatest burdens while prevalence in Mangochi, Chikwawa and Nsanje Districts was comparatively low [20]. Our observations suggest that these infection trends will not remain the case with a new alien host of liver fluke present.
To summarize, our report of P. columella considerably expands the known geographical range of this alien intermediate host snail species across southern Africa. We add to the malacological list of alien freshwater snails in Lake Malawi and Lower Shire River, contributing to a growing list of evidence for wider ecological change. We outline a new and pressing need for further and more thorough surveillance of human and animal fascioliasis in Malawi.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Malatji MP, Mukaratirwa S. Molecular detection of natural infection of Lymnaea (Pseudosuccinea) columella (Gastropoda: Lymnaeidae) with Fasciola gigantica (Digenea: Fasciolidae) from two provinces of South Africa. J Helminthol. 2019;94:e38. https://doi.org/10.1017/S0022149X19000129.
Malatji MP, Pfukenyi DM, Mukaratirwa S. Fasciola species and their vertebrate and snail intermediate hosts in East and Southern Africa: a review. J Helminthol. 2019;94:e63. https://doi.org/10.1017/S0022149X19000531.
Malatji MP, Lamb J, Mukaratirwa S. Molecular characterization of liver fluke intermediate host lymnaeids (Gastropoda: Pulmonata) snails from selected regions of Okavango Delta of Botswana, KwaZulu-Natal and Mpumalanga provinces of South Africa. Vet Parasitol Region Stud Reports. 2019;17:100318. https://doi.org/10.1016/j.vprsr.2019.100318.
Nyagura I, Malatji MP, Mukaratirwa S. Occurrence of Fasciola (Digenea: Fasciolidae) species in livestock, wildlife and humans, and the geographical distribution of their intermediate hosts in South Africa—a scoping review. Front Vet Sci. 2022;9:93542. https://doi.org/10.3389/fvets.2022.935428.
Schols R, Carolus H, Hammoud C, Muzarabani KC, Barson M, Huyse T. Invasive snails, parasite spillback, and potential parasite spillover drive parasitic diseases of Hippopotamus amphibius in artificial lakes of Zimbabwe. BMC Biol. 2021;19:160. https://doi.org/10.1186/s12915-021-01093-2.
Alharbi MH, Condemine C, Hesketh J, Kayuni SA, Arme TM, Archer J, et al. Biomphalaria pfeifferi (Gastropoda: Planorbidae) in Lake Malawi and Upper Shire River, Mangochi District, Malawi: distribution, genetic diversity and pre-patent schistosome infections. Trop Med Infect Dis. 2023;8:126.
Brown DS. Freshwater snails of Africa and their medical importance. Revised 2nd edition, London: Taylor & Francis. 1994
Abbasi I, King CH, Sturrock RF, Kariuki C, Muchiri E, Hamburger J. Differentiation of Schistosoma haematobium from related schistosomes by PCR amplifying an inter-repeat sequence. Am J Trop Med Hyg. 2007;76:950–5.
Remigio E. Molecular phylogenetic relationships in the aquatic snail genus Lymnaea, the intermediate host of the causative agent of fascioliasis: insights from broader taxon sampling. Parasitol Res. 2002;88:687–96. https://doi.org/10.1007/s00436-002-0658-8.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022-7. https://doi.org/10.1093/molbev/msab120.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. https://doi.org/10.1016/S0022-2836(05)80360-2.
Alasaad S, Soriguer RC, Abu-Madi M, El Behairy A, Jowers MJ, Baños PD, et al. A TaqMan real-time PCR-based assay for the identification of Fasciola spp. Vet Parasitol. 2011;179:266–71. https://doi.org/10.1016/j.vetpar.2011.01.059.
Medeiros C, Silva Scholte LL, Marques Cardoso PC, Pointier J-P, Rumi A, Rocha Oliveira IH, et al. An integrative approach for the identification of native and exotic Lymnaeids from Brazil. Malacologia. 2022;65:18. https://doi.org/10.4002/040.065.0102.
Mahulu A, Clewing C, Stelbrink B, Chibwana FD, Tumwebaze I, Stothard J Russell, et al. Cryptic intermediate snail host of the liver fluke Fasciola hepatica in Africa. Parasit Vectors. 2019;12:573. https://doi.org/10.1186/s13071-019-3825-9.
Kane RA, Stothard JR, Rollinson D, Leclipteux T, Evraerts J, Standley CJ, et al. Detection and quantification of schistosome DNA in freshwater snails using either fluorescent probes in real-time PCR or oligochromatographic dipstick assays targeting the ribosomal intergenic spacer. Acta Trop. 2013;128:241–9. https://doi.org/10.1016/j.actatropica.2011.10.019.
Ngcamphalala PI, Malatji MP, Mukaratirwa S. Geography and ecology of invasive Pseudosuccinea columella (Gastropoda: Lymnaeidae) and implications in the transmission of Fasciola species (Digenea: Fasciolidae)—a review. J Helminthol. 2022;96:e1. https://doi.org/10.1017/s0022149x21000717.
Howell A, Mugisha L, Davies J, LaCourse EJ, Claridge J, Williams DJL, et al. Bovine fasciolosis at increasing altitudes: parasitological and malacological sampling on the slopes of Mount Elgon, Uganda. Parasites Vectors. 2012;5:196. https://doi.org/10.1186/1756-3305-5-196.
De Kock KN, Joubert PH, Pretorius SJ. Geographical distribution and habitat preferences of the invader freshwater snail species Lymnaea columella (Mollusca: Gastropoda) in South Africa. Onderstepoort J Vet Res. 1989;56:271–5.
Madsen H, Stauffer JR. Schistosomiasis control under changing ecological settings in Lake Malawi. EcoHealth. 2022;19:320–3. https://doi.org/10.1007/s10393-022-01606-7.
Mzembe SA, Chaudhry MA. The epidemiology of fascioliasis in Malawi. Part II. Epidemiology in the definitive host. Trop Anim Health Prod. 1981;13:27–33. https://doi.org/10.1007/bf02237882.
Acknowledgements
The Wellcome Trust directly funds the HUGS project with salary support for SJ, AJ, LJC, PM, SAK, GN, DRK, PC and DL, alongside financial backing for our malacological surveys. This assistance is in conjunction with funding from the National Institute for Health Research (NIHR) (using the UK’s Official Development Assistance [ODA] funding) and from the Wellcome Trust (220818/Z/20/Z) under the NIHR-Wellcome Partnership for Global Health Research. CN is supported by the Wellcome Trust (223660/Z/21/Z) under the NIHR-Wellcome Partnership for Global Health Research. We acknowledge collaborative support from Dr. Chris Jones, LSTM and Dr. Themba Mzilahowa, Malaria Alert Centre, as part of the NIHR Shire_Vec project. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the NIHR or the Department of Health and Social Care. JA receives a MRC-DTP PhD Fellowship held at the LSTM.
Funding
Funding was provided by Wellcome Trust (Grant Nos. 220818/Z/20/Z, 223660/Z/21/Z).
Author information
Authors and Affiliations
Contributions
SJ, AJ, PM, LJC, JA, CN, GN, EK, DL, DRK, PC, SAK, JM and JRS were responsible for conceptualization and sample collection. AJ and JRS performed morphological identification of samples. SJ and LJC conducted molecular identification of samples. SJ, AJ and JRS wrote the main manuscript text and AJ and JRS prepared Figs. 1 and 2. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The “Hybridisation in UroGenital Schistosomiasis (HUGS)” study was approved in the UK by the Research Ethics Committee of the Liverpool School of Tropical Medicine (study protocol 22-028) and in Malawi by the College of Medicine Research and Ethics Committee (COMREC; study protocol P.08/21/3381).
Competing interests
The authors report that there are no competing interests.
Consent for publication
All authors have read and agree to the published version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jones, S., Juhász, A., Makaula, P. et al. A first report of Pseudosuccinea columella (Say, 1817), an alien intermediate host for liver fluke, in Malawi. Parasites Vectors 17, 186 (2024). https://doi.org/10.1186/s13071-024-06241-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06241-5