Abstract
Background
Long-lasting insecticidal nets (LLINs) may have different impacts on distinct mosquito vector species. We assessed the efficacy of pyrethroid-pyriproxyfen and pyrethroid-chlorfenapyr LLINs on the density of Anopheles gambiae s.s. and An. coluzzii compared to pyrethroid-only nets in a three-arm cluster randomised control trial in Benin.
Methods
Indoor and outdoor collections of adult mosquitoes took place in 60 clusters using human landing catches at baseline and every 3 months for 2 years. After morphological identification, around 15% of randomly selected samples of An. gambiae s.l. were dissected to determine parity, species (using PCR).
Results
Overall, a total of 46,613 mosquito specimens were collected at baseline and 259,250 in the eight quarterly collections post-net distribution. Post-net distribution, approximately 70% of the specimens of An. gambiae s.l. speciated were An. coluzzii, while the rest were mostly composed of An. gambiae s.s. with a small proportion (< 1%) of hybrids (An. gambiae/coluzzii). There was no evidence of a significant reduction in vector density indoors in either primary vector species [An. coluzzii: DR (density ratio) = 0.62 (95% CI 0.21–1.77), p = 0.3683 for the pyrethroid-pyriproxyfen LLIN and DR = 0.56 (95% CI 0.19–1.62), p = 0.2866 for the pyrethroid-chlorfenapyr LLIN, An. gambiae s.s.: DR = 0.52 (95% CI 0.18–1.46), p = 0.2192 for the pyrethroid-pyriproxyfen LLIN and DR = 0.53 (95% CI 0.19–1.46), p = 0.2222 for the pyrethroid-chlorfenapyr]. The same trend was observed outdoors. Parity rates of An. gambiae s.l. were also similar across study arms.
Conclusions
Compared with pyrethroid-only LLINs, pyrethroid-chlorfenapyr LLINs and pyrethroid-pyriproxyfen LLINs performed similarly against the two primary mosquito species An. gambiae s.s. and An. coluzzii in Benin.
Graphical Abstract

Similar content being viewed by others
Background
After 2 decades of success in reducing the malaria burden in sub-Saharan Africa, cases are now increasing in many countries [1]. Some of the factors explaining this resurgence are widespread pyrethroid resistance in Anopheles vectors of malaria and more recently the disruptions caused by the COVID-19 pandemic [1]. Given that pyrethroid-only long-lasting insecticidal nets (LLINs) were the sole class of nets recommended for community use by the World Health Organization (WHO) until recently, and the increasingly worrying epidemiological situation of malaria globally, urgent actions aiming to develop a new generation of LLINs are needed.
LLINs incorporating a mixture of a pyrethroid insecticide plus piperonyl butoxide demonstrated better efficacy on malaria than standard pyrethroid-only LLINs [2, 3] and were the first new second-generation LLINs to receive a WHO policy recommendation in 2017 [4]. Two other types of LLIN incorporating a pyrethroid and a second insecticide with a different mode of action, either pyriproxyfen (an insect growth regulator that inhibits fertility) or chlorfenapyr (a pyrrole insecticide which disrupts mitochondrial oxidative phosphorylation), were assessed in randomized controlled trials (RCTs) in Tanzania [5] and Benin [6]. In both trials, Interceptor G2® LLINs (mixture of alpha-cypermethrin-chlorfenapyr) provided clear additional protection against malaria compared to standard LLINs with 44 and 46% reductions in malaria case incidence after 2 years of follow-up in Tanzania and Benin, respectively. The effect of Royal Guard® LLINs (mixture of alphacypermethrin-pyriproxyfen) was not as evident and reductions in malaria incidence was marginal in both countries [5, 6]. In March 2023, Interceptor® G2, the first pyrethroid-chlorfenapyr LLIN in class product, received a full recommendation from the WHO, while the pyrethroid-pyriproxyfen LLIN, Royal Guard®, was given a conditional recommendation pending additional evidence on efficacy [7].
Entomological indicators play a crucial role in understanding epidemiological results, as the impact of vector control interventions may vary depending on vector species composition, behaviours (outside/inside biting or resting) and insecticide resistance [8]. Some insecticides may be more effective on secondary vectors rather than primary ones in an area; a better understanding of these phenomena will help refine future prevention strategies. In the Tanzania RCT, the pyrethroid-chlorfenapyr LLINs were the most effective against Anopheles funestus s.l. for 3 years, with PBO LLINs remaining effective for 2 years. The same authors also showed that neither of the dual active-ingredient (ai) LLINs succeeded in controlling Anopheles arabiensis [9]. The main entomological outcomes of the trial in Benin were reported for three malaria vector complexes (Anopheles gambiae s.l., An. funestus and An. nili) pooled together [6]. In Benin, the two primary vectors are Anopheles gambiae s.s. and An. coluzzii (both part of the An. gambiae s.l complex) with composition and insecticide resistance frequencies varying across the country [10, 11].
The present study reports a secondary analysis of the RCT entomological data investigating the efficacy of Royal Guard® LLINs and Interceptor® G2 LLINs compared to pyrethroid-only LLINs on the two primary vectors found in the study area, An. coluzzii and An. gambiae s.s.
Methods
Study area and design
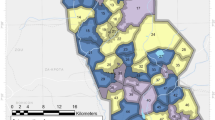
The present three-arm cluster RCT was conducted in Cove, Ouinhi and Zagnanado districts, located in the Zou region, Central Benin. Malaria endemicity was high, with transmission occurring year-round. Deployment of LLINs every 3 years remained the principal vector control strategy in this region where An. coluzzii and An. gambiae s.s., the main malaria vector species, displayed high pyrethroid resistance intensity [12]. The study area and trial design have been described previously [13] and the primary analyses of the trial were also published previously [6]. Briefly, the region consisted of 123 villages divided into 60 clusters, each formed from a village or a group of villages. Restricted randomization was used to randomly assign 20 clusters to each of the three study LLINs (Fig. 1). These were: (i) Royal Guard® LLIN, a 120-denier polyethylene net incorporating a mixture of 220 mg/m2 alpha-cypermethrin and 220 mg/m2 pyriproxyfen (Disease Control Technologies, Greer, SC, USA), (ii) Interceptor® G2® LLIN, a 100-denier polyester net coated with 200 mg/m2 chlorfenapyr and 100 mg/m2 alpha-cypermethrin (BASF SE, Ludwigshafen, Germany), and (iii) Interceptor® LLIN, a 100-denier polyester netting that incorporates 200 mg/m2 of alpha-cypermethrin (BASF SE, Ludwigshafen, Germany).
Procedures
Written consent to participate in the trial was sought from the household heads, and the adult volunteers that collected mosquitoes through human landing catches (HLCs), after being vaccinated against yellow fever. Prior to the study net distribution, one round of mosquito collection occurred between September and October 2019. The net distribution was conducted in March 2020 with support from the National Malaria Control Programme, with a ratio of one net for every two people. Due to the COVID-19 pandemic, there was no data collection between April and May 2020; then entomological collections using HLCs in each cluster were conducted every 3 months leading to eight collection rounds between June 2020 and April 2022 [6,7,8,9,10,11,12,13]. A total of four houses were surveyed in the core area of each of the 60 clusters per round (total 240 collection nights indoors and 240 outdoors per round), with the first randomly selected and the three others chosen in a 20-m radius around the first. In each house, collections were done indoors and outdoors from 19:00 to 7:00. Each night a first group of trained collectors worked between 7:00 p.m. and 01:00 a.m. and were substituted by a second group between 01:00 and 07:00 a.m. They used haemolysis tubes and flashlights to collect all mosquitoes on their lower limbs before they received any bites. Collected mosquitoes were morphologically identified to species level using a binocular microscope and the taxonomic identification key of Gillies et al. [14]. About 15% of An. gambiae s.l. randomly sampled across collection hours and locations (indoor and outdoor) were dissected to assess the parity rate [15]. Molecular species identification was also performed using PCR [16]. The trial profile is provided in Fig. 2.
Trial profile for the vector density. HHs household visits. LLIN long-lasting insecticidal net. PY pyrethroid. PPF pyriproxyfen. # For each cluster, four households were randomly selected for each collection rounds. *HH excluded from the analysis are those belonging to clusters with: → no mosquito speciated while number collected ≥ 1; → 1 ≤ number collected ≤ 10 and 0% < % speciated < 30%; → number collected > 10 and 0 < number speciated < 5. Four consecutive collection rounds were performed in each of the 2 post-intervention study years
Outcomes
The primary entomological outcome was measured indoors and outdoors for both An. gambiae s.s. and An. coluzzii. The density of vectors was defined as the estimated mean number of each mosquito species collected per person per night. This indicator, measured at the cluster-visit level, was calculated at baseline and averaged across collections for year 1 and year 2 post-net distribution. Density was compared between each intervention arm and the pyrethroid LLIN arm (control arm). Secondary entomological outcomes included were mosquito species composition, relative proportion of each molecular species infected and parity rate (the proportion of An. gambiae s.l. found parous).
Statistical analysis
A double entry of the entomological monitoring data was performed in CS Pro 7.2 software-designed databases. The datasets were cleaned with Stata 15.0 (Stata Corp., College Station, TX). As only a proportion (around 15%) of the total Anopheles collected were speciated, molecular species density was calculated at cluster level by multiplying the mean number of An. gambiae s.l. per cluster visited by the proportion of molecular species (An. coluzzii and An. gambiae s.s.). Some household visit data were excluded from the analysis using the following criteria:
-
No mosquito speciated while number collected ≥ 1,
-
1 ≤ Number of collected mosquitoes ≤ 10, and 0% < % of speciated mosquitoes < 30%,
-
Number of collected mosquitoes > 10, and 0 < number of mosquito speciated < 5.
The parity rate per species was calculated by dividing the number of parous mosquitoes by the total mosquitoes dissected.
Vector density, and parity rate were calculated at the cluster level. To analyse the vector density, a mixed-effect generalised linear model with a negative binomial distribution was used, while a mixed-effect logistic regression was used for parity rate. Cluster was included as a random effect.
Results
Baseline characteristics of the study area: mosquito species composition, vector density and parity rate
At baseline, a total of 46,613 mosquito specimens were collected, with 51.6% collected outdoors and the rest indoors. Overall, Anopheles mosquitoes accounted for 32.2% (n = 7264) of the mosquitoes collected indoors and varied between 24.9 and 37% of the total caught according to trial arm (Fig. 3). The majority of Anopheles were An. gambiae s.l. (87.7%). Anopheles mosquito species found in lower proportions included: Anopheles ziemanni (1.5%), followed by An. pharoensis (1.2%), An. funestus (0.9%) and An. nili (0.2%). Other mosquito species found by order of abundance were: Mansonia spp. (arm level range: 33.4–36.8%) Culex spp. (arm level range: 26.2–41.4%), Aedes spp. (arm level range: 0.3–2.0) and Coquillettidia spp. and Eretmapodites spp. (< 0.1%). Trends were similar indoors and outdoors (Fig. 3).
During this period, a total of 1797 An. gambiae s.l. (sporozoite positive samples plus a subset of randomly selected negative ones) were tested by PCR to identify sibling species. Overall, we found 53.9% An. coluzzii, and the rest were An. gambiae s.s. The relative proportion of An. coluzzii was usually the highest in all the arms, indoors and outdoors, except for the pyrethroid-CFP LLIN indoors where An. gambiae s.s. was found in the majority (Table 1).
The estimated density of An. coluzzii ranged between 10.6 and 17.6 bites/person/night (b/p/n) indoors and between 10.4 and 14.6 b/p/n outdoors according to arms. Anopheles gambiae s.s. estimated density varied between 9.3 and 11.4 b/p/n indoors and between 6.8 and 10.2 b/p/n outdoors (Table 1).
Overall, 82.1% of the total An. gambiae s.l. collected indoors and dissected (1461/1780) were found parous and 81.5% (866/1063) of those collected outdoors. Those proportions were similar among the three study arms (Table 1).
Post-intervention
Mosquito species composition
In the first year of the trial (between June 2020 and March 2021), a total of 161,569 mosquitoes were collected with a higher density outdoors (58.2%). Overall, the proportion of Anopheles collected was 23.6% (15,926/67,497) indoors and 13.8% (15,926/94,072) outdoors, with the lowest proportion found in the pyrethroid-CFP LLIN arm (28.5%) compared to pyrethroid-PPF LLIN arm (37.1%) and pyrethroid-only LLIN arm (34.4%). The proportion of Culex spp. caught was lower compared to baseline, whilst Mansonia spp. was higher across the study arms. As observed in baseline, Aedes spp. (< 3%) and other mosquitoes including both Coquillettidia spp. and Eretmapodites spp. (≤ 0.02%) were collected at lower proportions (Fig. 4).
In the second year of the trial, a total of 97,681 mosquito specimens were collected between April 2021 and April 2022. Overall, 28.8% (n = 28,084) of the total mosquitoes collected were Anopheles, with 14,876 sampled indoors and the rest outdoors. Relative proportions of Anopheles spp. and Aedes spp., both indoors and outdoors, were higher than those observed in year 1. The opposite trend was observed in Mansonia spp., and to a lesser extent in Culex spp. (Fig. 4).
A total of 8185 of the 53,723 An. gambiae s.l. collected both indoors and outdoors were tested for molecular species identification during the 2 years post-intervention. On average, An. coluzzii accounted for 71.9% of the An. gambiae s.l. indoors and outdoors over the 2 years. Across both years, indoor proportions were similar between arms and ranged between 70.5 and 73.0%, while the outdoor proportions ranged between 69.3 and 73.3% (Table 2). The majority of the remaining mosquitoes were An. gambiae s.s. with a small proportion (< 1%) of hybrids (An. gambiae/coluzzii) (Table 2). Species composition changed according to season and the relative proportion of An. coluzzii increased and peaked during the dry season between December and April each year and was the lowest in September–October during the rainy season (Additional file 1: Figure. S1).
Density in An. gambiae s.s. and An. coluzzii
Overall (year 1 + year 2), indoor estimated density of An. coluzzii in the pyrethroid-only LLIN arm was 18.6 b/p/n compared to 8.1 b/p/n in the pyrethroid-chlorfenapyr LLIN arm (DR = 0.56 95% CI (0.19–1.62); p = 0.2866) and 10.4 b/p/n in the pyrethroid-pyriproxyfen LLIN arm (DR = 0.62 95% CI (0.21–1.77); p = 0.3683). A non-significant reduction in density was observed in years 1 and 2 post-net distribution (indoors and outdoors) (Table 3).
Anopheles gambiae s.s. estimated indoor density was overall lower in the pyrethroid-pyriproxyfen LLIN arm [3.3 b/p/n, DR = 0.52 95% CI (0.18–1.46); p = 0.2192] and the pyrethroid-chlorfenapyr LLIN arm [1.9 b/p/n, DR = 0.53 95% CI (0.19–1.46); p = 0.2222] compared to the pyrethroid LLIN arm (4.1 b/p/n); however, this difference was not significant. This was also observed in each of the 2 years post-net distribution. Similar trends were found outdoors (Table 3).
Parity rate (PR) in An. gambiae s.l.
Overall, there was no evidence of a reduction in the parity rate in the two intervention arms compared to the control arm both indoors [PR = 81.6%, OR = 1.3 (95% CI 0.9–1.8), p = 0.2014 in the pyrethroid-pyriproxyfen LLIN arm, and PR = 79.8%, OR = 1.1 (95% CI 0.7–1.5), p = 0.7532 in the pyrethroid-chlorfenapyr LLIN arm, versus PR = 78.6% in the pyrethroid LLIN arm] and outdoors [PR = 80.2%, OR = 1.2 (95% CI 0.9–1.6), p = 0.2717 in the pyrethroid-pyriproxyfen LLIN arm, and PR = 80.2%, OR = 1.1 (95% CI 0.8–1.5), p = 0.5642 in the pyrethroid-chlorfenapyr LLIN arm, versus PR = 78.6% in the pyrethroid LLIN arm] in the first year of the trial. The same trend was observed during the first and the second year of the trial (Table 4).
Discussion
This secondary analysis provides further insights on the impact of pyrethroid-chlorfenapyr and pyrethroid-pyriproxyfen LLINs on the two main malaria vectors found in the Zou region, Southern Benin. Anopheles coluzzii and An. gambiae s.s. are commonly found circulating sympatrically across West Africa, but differ in their larval ecology, behaviour, migration, aestivation, and insecticide resistance mechanisms [17,18,19,20,21,22]. There is some indication that the impact of pyrethroid-chlorfenapyr LLIN was similar on both species (An. gambiae s.s. and An. coluzzii) with a slight reduction in year 2 on An. gambiae s.s. especially outdoors. Similar observation was found with pyrethroid-pyriproxyfen LLINs with some indication that the effect might be less than for the pyrethroid-chlorfenapyr LLIN.
One of the key factors for the acceptance of a vector control tool by a community is its ability to reduce the mosquito biting frequency. In the present trial, though there was not strong evidence, both pyrethroid-chlorfenapyr LLINs and pyrethroid-pyriproxyfen LLINs were found to reduce the density of An. coluzzii and An. gambiae s.s. at a broadly similar magnitude, both indoors and outdoors. By comparison, a clear differential effect was observed between the two LLINs after aggregating data of the three main malaria vector complexes (An. gambiae s.l. + An. funestus + An. nili) encountered in the study area, as the pyrethroid-pyriproxyfen LLINs reduced the indoor vector density by 42% (p = 0.11), while the pyrethroid-chlorfenapyr LLINs did significantly by 56% (p = 0.014) over the 2 first years of the trial [6]. The same trend was also observed with An. funestus, with the chlorfenapyr-pyrethroid LLIN controlling this vector species over 3 years, while the two dual a.i. LLINs had no impact on density of An. arabiensis in Tanzania [9]. The weak evidence (p > 0.05) for the reductions induced by the two dual a.i. LLINs on the density of the two primary vectors in the present trial could be partly due to data scarcity. The density was estimated at the cluster-visit level rather than at a household level to reduce bias from estimating proportions in small samples. This will have resulted in less power. In addition, from our observations in the field, the pyrethroid-pyriproxyfen LLINs were frequently used outside households for other purposes (fishing, plant protection) given its high shrinkage observed in the field as well as its ability to tear quickly. These factors may have limited the exposure time of vectors to the intervention tool, resulting in reduced sterilization effects of pyriproxyfen on vectors and a lack of effectiveness of this LLIN. Similarly, a trial previously conducted in Burkina Faso also revealed that a pyrethroid-pyriproxyfen LLIN successfully halved the entomological inoculation rate (EIR) but induced a weak reduction in clinical malaria incidence of 12% [23]. When comparing the indoor and outdoor impact of the two dual ai-LLINs, it appeared to have a greater effect indoors than outdoors, thus emphasizing the need for outdoor complementary vector control tools. Furthermore, the broadly similar impact that each of the two dual a.i. LLINs tended to have on the density of An. gambiae s.s. and An. coluzzii suggests that combining these insecticides (chlorfenapyr and pyriproxyfen) with the pyrethroid insecticide (alpha-cypermethrin) in the LLINs had a similar effect on the density of the two primary vectors.
Over the 2 years after the net distribution, about three quarters of the collected specimens of An. gambiae s.l. was An. coluzzii, while the rest was composed of An. gambiae s.s. with a small proportion (< 1%) of hybrids (An. gambiae/coluzzii). By comparison, the two predominant molecular species were previously found in similar proportions at baseline (50.9% for An. coluzzii vs. 49.1% for An. gambiae s.s.) [12]. The changes observed in proportions of these two primary species between the post-net distribution period and baseline could be due to the seasonality and/or the differential selection induced by the interventions. Indeed, the baseline collection occurred during only one round performed over the short rainy season (September–October 2019) so could not provide a representative image of the molecular species composition compared to the four rounds of collection (covering all seasons) of each of the two post-net distribution years. Furthermore, during the whole study period, An. coluzzii was found to peak over the dry seasons, which corroborates previous works by Salako et al. [24] in the northern regions of Atacora and Donga in Benin. This could be because, during that period of the year, there were many permanent/semi-permanent breeding sites created by rice paddies as well as tributaries of the Oueme and Zou rivers that irrigate the study area, the temporary breeding sites being only found during the rainy season. Indeed, according to Diabate et al. [25], permanent/semi-permanent and temporary breeding sites were conducive to the emergence of An. coluzzii and An. gambiae s.s., respectively.
Limitations of the present study include the lack of data on both entomological inoculation rate and rainfall, which influence vector density.
After dissecting a subsample of An. gambiae s.l., the parity rate, which shows the physiological age of mosquito populations, was similar across the three study arms, suggesting that this malaria vector complex has passed through approximately the same number of gonotrophic cycles in the three study arms. This finding corroborates previous results from Accrombessi et al. [6], who showed similar sporozoite rates across the three study arms. This conflicting trend might be due to the fact that, apart from the interventions deployed, parity rates could have been strongly influenced by other factors such as climate conditions (temperature, relative humidity), which can vary from place to place and over time, as previously mentioned by Adugna et al. [26], whoch we did not account for in our analysis. Thus, in a trial evaluating the efficacy of vector control tools, data on parity rates should be interpreted cautiously, given the existence of confounding factors.
Conclusions
The lack of a significant reduction in the density of primary vectors by either of the two dual active-ingredients LLINs could be because of the low sample size of mosquito speciated. Thus, both pyrethroid-chlorfenapyr LLINs and pyrethroid-pyriproxyfen LLINs appeared to have a similar impact on An. coluzzii and An. gambiae s.s. in this study.
Availability of data and materials
The data supporting the findings of the study must be available within the article and/or its supplementary materials, or deposited in a publicly available database.
References
World Health Organization. World malaria report 2022. Geneva; 2022. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789240064898
Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, et al. Effectiveness of a long lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391:5.
Staedke SG, Gonahasa S, Dorsey G, Kamya MR, Maiteki-Sebuguzi C, et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): a pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet. 2020;395:6.
World Health Organization 2017. Conditions for deployment of mosquito nets treated with a pyrethroid and piperonyl butoxide. Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/bitstream/handle/10665/258939/WHO-HTM-GMP-2017.17-eng.pdf
Mosha JF, Kulkarni MA, Lukole E, Matowo NS, Pitt C, Messenger LA, et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: a four-arm, cluster-randomised trial. Lancet. 2022;399:1227–41.
Accrombessi M, Cook J, Dangbenon E, Yovogan B, Akpovi H, Sovi A, et al. Efficacy of pyriproxyfen-pyrethroid long-lasting insecticidal nets (LLINs) and chlorfenapyr-pyrethroid LLINs compared with pyrethroid-only LLINs for malaria control in Benin: a cluster-randomised, superiority trial. Lancet. 2023;401:435–46.
World Health Organization. WHO Guidelines for malaria. Geneva; 2023 (WHO/UCN/GMP/2023.01). License: CC BY-NC-SA 3.0 IGO.
Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo. Uganda Malar J. 2019;18:445.
Matowo NS, Kulkarni MA, Messenger LA, Jumanne M, Martin J, Mallya E, et al. Differential impact of dual-active ingredient long-lasting insecticidal nets on primary malaria vectors: a secondary analysis of a 3-year, single-blind, cluster-randomised controlled trial in rural Tanzania. Lancet Planet Health. 2023;7:e370–80.
Salako AS, Ahogni I, Aïkpon R, Sidick A, Dagnon F, Sovi A, et al. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasit Vectors. 2018;11:618.
Kpanou CD, Sagbohan HW, Dagnon F, Padonou GG, Ossè R, Salako AS, et al. Characterization of resistance profile (intensity and mechanisms) of Anopheles gambiae in three communes of northern Benin, West Africa. Malar J. 2021;20:328.
Yovogan B, Sovi A, Padonou GG, Adoha CJ, Akinro B, Chitou S, et al. Pre-intervention characteristics of the mosquito species in Benin in preparation for a randomized controlled trial assessing the efficacy of dual active-ingredient long-lasting insecticidal nets for controlling insecticide-resistant malaria vectors. PLoS ONE. 2021;16:e0251742.
Accrombessi M, Cook J, Ngufor C, Sovi A, Dangbenon E, Yovogan B, et al. Assessing the efficacy of two dual-active ingredients long-lasting insecticidal nets for the control of malaria transmitted by pyrethroid-resistant vectors in Benin: study protocol for a three-arm, single-blinded, parallel, cluster-randomized controlled trial. BMC Infect Dis. 2021;21:194.
Gillies M, De Meillon B. The Anophelinae of Africa south of the Sahara. Publ South Afri Inst Med Res. 1968;54:343.
Detinova TS. The determination of the physiological age of the females of Anopheles gambiae by changes in the tracheal system of the ovaries. Med Parazit. 1945;14:45–9.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, Torre della A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163.
Collins E, Vaselli NM, Sylla M, Beavogui AH, Orsborne J, Lawrence G, et al. The relationship between insecticide resistance, mosquito age and malaria prevalence in Anopheles gambiae s.l. from Guinea. Sci Rep. 2019;9:8846.
The Anopheles gambiae 1000 Genome Consortium. Genetic diversity of the African malaria vector Anopheles gambiae. Nature. 2017; 552:96–100.
Tene Fossog B, Ayala D, Acevedo P, Kengne P, Ngomo Abeso Mebuy I, Makanga B, et al. Habitat segregation and ecological character displacement in cryptic African malaria mosquitoes. Evol Appl. 2015;8:326–45.
Gimonneau G, Bouyer J, Morand S, Besansky NJ, Diabate A, Simard F. A behavioral mechanism underlying ecological divergence in the malaria mosquito Anopheles gambiae. Behav Ecol. 2010;21:1087–92.
Dao A, Yaro AS, Diallo M, Timbiné S, Huestis DL, Kassogué Y, et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature. 2014;516:387–90.
Müller P, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008;4:e1000286.
Tiono AB, Ouédraogo A, Ouattara D, Bougouma EC, Coulibaly S, Diarra A, et al. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: a cluster-randomised controlled trial. Lancet. 2018;392:569–80.
Salako AS, Dagnon F, Sovi A, Padonou GG, Aïkpon R, Ahogni I, et al. Efficacy of actellic 300 CS-based indoor residual spraying on key entomological indicators of malaria transmission in Alibori and Donga, two regions of northern Benin. Parasit Vectors. 2019;12:612.
Diabate A, Dabire RK, Heidenberger K, Crawford J, Lamp WO, Culler LE, et al. Evidence for divergent selection between the molecular forms of Anopheles gambiae: role of predation. BMC Evol Biol. 2008;8:5.
Adugna T, Getu E, Yewhelew D. Parous rate and longevity of anophelines mosquitoes in bure district, northwestern Ethiopia. PLoS ONE. 2022;17:e0263295.
Acknowledgements
We are grateful to the Benin NMCP, population from Cove, Ouinhi and Zagnanado districts as well as their leaders for their commitment during the implementation of the present study. We also acknowledge the technicians who performed the mosquito processing, and the LSHTM ODK support team for providing electronic data solutions through LSHTM Open Research Kits (http://odk.lshtm.ac.uk/).
Funding
This research is supported by a grant to the London School of Hygiene and Tropical Medicine from UNITAID and the Global Fund via the Innovative Vector Control Consortium (IVCC). This cluster-randomized clinical trial is part of a larger project, “The New Nets Project”. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
AS, AAM, LAM, CN, JC, MA, MCA, and NP conceived the study. BY, AS, AD, CA, CJA, TFT, JC, LA, and NP participated in the design of the study. Entomological data was collected by BY, CJA, CZK, ZKA, PAA, CDK, and AS. Laboratory analyses were carried out by BY, CJA, and AS. The original draft of the manuscript was written by B.Y. and A.S. Data management and statistical analysis were conducted by BY, CJA, ED, BA, JC, and NP. GGP and MCA provided administrative support to the trial. AD, MA, PAA, AAM, TFT, CA, GGP, LAM, CN, JC, MCA, LA, and NP critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by Benin's National Ethics Committee for Health Research (no. 30/MS/DC/SGM/DRFMT/CNERS/SA, approval no. 6 of 04/03/2019) and the Ethics Committee of the London School of Hygiene and Tropical Medicine (16237-1). All participants gave their informed consent, before their involvement in the study. All collectors were trained to capture mosquitoes before they got bitten. They were vaccinated against yellow fever prior to the study and cared for at the local health facility when they suffered from malaria or had similar symptoms during the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figure S1. Seasonal variation of proportion of Anopheles coluzzii indoors and outdoors in the study area. Rs: rainy season, Ds: Dry season.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yovogan, B., Sovi, A., Djènontin, A. et al. The impact of pyrethroid-pyriproxyfen and pyrethroid-chlorfenapyr long-lasting insecticidal nets on density of primary malaria vectors Anopheles gambiae s.s. and Anopheles coluzzii in Benin: a secondary analysis of a cluster randomised controlled trial. Parasites Vectors 17, 7 (2024). https://doi.org/10.1186/s13071-023-06104-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06104-5