Abstract
Background
Infection with parasitic nematodes (helminths), particularly those of the order Strongylida (such as Haemonchus contortus), can cause significant and burdensome diseases in humans and animals. Widespread drug (anthelmintic) resistance in livestock parasites, the absence of vaccines against most of these nematodes, and a lack of new and effective chemical entities on the commercial market demands the discovery of new anthelmintics. In the present study, we searched the Global Health Priority Box (Medicines for Malaria Venture) for new candidates for anthelmintic development.
Methods
We employed a whole-organism, motility-based phenotypic screening assay to identify compounds from the Global Health Priority Box with activity against larvae of the model parasite H. contortus, and the free-living comparator nematode Caenorhabditis elegans. Hit compounds were further validated via dose–response assays, with lead candidates then assessed for nematocidal activity against H. contortus adult worms, and additionally, for cytotoxic and mitotoxic effects on human hepatoma (HepG2) cells.
Results
The primary screen against H. contortus and C. elegans revealed or reidentified 16 hit compounds; further validation established MMV1794206, otherwise known as ‘flufenerim’, as a significant inhibitor of H. contortus larval motility (half-maximal inhibitory concentration [IC50] = 18 μM) and development (IC50 = 1.2 μM), H. contortus adult female motility (100% after 12 h of incubation) and C. elegans larval motility (IC50 = 0.22 μM). Further testing on a mammalian cell line (human hepatoma HepG2 cells), however, identified flufenerim to be both cytotoxic (half-maximal cytotoxic concentration [CC50] < 0.7 μM) and mitotoxic (half-maximal mitotoxic concentration [MC50] < 0.7 μM).
Conclusions
The in vitro efficacy of MMV1794206 against the most pathogenic stages of H. contortus, as well as the free-living C. elegans, suggests the potential for development as a broad-spectrum anthelmintic compound; however, the high toxicity towards mammalian cells presents a significant hindrance. Further work should seek to establish the protein–drug interactions of MMV1794206 in a nematode model, to unravel the mechanism of action, in addition to an advanced structure–activity relationship investigation to optimise anthelmintic activity and eliminate mammalian cell toxicity.
Graphical Abstract

Similar content being viewed by others
Background
Infections and diseases (nematodiases) caused by gastrointestinal parasitic nematodes are a significant strain on both human and animal health [1,2,3]. The control of soil-transmitted helminths (STHs) was included in the World Health Organization (WHO)’s neglected tropical disease road map for 2021–2030 [3], with the core strategic interventions and critical actions focused on adequate hygiene, education, preventative chemotherapy and the development of more effective medicines in the case of emerging drug resistance [3]. In 2019, ~ 909 million people were estimated to be infected with intestinal nematodes (Ascaris, Trichuris, Ancylostoma and Necator), accounting for 1.97 million disability-adjusted life years [1], disproportionately affecting communities facing poverty. On the other hand, livestock helminth infections are ubiquitous, with parasites such as Haemonchus, Cooperia, Ostertagia, Teladorsagia and Trichostrongylus impacting producers globally [2, 4, 5]. In Australia alone, the annual cost due to nematodiases (in sheep and cattle) is estimated at USD 450 million [5]—a significant economic burden. Globally, the market for anti-parasitic drugs for livestock animals is estimated at USD 2.5 billion per annum [4], indicative of the impact of parasitic worms.
Central to the management of such parasites are knowledge and understanding of epidemiology (reviewed by [6, 7]), regular monitoring and accurate diagnoses of infections [8,9,10] and implementation of an anthelmintic regimen to achieve control. Although vaccination would be a preferred method to prevent infections and disease, vaccines have been challenging to develop [11], and only two have been commercialised for use in livestock (Bovilis Huskvac® and Barbervax® to prevent bovine dictyocaulosis and haemonchosis, respectively). Thus, anthelmintic treatment remains a key component of most control programs. However, unfortunately, drug resistance in nematodes of livestock is now widespread due to the excessive and/or widespread use of commercially available anthelmintics [2, 12,13,14,15,16,17]. As monepantel (Zolvix™) [18] and derquantel (Startect®) [19,20,21] are the only new anthelmintic drug classes (for use in livestock) introduced into the commercial market since 2000, there is an urgent need to discover and develop new anthelmintic compounds with novel mechanisms/modes of action.
Historically, anthelmintic drug discovery has been hindered by a lack of adequate screening/drug development technologies, and the economic costs associated with the drug discovery process (reviewed by [22, 23]). Early drug development campaigns relied on the testing of anthelmintic candidates in animal models [24,25,26,27]. Whilst effective, these assays are low-throughput, high-cost and labour-intensive. Today, anthelmintic drug discovery focuses on the use of in vitro methods to quantitatively assess test compounds for antiparasitic activity. A number of practical, time-efficient and relatively low-cost phenotypic (whole-worm) screening methods have been developed for the discovery of novel anthelmintic candidates. Methods vary in the species assessed, life cycle stage used, technology used for assessment, phenotypic characteristics assessed and the throughput potential (reviewed by [23]). Medium- to high-throughput assays reported to date utilise heat flow- [28, 29], impedance- [30], imaging- [31,32,33] or photometry-based [34,35,36,37,38,39] techniques to evaluate test compounds.
Anthelmintic discovery is being enabled through the use of multi-omic tools and resources for key parasitic nematodes such as Haemonchus contortus (order Strongylida; clade V) [40,41,42,43,44] and its free-living relative, Caenorhabditis elegans (reviewed by [45, 46]). Drug discovery efforts have also been enhanced by the development of proteomic-driven target deconvolution tools (reviewed by [47, 48]), allowing for the identification of novel drug–protein interactions. Thus, early-stage broad-spectrum nematocidal development may be achieved, first, through the discovery of new drug candidates via a phenotypic screening platform (utilising one or more model nematode organisms), and subsequent target deconvolution using advanced workflows, assisted by bioinformation.
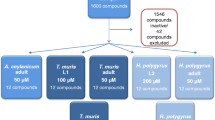
In the further pursuit of a new and effective anthelmintic entity, together with the Medicines for Malaria Venture (MMV; Geneva, Switzerland), we previously screened a number of small compound collections, including the Pathogen, Stasis, and Pandemic Response Boxes [49,50,51] for anthelmintic activity against H. contortus and/or C. elegans. Here, as part of an ongoing collaboration with MMV, we screened the recently curated Global Health Priority Box in a whole-organism, motility-based phenotypic assay [38, 39] for anthelmintic activity on the larvae H. contortus and C. elegans. This Box contains 240 compounds which are at various stages of development and have demonstrated activity against drug-resistant Plasmodium (n = 80 entities), neglected and zoonotic pathogens (n = 80) and various vectors (such as species of mosquitoes, ticks and mites) (n = 80). Then, we estimated the anthelmintic potency of (hit) compounds, evaluated the toxicity of hit compounds against HepG2 cells and worked toward identifying candidates for future medicinal chemistry optimisation and mechanism/mode of action studies.
Methods
Preparation of compounds for screening
The Global Health Priority Box compound collection from MMV, Geneva, Switzerland, contains 240 chemical entities, at various stages of development, with activity against drug-resistant Plasmodium, neglected and zoonotic pathogens (such as Leishmania, Mycobacterium and Trypanosoma) and vectors (such as species of mosquitoes, ticks and mites) (https://www.mmv.org/mmv-open/global-health-priority-box/about-global-health-priority-box; accessed 18 September 2023). Individual compounds were supplied as solid samples; each compound was resuspended in 10 μl of (100%) dimethyl sulfoxide (DMSO) to give a final concentration of 10 mM. Prior to screening, test compounds were each diluted to 40 μM in sterile lysogeny broth (LB; cf. [38, 52]) containing 100 IU/ml of penicillin, 100 µg/ml of streptomycin and 0.25 µg/ml of amphotericin B (Fungizone®, Thermo Fisher Scientific, Waltham, MA, USA); this supplemented LB was designated as LB*.
Production, storage and preparation of H. contortus larvae and adults
Haemonchus contortus (Haecon-5 strain; cf. [53]) was produced in experimental sheep as described previously [54] and in accordance with the institutional animal ethics guidelines (permit no. 23983-2811-4; The University of Melbourne, Parkville, Victoria, Australia). Helminth-free Merino sheep (6 months of age; male) were orally inoculated with 7000 third-stage larvae (L3s) of H. contortus. Four weeks after inoculation, faecal samples were collected from sheep with patent H. contortus infection. These samples were incubated at 27 °C and > 90% relative humidity for 1 week to yield larvae [54], which were then collected in tap water and allowed to migrate through two layers of nylon mesh (pore size: 20 μm; Rowe Scientific, Doveton, Victoria, Australia) to remove debris. Clean L3s were stored in the dark at 11 °C for up to 6 months [54]. Immediately prior to use in assays, H. contortus L3s were artificially exsheathed via exposure to 0.15% (v/v) sodium hypochlorite for 20 min at 38 °C [54], achieving an exsheathment rate of 90%. The larvae were then immediately washed five times with 50 ml of sterile physiological saline solution by centrifugation at 2000×g (5 min) and resuspended in LB* at a concentration of 200 xL3s per 50 µl (for the primary screen) or 300 xL3s per 50 µl (for the dose–response assays).
Adult H. contortus were collected from the abomasa of sheep infected for 10 weeks, then washed extensively in RPMI 1640 media supplemented with final concentrations of 2 mM L-glutamine, 100 IU/ml of penicillin, 100 µg/ml of streptomycin and 0.25 µg/ml of amphotericin B (Thermo Fisher Scientific, Scoresby, VIC, Australia; this supplemented RPMI was designated as RPMI*). Using a dissecting microscope, female and male worms were separated (38 °C in RPMI*) immediately prior to compound testing.
Production, storage and preparation of C. elegans larvae
Caenorhabditis elegans (N2—wild-type Bristol strain) was maintained in the laboratory under standard conditions at 20 °C on nematode growth media (NGM) agar plates, with Escherichia coli OP50 as a food source (Stiernagle, 2006). Gravid adult worms were collected from NGM plates, washed with sterile M9 buffer and then treated with a solution containing 0.4% (v/v) sodium hypochlorite and 170 mM sodium hydroxide for 4–8 min at 22–24 °C (room temperature) to release eggs [55, 56]. The eggs were then washed five times with 15 ml of sterile M9 buffer (centrifugation at 500×g, 2 min). After washing, the egg pellet was suspended in 8 ml of M9 buffer in a 15 ml tube and gently agitated for 24 h at 22–24 °C to produce first-stage larvae (L1s); 45 h prior to screening, synchronised C. elegans L1s were inoculated onto NGM plates containing 500 µl of E. coli OP50 (~ 3000 larvae per plate) and allowed to develop to fourth-stage larvae (L4s) at 20 °C. L4s were collected from plates and washed twice with sterile M9 buffer by centrifugation (500×g, 2 min) to remove E. coli OP50, and then resuspended in LB* at a concentration of 125 L4s per 50 µl (for the primary screen) or 100 L4s per 50 µl (for the dose–response assays).
Screening for anthelmintic activity against H. contortus larvae
An established high-throughput phenotypic screening assay [38] was used to test the anthelmintic activity of compounds on H. contortus xL3s. Compounds were assessed for motility inhibition at a concentration of 20 µM in LB*. Four compounds—monepantel (Zolvix™; Elanco, Australia), monepantel/abamectin (Zolvix Plus™; Elanco, Australia), moxidectin (Cydectin®; Virbac, France) and compound MIPS-0018666 (abbreviated herein as M-666; cf. [57])—were used as positive controls (final concentration of 20 µM in LB*). A solution of LB* + 1% (v/v) DMSO was used as a negative control. Test compounds were distributed amongst one flat-bottom, 384-well microplate (cat no. 3860; Corning, Corning, NY, USA). Eighty xL3s of H. contortus in 20 µl of LB* were added to each well to give a final volume of 40 µl. The plate was then placed in a CO2 incubator (10% [v/v] CO2, 38 °C, > 90% humidity). At 90 h, worm activity was captured using a WMicroTracker ONE unit (Phylumtech, Sunchales, Santa Fe, Argentina). Over a period of 15 min, disturbance of an infrared beam in individual wells was recorded as a worm ‘activity count’. Activity counts were then normalised to the positive and negative controls using the GraphPad Prism program (v.9.1.0 GraphPad Software, San Diego, CA, USA). The screening plate was then returned to the incubator (10% [v/v] CO2, 38 °C, > 90% humidity) for an additional 72 h. At 168 h, worms were fixed with 40 µl of Lugol solution (Sigma-Aldrich, St. Louis, MO, USA). Compounds were then assessed (microscopically) for inhibition of larval development and/or induction of a non-wild-type phenotype in H. contortus worms. A compound that reduced xL3 motility by ≥ 70% and/or inhibited larval development or induced an abnormal phenotype (relative to the negative control) was recorded as a ‘hit’ compound. The performance of the assay was monitored using the Z′-factor [58] calculated using data for the negative (DMSO) and positive (M-666) control compounds on individual plates.
Screening for anthelmintic activity against C. elegans
An established assay [39] was employed to test the anthelmintic activity of compounds on C. elegans. Test compounds and positive and negative controls were prepared in a flat-bottom, 384-well microplate (cf. [39]). Fifty L4s of C. elegans in 20 µl of LB* were added to each well to give a final volume of 40 µl. The plate was then placed in an incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 20 °C for 40 h. At 40 h, the worm (in transition from L4s to young adults) activity was captured (over a period of 15 min) utilising the WMicroTracker ONE unit. Activity counts were then normalised to the positive and negative controls using the GraphPad Prism software program (v.9.1.0). A compound that reduced worm motility by ≥ 70% (relative to the negative control) was recorded as a hit compound. The Z′ factor was calculated in the same manner as described previously [58].
Haemonchus contortus dose–response assay
The dose–response assay for H. contortus followed a well-established protocol [38]; it was employed to evaluate the potency of hit compounds against this nematode. Test compounds were assessed individually for an effect on the motility of xL3s (10-point, twofold serial dilution in LB*, 40–0.16 μM). One compound, monepantel (prepared in the same manner as the test compounds), was used as a positive control. A solution of LB* was used as a negative control. The test and positive-control compounds were arrayed in triplicate across individual flat-bottom 96-well microplates, with six wells on each plate containing the negative control. Three hundred xL3s of H. contortus in 50 μl of LB* were added to each well to give a final volume of 100 μl. Plates were then placed in a CO2 incubator (10% [v/v] CO2, 38 °C, > 90% humidity). After 168 h of incubation, worm activity was captured using a WMicroTracker ONE unit. Over a period of 15 min, disturbance of an infrared beam in individual wells was recorded as a worm activity count. Raw activity counts for each well were normalised to the negative controls. The compound concentrations were log10-transformed and fitted using a variable-slope four-parameter equation, using the ordinary least squares fit model, employing GraphPad Prism (v.9.1.0). Larval development was established at 168 h of incubation with compound, as described previously [54]. The development inhibition and phenotypes of larvae were examined microscopically [54]. A one-way analysis of variance (ANOVA) with a Tukey multiple comparison test or an unpaired t-test was used to establish statistically significant differences in larval motility or development.
Caenorhabditis elegans dose–response assay
The dose–response assay for C. elegans followed a well-established protocol [39] and was employed to evaluate the potency of hit compounds against this nematode. Test and positive-control compounds as well as negative controls were prepared in 96-well microplates (cf. [39]). One hundred C. elegans in 50 μl of LB* were added to each well to give a final volume of 100 μl. Plates were then placed in an incubator at 20 °C for 40 h. At 40 h, worm activity was captured using a WMicroTracker ONE unit. Raw activity counts for each well were normalised to the negative controls. The compound concentrations were log10-transformed and fitted using a variable-slope four-parameter equation, using the ordinary least squares fit model, employing GraphPad Prism (v.9.1.0). A one-way ANOVA with a Tukey multiple comparison test or an unpaired t-test was used to establish statistically significant differences in larval motility.
Assessment of the activity of selected compounds on H. contortus adults
The activity of two test compounds was assessed on adult female specimens of H. contortus in an established assay [59]. The compound was added in triplicate to the wells of a 24-well plate (Corning, USA) at a concentration of 40 μM in 500 μl of RPMI*. Two positive-control compounds, monepantel and moxidectin, and a negative control containing 1% (v/v) DMSO only were included in triplicate on the same plate. Three adult females were added to each of the triplicate wells containing either the test compound or the controls and placed in a CO2 incubator (10% [v/v] CO2, 38 °C, > 90% relative humidity) for 24 h. A video recording (30 s) of each well was taken at 3 h, 6 h, 12 h and 24 h during the total incubation period to assess the reduction in worm motility, which was scored as 3 (‘good’), 2 (‘low’), 1 (‘very low’) or 0 (‘no movement’; cf. [59]). For each test or control compound, the motility scores for each of the triplicate wells were calculated, normalised with reference to the negative control (100% motility) and recorded as a percentage. A two-way ANOVA with a Tukey multiple comparison test was used to establish statistically significant differences in worm motility.
Evaluation of the cellular and mitochondrial toxicity using HepG2 cells
The cytotoxic and mitotoxic activity of MMV1794206 on HepG2 human hepatoma cells was evaluated using an established protocol [60,61,62,63]. The test compounds were serially diluted (seven-point, twofold serial dilution, 40–0.63 µM) in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, USA), with GlutaMax™ supplemented with 25 mM D-glucose (cytotoxicity) or D-galactose (mitotoxicity), 10% heat-inactivated foetal bovine serum (FBS), 100 IU/ml of penicillin, 100 µg/ml of streptomycin and 0.25 µg/ml of amphotericin B (denoted as DMEM*). Monepantel and moxidectin (prepared in the same manner as the test compound) were included as reference compounds. Two compounds, doxorubicin (cytotoxic; Sigma-Aldrich, USA) and M-666 (mitotoxic; [57]), were used as positive controls at a single concentration of 10 µM. A solution of DMEM* + 0.25% (v/v) DMSO was used as a negative control. HepG2 cells were seeded into wells of a 96-well plate in 80 µl of DMEM* (at 1 × 105 cells per well) and allowed to adhere for 16 h at 37 °C and 5% (v/v) CO2 at > 90% humidity prior to incubation with individual compounds, at a final volume of 100 µl. For the assessment of mitochondrial toxicity, cells were starved of serum (DMEM* without FBS) for 4 h prior to the incubation with compounds [60, 61]. Following 48 h of incubation with compounds, cell viability was determined by crystal violet staining [62]. The absorbance (595 nm) of treated cells was normalised using the negative controls (viability: 100%) to calculate the cell viability. All compounds and controls were tested in triplicate. To determine the half-maximal cytotoxic concentration (CC50) and half-maximal mitotoxic concentration (MC50) values, compound concentrations were log10-transformed, baseline-corrected using a respective positive control (doxorubicin or M-666) and fitted using a variable-slope four-parameter equation and ordinary least squares fit model using GraphPad Prism (v.9.1.0).
Results
A primary screen identifies 16 compounds with anthelmintic activity
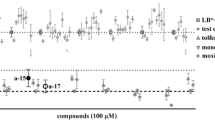
Sixteen compounds (Table 1; Fig. 1A) were shown to significantly inhibit the motility (90 h) and/or development (168 h) of exsheathed L3s (xL3s) of H. contortus. MMV1577458, MMV688934, MMV002519 and MMV1794206 reduced larval motility by 72–100% and development by 100%, and each of these compounds induced an abnormal phenotype (Str or Cur; Table 1). Compounds MMV1794214, MMV1577463 and MMV027339 reduced larval motility by 73–87%, but did not inhibit development or induce an abnormal phenotype. Nine compounds (i.e. MMV1577454, MMV1633829, MMV1578924, MMV1634081, MMV1577467, MMV1633828, MMV672931, MMV1633823 and MMV002231) inhibited larval motility by < 70% after 90 h, and inhibited development by ≥ 90% after 168 h.
Results of the primary screen of the Medicines for Malaria Venture (MMV) Global Health Priority Box (n = 240) against (A) exsheathed third-stage larvae (xL3s) of Haemonchus contortus and (B) young adults of Caenorhabditis elegans with reference to four distinct control compounds (monepantel, monepantel/abamectin, M-666 and moxidectin) and a negative (LB* + 1% DMSO) control. All test and positive-control compounds were tested at 20 μM. Each grey dot represents an individual test compound. Mean ± standard error of the mean (SEM) indicated for positive-control compounds (four data points) and negative controls (32 data points for LB* + 1% DMSO). For all screens, the Z′ factor ranged between 0.76 and 0.92
Eight of the 16 compounds (Table 1; Fig. 1B) were found to have activity against young adults of C. elegans (40 h). MMV1577458 and MMV1794206 both inhibited motility by 100%, whereas compounds MMV1633828, MMV672931, MMV002231, MMV1578924, MMV1577454 and MMV1633829 inhibited larval motility in the range of 85–94%. Throughout the primary screens on H. contortus and C. elegans, the Z′-factor ranged between 0.76 and 0.92.
Potency and toxicity assessment reveals MMV1794206 as an anthelmintic candidate
Of the seven compounds that inhibited H. contortus xL3 motility by ≥ 70%, three compounds, MMV1577458 (chlorfenapyr), MMV688934 (tolfenpyrad) and MMV0002519 (rotenone), had been previously assessed for motility inhibition on H. contortus larvae (cf. [49, 63, 64]). Thus, the remaining four compounds (MMV1794214, MMV1794206, MMV1577463 and MMV027339) were prioritised for further potency assessment on H. contortus larval motility inhibition. Following incubation for 90 h (Fig. 2A), MMV1794214 displayed a half-maximal inhibitory concentration (IC50) of 4.5 ± 1.1 μM (maximum motility inhibition, MMI: 70%), whereas compound MMV1794206 had an IC50 of 18 ± 4 μM (MMI: 98%)—both compounds were less active than the positive-control compound, monepantel (0.33 ± 0.12 μM, MMI: 95%). Of note, compounds MMV1577463 and MMV027339 both displayed motility inhibition (IC50) > 40 μM. Subsequently, the potency of MMV1794214 and MMV1794206 to inhibit larval development of H. contortus following 168 h of incubation (Fig. 2B) was assessed. MMV1794214 displayed an IC50 of > 40 μM, whereas compound MMV1794206 had an IC50 of 1.2 ± 0.1 μM, compared to that of monepantel, which displayed an IC50 of 0.26 ± 0.03 μM. Both MMV1794214 and MMV1794206 were further evaluated for the inhibition of the motility of adult females of H. contortus (at a single concentration of 40 μM; Fig. 2C). While MMV1794214 did not markedly reduce motility (~ 0%, 0%, 23% and 15% at 3 h, 6 h, 12 h and 24 h, respectively), MMV1794206 reduced motility by ~ 33% (3 h), 89% (6 h), 100% (12 h) and 100% (24 h). The monepantel control reduced motility by ~ 0%, 23%, 66% and 100% at 3 h, 6 h, 12 h and 24 h, respectively.
The potency of four active test compounds (MMV1794214, MMV1794206, MMV1577463 and MMV027339) against exsheathed third-stage larvae (xL3s) of Haemonchus contortus, the potency of two active test compounds (MMV1794214 and MMV1794206) on adult females of H. contortus, and the potency of one active test compound (MMV1794206) on young adults of Caenorhabditis elegans, with reference to monepantel and/or moxidectin (positive controls). Each curve shows (A) the inhibition of H. contortus larval motility at 90 h, (B) the inhibition of H. contortus larval development at 7 days, (C) the in vitro motility inhibition (%) of H. contortus adult females over a period of 24 h (motility scores assessed at 3-, 6-, 12- and 24 h time points; cf. Taki et al. [59]) and (D) the reduction of C. elegans motility at 40 h. Data points represent either one (C) or three (A, B and D) experiments conducted in triplicate; the mean ± standard deviation (SD, C) or standard error the mean (SEM, A, B and D). Statistical significance was evaluated with reference to a negative control (C); **** denotes P ≤ 0.0001
Eight compounds were found to inhibit C. elegans young adult motility by ≥ 70%, and one of them (MMV1794206; Fig. 2D) was prioritised for further validation (the remaining seven were already known nematocides). Following incubation for 40 h, MMV1794206 exhibited an IC50 of 0.22 ± 0.09 μM (MMI: 100%). In comparison, the monepantel control displayed an IC50 of 0.03 ± 0.01 μM (MMI: 95%).
Given that compound MMV1794206 displayed significant activity on H. contortus larvae and adult worms as well as C. elegans young adults, its toxicity was assessed by measuring cell death using crystal violet staining. This compound was shown to be both cytotoxic (CC50 < 0.7 μM; Fig. 3A) and mitotoxic (MC50 < 0.7 μM; Fig. 3B) against HepG2 (human hepatoma) cells.
Toxicity assessment of MMV1794206, monepantel and moxidectin on human hepatoma (HepG2) cells, with reference to two positive controls, doxorubicin (Dox; cytotoxic) and M-666 (mitotoxic). For each compound, the (A) half-maximal cytotoxic concentration (CC50) and (B) half-maximal mitotoxic concentration (MC50) were established via a cell viability assay after 48 h of incubation. Crystal violet staining was used to measure the absorbance (595 nm) of treated cells, which was negative- (blank) and baseline- (100% cell viability) corrected. Data points represent triplicates and are presented as a mean ± standard deviation (SD)
Discussion
The screening of the Global Health Priority Box, a collection of 240 molecules with activity against drug-resistant malaria, neglected tropical diseases or vector species, revealed and/or reidentified several compounds with in vitro activity on H. contortus and/or C. elegans.
Compounds with previously identified activity on H. contortus—chlorfenapyr (MMV1577458) [64], tolfenpyrad (MMV688934) [49] and rotenone (MMV0002519) [63]—were all reidentified as hit compounds in this study. Two compounds—MMV1577463 and MMV027339—were initially identified as hits (83 and 72% motility reduction, respectively), but upon further potency evaluation were found to display IC50 values (against H. contortus) of > 40 μM. Of note, two compounds, MMV1794214 and MMV1794206, were confirmed as inhibitors of H. contortus larval motility (IC50 values of 4.5 and 18 μM, respectively), with MMV1794206 also showing a significant reduction of larval development and motility of adult females of H. contortus in vitro. Although monepantel was more potent at reducing larval motility and development, compound MMV1794206 inhibited the motility of adult females (100% at 12 h) before monepantel did (66% and 100% at 12 and 24 h, respectively).
Of the eight compounds identified as hits based on motility reduction in C. elegans, seven—i.e. chlorfenapyr (MMV1577458), moxidectin (MMV1633828), ivermectin (MMV672931), selamectin (MMV002231), milbemectin (a mixture of milbemycin A3 and milbemycin A4; MMV1578924), abamectin (MMV1577454) and eprinomectin (MMV1633829)—had been investigated previously for antiparasitic activity and/or are commercially available nematocides. Ivermectin, selamectin, abamectin and eprinomectin are all well-known macrocyclic lactones belonging to the chemical family of avermectins [65]; moxidectin and milbemectin are milbemycin macrocyclic lactones [66]. One compound, MMV1794206, was also identified as a hit against C. elegans, and validated as a potent motility inhibitor with an IC50 of ~ 0.22 μM, compared with 0.03 μM for monepantel. However, MMV1794206 was shown to be toxic to a human cell line (HepG2 cells), suggesting likely adverse effects in mammals administered with this compound.
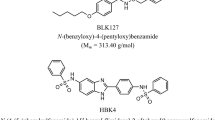
The potent effect of MMV1794206—herein referred to as ‘flufenerim’—against most of the developmental stages of H. contortus (see [67]), and the activity on the related free-living worm C. elegans, indicates that this compound is a potential candidate for nematocide development—however, the demonstrated cell toxicity to HepG2 cells presents a barrier for future development. Structurally, flufenerim (Fig. 4)—a 4-aminopyrimidine derivative—is primed for a diverse range of structural alterations via a concise synthetic route [68]. Thus, a structure–activity relationship investigation of flufenerim would be both feasible and cost-effective, but would need to focus on both the optimisation of anthelmintic activity and the elimination of toxicity to mammalian cells, a significant challenge.
Flufenerim has been previously developed as a pesticide against agricultural pests, including aphids (Aphis medicaginis and Myzus persicae), a fungus (Pseudoperonospora cubensis), moths (Mythimna separata and Spodoptera littoralis) and whiteflies (Bemisia tabaci) [68,69,70]. The mode of action of flufenerim is unclear. Ghanim et al. [69] showed a decrease in acetylcholine esterase (AChE) activity in whiteflies (in vitro and in vivo) following treatment with flufenerim, but it was not confirmed whether this was a result of a direct or indirect effect; Liu et al. [68] reported a similar decrease in AChE activity in aphids. Notably, one drug, naphthalophos (an organophosphate), which inhibits nematode AChE activity was commercially available (in Australia) for use in livestock (as an anthelmintic). Although relatively rare, resistance to naphthalophos in parasitic worms has been reported [71, 72], yet the use of organophosphates is unwarranted due to a relatively narrow safety margin [73]. In contrast, although the Insecticide Resistance Action Committee (IRAC) has not assigned flufenerim’s mode of action, another structurally related acaricide, pyrimidifen, is classified as a mitochondrial complex I electron transport inhibitor (METI) [74]. The conserved 5-chloropyrimidin-4-amine motif does suggest that pyrimidifen and flufenerim share a similar mode of action, namely, as METIs. Several METI insecticides have been previously identified to display anthelmintic activity—notably, the pyrazole compounds tebufenpyrad and tolfenpyrad [75], and the rotenoid compound, rotenone [64]. In addition, a preliminary study [75] suggested that tolfenpyrad may indeed disrupt or interrupt mitochondrial function in H. contortus. However, tolfenpyrad was further shown to inhibit respiration in rat hepatoma (FAO cell line) cells, with the level of inhibition of oxygen consumption in FAO cells being positively correlated with compound-induced murine toxicity [76]. Tolfenpyrad had also been previously developed and commercialised as an acaricide ear-tag (for use in cattle; Tolfenpro®), but was recalled due to ocular inflammation associated with treatment [77]. Thus, in the case of tolfenpyrad and other METI parasiticides, concerns of mammalian toxicity associated with drug treatment have prevented their development. Indeed, given the high toxicity associated with flufenerim, it will be critical for future compound optimisation and lead development efforts to include rigorous toxicity testing.
If flufenerim were to achieve anthelmintic action via AChE inhibition, mitochondrial interruption and/or another yet unknown pathway, significant laboratory-based target identification and validation experiments would need to be conducted. Importantly, a significant delineation in structure between the nematode and mammalian protein target(s) would be critical in achieving selectivity for the parasite. Anthelmintic target identification and validation (in parasitic species and the free-living C. elegans; cf. [45]) has been achieved via competition assays [78,79,80,81], electrophysiological studies [82, 83], resistance studies [18, 84,85,86,87] and, more recently, RNA interference (RNAi) (e.g., [88]). Recent technological advances have also paved the way for mass spectrometry-based investigations of protein–drug interactions (reviewed by [47, 48]). Methods such as thermal proteome profiling (TPP) have been utilised to identify protein targets for several therapeutics [89,90,91,92,93,94]—importantly, this method has been used to uncover candidate drug targets of novel nematocides in H. contortus (see [95]). Such an approach could also be applied to flufenerim, possibly identifying the drug–protein interactions in this and related nematodes, and providing insight into the anthelmintic mode of action of this candidate.
Conclusion
To address the global socioeconomic impacts of widespread drug resistance in parasitic nematodes of livestock animals, new anthelmintics with novel modes of action are needed. Using two model nematodes, H. contortus and C. elegans, we screened a small collection of chemical entities, the Global Health Priority Box, for nematocidal and nematostatic activity. We identified one compound, MMV1794206 (flufenerim), which displayed in vitro anthelmintic activity against key developmental stages of the parasitic worm H. contortus, and also inhibited the motility of the related, free-living worm C. elegans. However, MMV1794206 was also shown to be toxic to a mammalian cell line (HepG2). Future work should focus on the identification and validation of flufenerim–nematode protein interactions and on undertaking a structure–activity relationship investigation to both optimise anthelmintic activity and eliminate mammalian cell toxicity.
Availability of data and materials
All data generated and analysed during this study are included in this published article.
Abbreviations
- AChE:
-
Acetylcholine esterase
- DMSO:
-
Dimethyl sulfoxide
- L3:
-
Third-stage larvae
- xL3:
-
Exsheathed third-stage larva
- L4:
-
Fourth-stage larva
- METI:
-
Mitochondrial complex I electron transport inhibitor
- MMI:
-
Maximum motility inhibition
References
Institutes for Health Metrics and Evaluation. Intestinal nematode infections – level 3 cause. 2020. https://www.healthdata.org/results/gbd_summaries/2019/intestinal-nematode-infections-level-3-cause. Accessed 12 Sep 2023.
Charlier J, Rinaldi L, Musella V, Ploeger HW, Chartier C, Rose Vineer H, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med. 2021;182:105103.
World Health Organization. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2021.
Selzer PM, Epe C. Antiparasitics in animal health: quo vadis? Trends Parasitol. 2021;37:77–89.
Shephard R, Ware JW, Blomfield B, Neithe G. Priority list of endemic diseases for the red meat industry—2022 update. North Sydney: Meat & Livestock Australia Limited; 2022.
Kahn LP, Woodgate RG. Integrated parasite management: products for adoption by the Australian sheep industry. Vet Parasitol. 2012;186:58–64.
Maqbool I, Wanil ZA, Shahardar RA, Allaie IM, Shah MM. Integrated parasite management with special reference to gastro-intestinal nematodes. J Parasit Dis. 2017;41:1–8.
Terrill TH, Miller JE, Burke JM, Mosjidis JA, Kaplan RM. Experiences with integrated concepts for the control of Haemonchus contortus in sheep and goats in the United States. Vet Parasitol. 2012;186:28–37.
Kearney PE, Murray PJ, Hoy JM, Hohenhaus M, Kotze A. The ‘toolbox’ of strategies for managing Haemonchus contortus in goats: what’s in and what’s out. Vet Parasitol. 2016;220:93–107.
Tinkler SH. Preventative chemotherapy and anthelmintic resistance of soil-transmitted helminths—can we learn nothing from veterinary medicine? One Health. 2020;9:100106.
Claerebout E, Geldhof P. Helminth vaccines in ruminants: from development to application. Vet Clin North Am Food Anim Pract. 2020;36:159–71.
Sargison ND. Keys to solving health problems in small ruminants: anthelmintic resistance as a threat to sustainable nematode control. Small Rumin Res. 2016;142:11–5.
Kotze AC, Prichard RK. Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv Parasitol. 2016;93:397–428.
Hodgkinson JE, Kaplan RM, Kenyon F, Morgan ER, Park AW, Paterson S, et al. Refugia and anthelmintic resistance: concepts and challenges. Int J Parasitol Drugs Drug Resist. 2019;10:51–7.
Kaplan RM. Biology, epidemiology, diagnosis, and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Vet Clin North Am Food Anim Pract. 2020;36:17–30.
Rose Vineer H, Morgan ER, Hertzberg H, Bartley DJ, Bosco A, Charlier J, et al. Increasing importance of anthelmintic resistance in European livestock: creation and meta-analysis of an open database. Parasite. 2020;27:69.
Charlier J, Bartley DJ, Sotiraki S, Martinez-Valladares M, Claerebout E, von Samson-Himmelstjerna G, et al. Anthelmintic resistance in ruminants: challenges and solutions. Adv Parasitol. 2022;115:171–227.
Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–80.
Lee BH, Clothier MF, Dutton FE, Nelson SJ, Johnson SS, Thompson DP, et al. Marcfortine and paraherquamide class of anthelmintics: discovery of PNU-141962. Curr Top Med Chem. 2002;2:779–93.
Little PR, Hodges A, Watson TG, Seed JA, Maeder SJ. Field efficacy and safety of an oral formulation of the novel combination anthelmintic, derquantel-abamectin, in sheep in New Zealand. N Z Vet J. 2010;58:121–9.
Little PR, Hodge A, Maeder SJ, Wirtherle NC, Nicholas DR, Cox GG, et al. Efficacy of a combined oral formulation of derquantel-abamectin against the adult and larval stages of nematodes in sheep, including anthelmintic-resistant strains. Vet Parasitol. 2011;181:180–93.
Nixon SA, Welz C, Woods DJ, Costa-Junior L, Zamanian M, Martin RJ. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Int J Parasitol Drugs Drug Resist. 2020;14:8–16.
Herath HMPD, Taki AC, Rostami A, Jabbar A, Keiser J, Geary TG, et al. Whole-organsim phenotypic screening methods used in early-phase anthelmintic drug discovery. Biotechnol Adv. 2022;57:107937.
Brown HD, Matzuk AR, Ilves IR, Peterson LH, Harris SA, Sarett LH, et al. Antiparasitic drugs. IV. 2-(4’-Thiazolyl)-benzimidazole, a new anthelmintic. J Am Chem Soc. 1961;83:1764–5.
Gordon HM. Thiabendazole: a highly effective anthelmintic for sheep. Nature. 1961;1961:1409–10.
Thienpont D, Vanparijs OFJ, Raeymaekers AHM, Vandenberk J, Demoen PJA, Allewijn FTN, et al. Tetramisole (R8299), a new, potent broad spectrum anthelmintic. Nature. 1966;209:1084–6.
Egerton JR, Ostlind DA, Blair LS, Eary CH, Suhayda D, Cifelli S, et al. Avermectins, a new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob Agents Chemother. 1979;15:372–8.
Theresia M, Braissant O, Haggenmüller Y, Keiser J. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J Clin Microbiol. 2011;49:1217–25.
Keiser J, Manneck T, Kirchhofer C, Braissant O. Isothermal microcalorimetry to study the activity of triclabendazole and its metabolites on juvenile and adult Fasciola hepatica. Exp Parasitol. 2013;133:265–8.
Rinaldi G, Loukas A, Brindley PK, Irelan JT, Smout MJ. Viability of developmental stages of Schistosoma mansoni quantified with xCELLigence worm real-time motility assay (xWORM). Int J Parasitol Drugs Drug Resist. 2015;5:141–8.
Storey B, Marcellino C, Miller M, Maclean M, Mostafa E, et al. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “the worminator.” Int J Parasitol Drugs Drug Resist. 2014;4:233–43.
Puckering T, Thompson J, Sathyamurthy S, Sukumar S, Shapira T, Ebert P. Automated wormscan. F1000Res. 2019;6:192.
Partridge FA, Brown AE, Buckingham SD, Willis NJ, Wynne GM, Forman R, et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int J Parasitol Drugs Drug Resist. 2018;8:8–21.
Aguiar PHN, Fernandes NMGS, Zani CL, Mourão MM. A high-throughput colorimetric assay for detection of Schistosoma mansoni viability based on tetrazolium salt XTT. Parasit Vectors. 2017;10:300.
Phiri AM, de Pomerai DI, Buttle DJ, Behnke JM. A novel assay for the detection of anthelmintic activity mediated by cuticular damage to nematodes: validation on Caenorhabditis elegans exposed to cysteine proteinases. Parasitology. 2017;144:583–93.
Abriola L, Hoyer D, Caffrey CR, Williams DL, Yoshino TP, Vermeire JJ. Development and optimization of a high-throughput screening method utilizing Ancylostoma ceylanicum egg hatching to identify novel anthelmintics. PLoS ONE. 2019;14:e0217019.
Cintra GAS, Neto BAD, Carvalho PHPR, Moraes CB, Freitas-Junior LH. Expanding the biological application of fluorescent benzothiadiazole derivatives: a phenotypic screening strategy for anthelmintic drug discovery using Caenorhabditis elegans. SLAS Discov. 2019;24:755–65.
Taki AC, Byrne JJ, Wang T, Sleebs BE, Nguyen N, Hall RS, et al. High-throughput phenotypic screen for anthelmintic activity on Haemonchus contortus. Pharmaceuticals. 2021;14:616.
Taki AC, Byrne JJ, Wang T, Boag PR, Jabbar A, Gasser RB. Practical high-throughput method to screen compounds for anthelmintic activity against Caenorhabditis elegans. Molecules. 2021;26:4156.
Wang T, Nie S, Ma G, Korhonen PK, Koehler AV, Ang C-S, et al. The developmental lipidome of Haemonchus contortus. Int J Parasitol. 2018;48:887–95.
Wang T, Ma G, Ang C-S, Korhonen PK, Xu R, Nie S, et al. Somatic proteome of Haemonchus contortus. Int J Parasitol. 2019;49:311–20.
Wang T, Ma G, Ang C-S, Korhonen PK, Koehler AV, Young ND, et al. High throughput LC-MS/MS-based proteomic analysis of excretory-secretory products from short-term in vitro culture of Haemonchus contortus. J Proteomics. 2019;204:103375.
Wang T, Ma G, Ang C-S, Korhonen PK, Stroehlein A, Young ND, et al. The developmental phosphoproteome of Haemonchus contortus. J Proteomics. 2020;213:103615.
Doyle SR, Tracey A, Laing R, Holroyd N, Bartley D, Bazant W, et al. Genomic and transcriptomic variation defines the chromosome-scale assembly of Haemonchus contortus, a model gastrointestinal worm. Commun Biol. 2020;3:656.
Hahnel SR, Dilks CM, Heisler I, Andersen EC, Kulke D. Caenorhabditis elegans in anthelmintic research—old model, new perspectives. Int J Parasitol Drugs Drug Resist. 2020;14:237–48.
Harris TW, Arnaboldi V, Cain S, Chan J, Chen WJ, Cho J, et al. WormBase: a modern model organism information resource. Nucl Acids Res. 2020;48:D762–7.
Hong KT, Lee J-S. Label-free proteome profiling as a quantitative target identification technique for bioactive small molecules. Biochemistry. 2020;59:213–5.
Ha J, Park H, Park J, Park SB. Recent advances in identifying protein targets in drug discovery. Cell Chem Biol. 2021;28:394–423.
Preston S, Jiao Y, Jabbar A, McGee SL, Laleu B, Willis P, et al. Screening of the ‘Pathogen Box’ identifies an approved pesticide with major anthelmintic activity against the barber’s pole worm. Int J Parasitol Drugs Drug Res. 2016;6:329–34.
Jiao Y, Preston S, Koehler AV, Stroehlein AJ, Chang BCH, Simpson KJ, et al. Screening of the ‘Stasis Box’ identifies two kinase inhibitors under pharmaceutical development with activity against Haemonchus contortus. Parasit Vectors. 2017;10:323.
Shanley HT, Taki AC, Byrne JJ, Jabbar A, Wells TNC, Samby K, et al. A high-throughput phenotypic screen of the ‘Pandemic Response Box’ identifies a quinoline derivative with significant anthelmintic activity. Pharmaceuticals. 2022;15:257.
Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300.
Schwarz EM, Korhonen PK, Campbell BE, Young ND, Jex AR, Jabbar A, et al. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013;14:R89.
Preston S, Jabbar A, Nowell C, Joachim A, Ruttkowski B, Baell J, et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int J Parasitol. 2015;45:333–43.
Stiernagle T. Maintenance of C. elegans. In: The C. elegans Research Community, editor. Wormbook. Pasadena: Wormbook; 2006. p. 1–11.
Porta-de-la-Riva M, Fontrodona L, Villanueva A, Cerón J. Basic Caenorhabditis elegans methods: synchronization and observation. J Vis Exp. 2012;64:4019.
Le TG, Kundu A, Ghoshal A, Nguyen NH, Preston S, Jiao Y, et al. Optimization of novel 1-methyl-1H-pyrazole-5-carboxamides leads to high potency larval development inhibitors of the barber’s pole worm. J Med Chem. 2018;61:10875–94.
Zhang J-H, Chung TDY, Oldenburg KR. A simple statistical parameter for use in the evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73.
Taki AC, Brkljača R, Wang T, Koehler AV, Ma G, Danne J, et al. Natural compounds from the marine brown alga Caulocystis cephalornithos with potent in vitro-activity against the parasitic nematode Haemonchus contortus. Pathogens. 2020;9:550.
Swiss RL, Will Y. Assessment of mitochondrial toxicity in HepG2 cells cultured in high-glucose- or galactose- containing media. Curr Protoc Toxicol. 2011;49:1–14.
Kamalian L, Chadwick AE, Bayliss M, French NS, Monshouwer M, Snoeys J, et al. The utility of HepG2 cells to identify direct mitochondrial dysfunction in the absence of cell death. Toxicol In Vitro. 2015;29:732–40.
Śliwka L, Wiktorska K, Suchocki P, Milczarek M, Mielczarek S, Lubelska K, et al. The comparison of MTT and CVS assays for the assessment of anticancer agent interactions. PLoS ONE. 2016;11:e0155772.
Herath HMPD, Preston S, Hofmann A, Davis RA, Koehler AV, Chang BCH, et al. Screening of a small, well-curated natural product-based library identifies two rotenoids with potent nematocidal activity against Haemonchus contortus. Vet Parasitol. 2017;244:172–5.
Herath HMPD, Song H, Preston S, Jabbar A, Wang T, McGee SL, et al. Arylpyrrole and fipronil analogues that inhibit the motility and/or development of Haemonchus contortus in vitro. Int J Parasitol Drugs Drug Res. 2018;8:379–85.
Batiha GE-S, Alqahtani A, Ilesanmi OB, Saati AA, El-Mleeh A, Hetta HF, et al. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals. 2020;2020:196.
Takiguchi YO, Mishima H, Okuda M, Terao M, Aoki A, Fukuda R. Milbemycins, a new family of macrolide antibiotics: fermentation, isolation and physico-chemical properties. J Antibiot. 1980;33:1120–7.
Besier RB, Kahn LP, Sargison ND, Van Wyk JA. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv Parasitol. 2016;93:95–143.
Liu X-H, Wen Y-H, Cheng L, Xu T-M, Wu N-J. Design, synthesis, and pesticidal activities of pyrimidin-4-amine derivatives bearing a 5-(trifluoromethyl)-1,2,4-oxadiazole moiety. J Agric Food Chem. 2021;69:6968–80.
Ghanim M, Lebedev G, Kontsedalov S, Ishaaya I. Flufenerim, a novel insecticide acting on diverse insect pests: biological mode of action and biochemical aspects. J Agric Food Chem. 2011;59:2839–44.
Wang L, Yang Z, Pan S, Zhu M, Guan A, Sun X, et al. A new potential aphicide against Myzus persicae: design, synthesis and 3D-QSAR of novel phenoxypyridine derivatives containing 4-aminopyridine. J Mol Struct. 2022;1262:132949.
Green PE, Forsyth BA, Rowan KJ, Payne G. The isolation of a field strain of Haemonchus contortus in Queensland showing multiple anthelmintic resistance. Aus Vet J. 1981;57:79–84.
Lamb J, Elliot T, Chambers M, Chick B. Broad spectrum anthelmintic resistance of Haemonchus contortus in Northern NSW of Australia. Vet Parasitol. 2017;241:48–51.
Povey AC. Gene-environmental interactions and organophosphate toxicity. Toxicol. 2010;278:294–304.
Perry AS, Yamamoto I, Ishaaya I, Perry RY. Compounds interfering with ATP synthesis. In: McNeal BL, Tardieu F, Van Keulen H, Van Vleck D, editors. Insecticides in agriculture and environment. Berlin: Springer-Verlag; 1998. p. 121–5.
Jiao Y, Preston S, Song H, Jabbar A, Liu Y, Baell J. Assessing the anthelmintic activity of pyrazole-5-carboxamide derivatives against Haemonchus contortus. Parasit Vectors. 2017;10:272.
Preston S, Garcia-Bustos J, Hall LG, Martin SD, Le TG, Kundu A, et al. 1-Methyl-1H-pyrazole-5-carboxamide derivatives exhibit unexpected acute mammalian toxicity. J Med Chem. 2021;64:840–4.
Bayer. Tolfenpro® insecticide ear tag recall. Kansas: Bayer; 2016.
Friedman PA, Platzer EG. Interaction of anthelmintic benzimidazoles and benzimidazole derivatives with bovine brain tubulin. Biochim Biophys Acta. 1978;544:605–14.
Robertson AP, Clark CL, Burns TA, Thompson DP, Geary TG, Trailovic SM, et al. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J Pharmacol Exp Ther. 2002;2002:853–60.
Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006;20:2606–8.
Abongwa M, Marjanovic DS, Tipton JG, Zheng F, Martin RJ, Trailovic SM, et al. Monepantel is a non-competitive antagonist of nicotinic acetylcholine receptors from Ascaris suum and Oesophagostomum dentatum. Int J Parasitol Drugs Drug Resist. 2018;8:36–42.
Puttachary S, Trailovic SM, Robertson AP, Thompson DP, Woods DJ, Martin RJ. Derquantel and abamectin: effects and interactions on isolated tissues of Ascaris suum. Mol Biochem Parasitol. 2013;188:79–86.
Baur R, Beech R, Sigel E, Rufener L. Monepantel irreversibly binds to and opens Haemonchus contortus MPTL-1 and Caenorhabditis elegans ACR-20 receptors. Mol Pharmacol. 2015;87:96–102.
Robertson AP, Bjorn HE, Martin RK. Resistance to levamisole resolved at the single-channel level. FASEB J. 1999;13:749–60.
Burns AR, Kwok TCY, Howard A, Houston E, Johanson K, Chan A, et al. High-throughput screening of small molecules for bioactivity and target identification in Caenorhabditis elegans. Nat Protoc. 2006;1:1906–14.
Burns AR, Luciani GM, Musso G, Bagg R, Yeo M, Zhang Y, et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat Commun. 2015;6:7485.
Gibson SB, Ness-Cohn E, Andersen EC. Benzimidazoles cause lethality by inhibiting the function of Caenorhabditis elegans neuronal beta-tubulin. Int J Parasitol Drugs Drug Resist. 2022;20:89–96.
Khan S, Nisar A, Yuan J, Luo X, Dou X, Liu F, et al. A whole genome re-sequencing based GWA analysis reveals candidate genes associated with ivermectin resistance in Haemonchus contortus. Genes. 2020;11:367.
Savitski MM, Reinhard FBM, Franken H, Werner T, Savitski MF, Eberhard D. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science. 2014;346:1255784.
Franken H, Mathieson T, Childs D, Sweetman GMA, Werner T, Tögel I, et al. Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat Protoc. 2015;10:1567–93.
Reinhard FBM, Eberhard D, Werner T, Franken H, Childs D, Doce C, et al. Thermal proteome profiling monitors ligand interactions with cellular membrane proteins. Nat Methods. 2015;12:1129–31.
Becher I, Werner T, Doce C, Zaal EA, Tögel I, Khan CA, et al. Thermal profiling reveals phenylalanine hydroxylase as an off-target of panobinostat. Nat Chem Biol. 2016;12:908–10.
Perrin J, Werner T, Kurzawa N, Rutkowska A, Childs DD, Kalxdorf M, et al. Identifying drug targets in tissues and whole blood with thermal-shift profiling. Nat Biotechnol. 2020;38:303–8.
Mateus A, Kurzawa N, Perrin J, Bergamini G, Savitski MM, et al. Drug target identification in tissues by thermal proteome profiling. Annu Rev Pharmacol Toxicol. 2022;6:465–82.
Taki AC, Wang T, Nguyen NH, Ang C-S, Leeming MG, Nie S, et al. Thermal proteome profiling reveals Haemonchus orphan protein HCO_011565 as a target of the nematocidal small molecule UMW-868. Front Pharmacol. 2022;13:1014804.
Acknowledgements
We would like to acknowledge the team at Medicines for Malaria Venture (MMV), for curating and supplying the Global Health Priority Box, and their ongoing support.
Funding
BES is a Corin Fellow. HTS was supported by a Melbourne Research Scholarship. We gratefully acknowledge the financial support from the Australian Research Council (LP220200614 and LP180101085), PhylumTECH and Oz Omics Pty Ltd.
Author information
Authors and Affiliations
Contributions
RBG, BES and HTS formulated the overarching research goals. ACT, JJB and HTS developed the methodology and conducted the research; ACT and HTS analysed and validated the data. HTS and RBG drafted the manuscript, with valuable revisions conducted by BES, ACT, NN, JJB, TNCW and AJ. This project was supervised and administrated by RBG, BES and ACT. RBG, AJ and BES acquired the funding for this project. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the institutional animal ethics guidelines (permit no. 23983-2811-4; The University of Melbourne).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shanley, H.T., Taki, A.C., Byrne, J.J. et al. A phenotypic screen of the Global Health Priority Box identifies an insecticide with anthelmintic activity. Parasites Vectors 17, 131 (2024). https://doi.org/10.1186/s13071-024-06183-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06183-y