Abstract
Background
Mosquitoes are the deadliest organisms in the world, killing an estimated 750,000 people per year due to the pathogens they can transmit. Mosquitoes also pose a major threat to other vertebrate animals. Culex territans is a mosquito species found in temperate zones worldwide that feeds almost exclusively on amphibians and can transmit parasites; however, little is known about its ability to transmit other pathogens, including fungi. Batrachochytrium dendrobatidis (Bd) is a topical pathogenic fungus that spreads through contact. With amphibian populations around the world experiencing mass die-offs and extinctions due to this pathogen, it is critical to study all potential modes of transmission. Because Cx. territans mosquitoes are in contact with their hosts for long periods of time while blood-feeding, we hypothesize that they can transmit and pick up Bd.
Methods
In this study, we first assessed Cx. territans ability to transfer the fungus from an infected surface to a clean one under laboratory conditions. We also conducted a surveillance study of Bd infections in frogs and mosquitoes in the field (Mountain Lake Biological station, VA, USA). In parallel, we determined Cx. territans host preference via blood meal analysis of field caught mosquitoes.
Results
We found that this mosquito species can carry the fungus to an uninfected surface, implying that they may have the ability to transmit Bd to their amphibian hosts. We also found that Cx. territans feed primarily on green frogs (Rana clamitans) and bullfrogs (Rana catesbeiana) and that the prevalence of Bd within the frog population at our field site varied between years.
Conclusions
This study provides critical insights into understanding the role of amphibian-biting mosquitoes in transmitting pathogens, which can be applied to disease ecology of susceptible amphibian populations worldwide.
Graphical Abstract

Similar content being viewed by others
Background
Mosquitoes are the deadliest animals in the world, spreading many pathogens to their hosts [1, 2]. Although most vectors feed on endothermic hosts such as mammals or birds, some species are known to be ectotherm specialists [3, 4]. A primary example is Culex territans Walker 1856, a species of mosquito found in temperate and subtropical zones across the Northern Hemisphere [5,6,7,8,9] that feeds preferentially on amphibians, especially anurans (i.e., frogs and toads) [10,11,12]. Despite its widespread distribution, little is known about this mosquito. Culex territans is a potential vector of pathogens such as giant anuran trypanosomes, hepatozoon parasites [12,13,14], and viruses [15, 16], but its role in transmitting other pathogens, such as fungi, is unknown. Batrachochytrium dendrobatidis (Bd) is a topical pathogenic fungus affecting the keratinized tissue in amphibians and is a major contributor to declines in amphibian populations worldwide [17,18,19,20]. Chytridiomycosis, the disease caused by Bd, affects anuran amphibians more than other amphibian orders, with pathogenic effects varying significantly among species [21, 22]. As an aquatic fungus, Bd can be spread environmentally through water or by physical contact with other infected frogs [23]. Fish, crustaceans, and reptiles are known carriers of Bd [24,25,26,27,28]; however, the roles that vectors might play in transmitting this deadly fungus remains poorly understood [29, 30]. Toledo et al. [29] found Bd DNA present on biting midges (Corethrella spp.), showing that arthropod vectors can play a role in the transmission of this fungus. Mosquitoes are known to harbor fungi [31, 32], and Cx. territans has a long period of contact time with the skin surface of the frog during blood-feeding (minimum of 30 min to feed to repletion [33]). This amount of time could allow the proboscis and the legs to acquire any fungal spores present on the skin surface of their host. Interestingly, a closely related mosquito species, Cx. quinquefasciatus, which feeds on birds and mammals, has been shown to be able to acquire the fungus and transport it to a new surface [30]. Our study tests the ability of Cx. territans, the northern frog-biting mosquito, to transfer Bd to amphibian hosts, as Cx. quinquefasciatus is not known to feed on amphibians.
In the present study, we hypothesized that Cx. territans could acquire Bd spores and transmit them to a new surface, thus demonstrating the ability to vector this fungal pathogen to anuran hosts through mechanical transmission (i.e., by spreading spores present on the mosquito legs to the skin of their hosts). To test this hypothesis, we first conducted blood meal analyses to determine the primary hosts for this specific population of Cx. territans. We then screened field-caught mosquitoes and frogs for the presence of Bd at our field site, Mountain Lake Biological Station (MLBS, Pembroke, VA, USA), and carried out a controlled experiment to evaluate the ability of Cx. territans to transmit the fungus from one medium surface to another. This work will provide a deeper insight into mosquito-borne disease ecology as well as understanding the potential Cx. territans has for vectoring Bd, which will enhance our understanding of the spread of this deadly fungus.
Methods
Mosquito sample collection
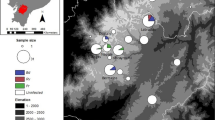
Mosquitoes were collected at MLBS (37.375654°–80.522140°, 1160 m above sea level [ASL]) in Giles County, VA, USA. Mosquitoes were collected while at rest around midday from May through September at Sylvatica Pond (37.377079, −80.522245) using a giant bug aspirator (John W. Hock Company, Gainesville, FL, USA) [34]. Following collection, the mosquitoes were brought to the MLBS laboratory, cold-anesthetized, and sorted under a microscope based on species, sex, and reproductive stage [6]. Blood-fed Cx. territans females were put in individual 2-ml Eppendorf tubes (Cat #24-283S, Genesee Scientific, Rochester, NY, USA) and were immediately frozen at −80 °C until use for blood meal analysis and pathogen screening for the presence of Bd (Fig. 1A), and any bycatch was released.
Blood meal analysis
Blood-fed mosquitoes collected in the field were thawed, and the abdomens (containing the blood meals) were removed using fine forceps. Abdomens were homogenized using a homogenizer and pestle, and DNA was extracted using Qiagen DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions (without the purification step, eluted in 20 µl AE buffer). We amplified the mitochondrial 16S ribosomal RNA (rRNA) gene from each mosquito blood meal via polymerase chain reaction (PCR) using a 50 µl solution containing 25 µl DreamTaq Green PCR Master Mix (2×) (Cat #FERK1081 Thermo Fisher Scientific, Waltham, MA, USA), 21 µl PCR-grade water (CAS: 7732-1-8-5, Sigma Aldrich, St. Louis, MO, USA), 1 µl of forward and reverse primers (L2513: 3′-GCCTGTTTACCAAAAACATCAC-5′; H2714: 3′-CTCCATAGGGTCTTCTCGTCTT-5′) [35] and 2 µl DNA. Amplification was carried out using a thermal cycler (MyCycler, Bio-Rad, Hercules, CA, USA) with 35 cycles of 95 °C for 30 s, 57 °C for 15 s, and 72 °C for 30 s. PCR products were run on a 1% agarose gel containing 0.1% GelRed Nucleic Acid Gel Stain (Biotium, Fremont, CA, USA) for 30 min at 110 V to confirm successful amplification (Bio-Rad), with the amplified product sent to Genewiz (South Plainfield, NJ, USA) for Sanger sequencing. Sequences were aligned using the Qiagen CLC Genomics Workbench (Qiagen, Hilden, Germany) [36], and analyzed using the National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST). Identification of the source of mosquito blood meals was determined for sequences with a sequence identity of 85% or higher [37] (Fig. 1A).
Anuran collection and swabbing
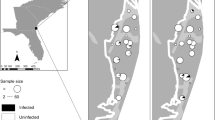
For taxonomic stability, we follow the nomenclature of AmphibiaWeb (https://amphibiaweb.org). Anurans (frogs) were collected by hand at three permanent artificial ponds (naturalized for over 50 years) at MLBS: Riopel (37.374552, −80.552109), Sylvatica (37.377079, −80.522245), and Horton (37.378483, −80.522078). The largest of these ponds, Riopel, is approximately 80 × 90× 4 m, whereas the smaller two fluctuate in depth (≤ 1 m) depending on rainfall and measure approximately 30 × 30 m. None of the ponds contain fish. Frog sampling was conducted under Virginia Department of Game and Inland Fisheries collection permit nos. 064750 and 070446 and Virginia Tech Institutional Animal Care and Use Committee (IACUC) protocols (#19-003 and #22-066). Based on the results obtained for the mosquito blood meal analysis, collections were limited to male and female Rana (Aquarana) clamitans and Rana (Aquarana) catesbeiana. Individuals were placed and kept in unused polyethylene bags (25.4 × 40.64 cm 4 Mil Industrial Poly Bags, Uline, Pleasant Prairie, WI, USA) with spring water and tied to prevent escape. Larger frogs were kept in individual terrariums (23.5 × 15.24 × 17.78 cm Kritter Keeper) (Lee’s Aquarium & Pet Products, San Marcos, CA, USA), which were cleaned using a 10% bleach solution between uses to prevent contamination. During handling, nitrile gloves were worn and changed between each individual to prevent potential cross-contamination. Adult frogs were swabbed for Bd (described below), measured (snout–vent length), weighed using a spring scale (Pesola, Schindellegi, Switzerland), and marked using the toe clipping method described by Martoff [38] prior to release.
Adult frogs of the two species and sexes were swabbed using rayon-tipped swabs (MW113 series urethral swab, Medical Wire & Equipment Co Ltd, Corsham, UK). The skin of each individual was swabbed five times each on the venter, each medial thigh, and between the digits of each hind leg (Fig. 2A). Swabs were frozen at −80 °C and sent for Bd screening at the Genetics Core Facility of the Sam Noble Museum, University of Oklahoma.
A Frogs were swabbed five times each on the (1) abdomen, (2) both medial hind thighs, and (3) between the hind digits of both feet. Swabs were (4) sent out for qPCR to confirm Bd presence or absence. B The (1) proboscis and legs of field-caught mosquitoes were removed and (2) placed on a sterile agar plate, which was divided to separate the two body parts. After 4 days, the plates were (3) swabbed and (4) sent out for qPCR and analysis. C Numbers of frogs caught and swabbed per species between 2020 and 2022. D Representation of the numbers of frogs that tested positive for Bd from 2020 to 2022
Experimental transmission assays
Culex territans mosquitoes were caught as larvae in the ponds at MLBS and reared in the laboratory under conditions as described in Reinhold et al. [39]. Female Cx. territans were tested for transmission using a modified experimental setup after Gould et al. [30]. All experiments were conducted in a biosafety cabinet at 25–27 °C using standard sterile practices to maintain the sterility of the plates. Batrachochytrium dendrobatidis Global Panzootic Lineage (GPL) strain JEL0423 (Collection of Zoosporic Eufungi at the University of Michigan [CZEUM], Ann Arbor, MI, USA) was plated on sterile 1%T media using CZEUM protocols (10 g Fisher BioReagents tryptone BP1421-500; Fisher Scientific, Waltham, MA, USA) and 10 g bacteriological-grade agar [IBI Scientific CAS #9002-18-0, Dubuque, IA, USA], in 1 L deionized water). Mosquitoes were briefly cold-anesthetized and tethered by the thorax to tungsten rods with ultraviolet (UV)-cured glue (Bondic, Niagara Falls, NY, USA), which held the mosquitoes stationary when exposed to a plate containing Bd (cell count: 5.251 × 105 ± 9.727 × 105) (50 × 11 mm petri dishes, #EW-14005-24, Advantech, Taipei City, Taipei, Taiwan). These plates were swabbed to confirm the presence of Bd after mosquito exposure. An average of ten mosquitoes (8 < n < 12) were held on the Bd containing plate for 30 min (N = 123, 13 replicates, with some mosquitoes lost during transfer). These mosquitoes were then transferred to new individual sterile 1%T agarose plates for 30 min of contact time to simulate the average time needed for this mosquito species to feed to repletion on a frog host [33]. Plates were incubated at room temperature for 96 h before being swabbed. Swabs were sent to the Genetics Core Facility of the Sam Noble Museum, University of Oklahoma, to confirm the transfer of Bd. To control for environmental or potential previous Bd exposure (during larval or pupal stage), the same experiment was conducted by exposing 50 mosquitoes (in 5 sets of 10) to sterile plates instead of Bd-inoculated plates (N = 50). These mosquitoes were also transferred to new individual plates after 30 min and allowed to maintain contact for 30 min. Plates were swabbed after 96 h and sent out for processing as described previously (Fig. 3A).
A Mosquitoes were (1) tethered to a tungsten rod and (2) placed on a Bd plate with legs touching the agar. After 30 min, the mosquitoes were (3) transferred to a new, individual sterile plate with limbs in contact with the agar for another 30 min and (4) let sit for 4 days. After 4 days, the plates were (5) swabbed and stored at −80 °C until they were (6) sent out for qPCR and analysis. B Representation of negative controls (n = 56). C Representation of Bd-infected plates to confirm the presence of Bd (n = 6). D The number of plates that were positive for Bd 4 days after contact with an inoculated mosquito (n = 123)
Field mosquito Bd sampling
A total of 82 field-caught, blood-fed Cx. territans mosquitoes [57 and 25 from 2021 and 2022, respectively] were sampled for Bd. Sterile 1%T agar plates (50 × 11 mm petri dishes, #EW-14005-24, Advantech, Taipei City, Taipei, Taiwan) were made following CZEUM instructions to simulate the landing onto the surface of an amphibian’s skin. Each plate was divided into 2 sides for the proboscis and legs respectively. The proboscis was clipped using fine-tipped forceps and put on one side of the plate, and the legs from the same mosquito were pulled and placed on the other side of the plate. Forceps were cleaned and dried before removing any parts of the mosquitoes and in between individuals. Plates were incubated at room temperature for 96 h before being swabbed with MW113 swabs. Swab samples were stored at −80 °C until being sent out for processing as described previously (Fig. 2B).
Screening samples using qPCR
Pathogen screening for Bd was conducted at the Genetics Core Facility of the Sam Noble Museum at the University of Oklahoma. Swabs from both agar plates and frogs were first extracted using the PrepMan Ultra Sample Preparation Reagent protocol [40] (Applied Biosystems, Thermo Fisher Scientific). Quantitative PCR (qPCR) techniques were used to determine the presence/absence of Bd genetic material and to estimate the number of gene copies per sample, or Bd load, using QuantStudio software v3 (Applied Biosystems, Thermo Fisher Scientific). The Bd assay primers target the internal transcribed spacer (ITS-1) rRNA gene (forward primer: ITS1-3 Chytr; reverse primer: 5.8S) from Boyle et al. [41]. All samples were run in triplicate, with positive controls of known Bd gene copies (gBlock DNA quantities 1e1–1e4) and a negative control (molecular grade sterile water). Samples were considered positive for Bd (Bd+) if amplification occurred in at least two of the three wells and if the mean Bd gene copy number was greater than 1.0, with samples rerun as needed [42, 43].
Results
Blood meal analysis
A total of 538 mosquitoes were collected from Sylvatica Pond at MLBS. Among them, 98 were blood-fed Cx. territans. We identified a total of five host species, including R. clamitans (61%) (green frog), R. catesbeiana (35%) (American bullfrog), Rana sylvatica (2%) (wood frog), H. versicolor (1%) (gray treefrog), and Nerodia sipedon (1%) (northern water snake). We noted that Cx. territans preferentially fed on R. clamitans (61%) and R. catesbeiana (35%) (Fig. 1B).
Frog collection and swabbing
A total of 127 R. clamitans and 87 R. catesbeiana were caught and swabbed between 2020 and 2022 (Fig. 2A, C). Among the individuals swabbed, 17.8% were confirmed as Bd+. The prevalence of Bd infection among the surveyed populations varied between years and species: 9.1% prevalence in R. catesbeiana and 6.1% in R. clamitans during 2020 dropped to 4.5% and 0% in R. catesbeiana and R. clamitans, respectively, in 2021. During 2022 we found 38% and 50% prevalence among R. catesbeiana and R. clamitans, respectively (Fig. 2D). None of the 82 field-caught mosquitoes screened for Bd were positive.
Transmission assays
Of the 123 experimental mosquitoes tested, 80 were positive for Bd (65%) (cell counts 5.251 × 105 ± 9.727 × 105; gene copies 1.94499 × 105 ± 3.37174 × 105) (Fig. 3D). None of the five initial control plates or the 50 test controls were positive for Bd, showing no evidence of prior exposure to the fungus (Fig. 3B). All Bd-inoculated plates contained Bd, confirming the experimental mosquitoes were exposed to the fungus (Fig. 3C).
Discussion
This study was conducted to assess the prevalence of the pathogenic fungus at our field site, MLBS, and to ascertain whether the amphibian-biting mosquito, Cx. territans, is capable of vector transmission of Bd to anurans. We determined the primary hosts of Cx. territans and tested two focal hypotheses: (1) Pathogen screening efforts would detect Bd on Cx. territans and their anuran blood meal hosts in the field, and (2) Cx. territans is capable of Bd transmission from one surface to another in a laboratory setting.
Although Cx. territans has been known to feed on a wide variety of hosts [10,11,12], our results show that the mosquito population at MLBS exhibits a strong preference for R. clamitans and R. catesbeiana (Fig. 1). Both anuran species are commonly observed around the MLBS ponds. A plethora of other potential hosts have been observed at the MLBS ponds, including R. sylvatica, Rana palustris (pickerel frog) H. versicolor, and Pseudacris crucifer (spring peeper). Additional potential hosts present at MLBS include N. sipedon (northern water snake), Crotalus adamanteus (eastern diamondback rattlesnake), and numerous salamander species (in particular Notophthalmus viridescens, and several Desmognathus and Plethodon spp.) [44]. The strong host preference for R. clamitans and R. catesbeiana exhibited by Cx. territans led us to focus our Bd screening on these two species.
Overall, we found that the current prevalence of Bd is relatively low among the R. clamitans and R. catesbeiana populations at MLBS. Previous studies at MLBS found several species of amphibians carrying Bd. Rothermel et al. [45] and Wimsatt et al. [46] found Bd present in all Notophthalmus viridescens (red-spotted newt) individuals tested at MLBS, which is present in all three ponds at the station where R. clamitans and R. catesbeiana are found. While these studies only tested one species, they demonstrate that Bd has been present in the MLBS ponds for more than a decade. Hughey et al. [47] also reported Bd-infected individuals of N. viridescens, P. crucifer, and R. catesbeiana, and found lower rates of infection among R. catesbeiana than the other two species. None of these studies associated Bd infections at MLBS with die-offs or significant population declines. Collectively, the present and previous studies show that Bd is established at MLBS. Our results also suggest that infection prevalence and detectability vary from year to year (Fig. 2D). This kind of variability is not uncommon in areas where Bd is considered endemic [47,48,49,50,51,52] and may be part of natural long-term oscillations described by Talley et al. [49]. Several factors could be playing a role in these oscillations. The presence of carriers in these areas, including (but not limited to) N. viridescens, R. catesbeiana, and crayfish (e.g., Cambarus spp.) may be acting as fungal reservoirs and may partially account for the yearly fluctuations we detected in anuran populations [24, 26, 27, 45,46,47, 53,54,55]. Environmental factors could also affect Bd prevalence, with colder and drier years potentially causing a shift in Bd prevalence [20, 23, 49, 51, 56, 57]. Despite the sudden increase in Bd in 2022, no mass die-offs of amphibians were observed at the time, which suggests that Bd is not an immediate concern for the amphibians at the site, but future observations and surveillance will provide more insight.
Our experimental transmission test confirmed that Cx. territans can transmit Bd from one surface to another (Fig. 3D). This suggests that Cx. territans can transfer the fungus to a new host after landing and feeding on an infected host. Ecologically, Cx. territans is a good candidate to vector a topical fungus, given that it feeds primarily on frogs and is very slow to feed to repletion [10,11,12, 33]. If the mosquito does not have the opportunity to feed to repletion (i.e., frog jumps into the water), they may seek a new host to complete their meal, allowing them to transmit Bd zoospores between hosts. They may also transmit the fungus between gonotrophic cycles, though further testing is needed to determine the maximum time the mosquito can carry the fungus. Because 35% of the mosquitoes did not transmit Bd to a new plate, there may be a threshold concentration necessary for transmission. The large range in cell counts on each inoculated Bd plate could also have contributed to the variability in individual mosquitoes’ ability to transmit zoospores between plates. Another source of variability could be the uneven nature of the way the Bd fungus grows. All mosquitoes were placed on visible fungus, but it was difficult to determine which plated area was actively releasing zoospores. This may provide a more ecologically relevant way of testing the ability of a mosquito to transmit Bd experimentally than has been previously shown in the literature [30], since the landing area of the mosquito on a frog may not be releasing zoospores. Because more than two-thirds of the mosquitoes transmitted Bd to uninfected surfaces, we conclude that Cx. territans can carry spores on their appendages and could thus be a competent vector of Bd in the field.
We hypothesized that we would find Bd on the mosquitoes caught in the field, but all field-caught mosquitoes were negative for Bd. These results are not unexpected, considering the low prevalence of the fungus in the frog population in 2020 and 2021 and the small mosquito sample size for 2022. However, based on the ability of other arthropod vectors to act as carriers of Bd in the wild [29] and the ability of Cx. territans to transmit the fungus in a laboratory setting, we would expect to find Bd-carrying mosquitoes in a larger sample or among samples from a more highly infected population of frogs. Because this mosquito species lives in close association with frogs throughout the Northern Hemisphere and given that Bd is also prevalent in these regions [5,6,7,8,9,10], transmission in areas of high Bd prevalence is likely. The lack of Bd found on field mosquitoes may also be due to the location of Bd on frogs. Rana clamitans and R. catesbeiana were often observed resting partially submerged in water, as noted in previous studies [38, 58]. This position results in their ventral surfaces being more likely to be exposed to the water-borne fungus, and consequently, unless the fungus has spread extensively, a mosquito feeding on the dorsum of a frog’s body may not make contact with the fungus. Mosquitoes were observed in the field clustering on the frogs’ back legs and lower back, possibly to avoid detection and antiparasitic behavior (e.g., grooming) from the host as seen in other mosquito species [59,60,61]. We also observed mosquitoes on the ventral surfaces of the body and on the toes when those areas were exposed. Culex territans is known to feed on more terrestrial amphibians and reptiles [10, 11]. It would therefore be beneficial for future studies to collect additional Cx. territans mosquitoes and test for field transmission, especially in areas where Bd prevalence is high.
Conclusion
In this study, we determined the host preference of Cx. territans, the prevalence of Bd at the MLBS field site, the ability of Cx. territans to transmit Bd in a laboratory setting, and Bd prevalence among blood-fed mosquitoes in the field. We found that, at MLBS, Cx. territans feeds preferentially on R. clamitans and R. catesbeiana. We observed varying Bd prevalence over the course of 3 years at MLBS within R. clamitans and R. catesbeiana populations. Most importantly, we found that Cx. territans can transmit the fungus to a clean surface. We did not, however, find mosquitoes in the field with Bd present on their proboscis or legs. Overall, the results of this study add to the growing understanding of Bd epidemiology and disease ecology both on a local and global scale. Future research will help determine whether mosquitoes in the field can carry the fungus and transmit it to a new host as well as the length of time the fungus can stay on the mosquito.
Availability of data and materials
The data supporting the findings of this study are available within the article.
Abbreviations
- Bd :
-
Batrachochytrium dendrobatidis
- Cx :
-
Culex
- R :
-
Rana
- MLBS:
-
Mountain Lake Biological Station
- H :
-
Hyla
- N :
-
Notophthalmus
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative polymerase chain reaction
- rRNA:
-
Ribosomal RNA
References
Center for Disease Control and Prevention (CDC). 2018. https://www.cdc.gov/globalhealth/stories/2019/world-deadliest-animal.html. Accessed 26 Jan 2023.
Gates B. The deadliest animal in the world mosquito week. Kirkland: The gates notes LLC; 2014.
Cebrián-Camisón S, Martínez-de la Puente J, Figuerola J. A literature review of host feeding patterns of invasive Aedes mosquitoes in Europe. Insects. 2020;11:848.
Reeves LE, Holderman CJ, Blosser EM, Gillett-Kaufman JL, Kawahara AY, Kaufman PE, et al. Identification of Uranotaenia sapphirina as a specialist of annelids broadens known mosquito host use patterns. Commun Biol. 2018;1:1–8.
Culverwell CL, Uusitalo RJ, Korhonen EM, Vapalahti OP, Huhtamo E, Harbach RE. The mosquitoes of Finland: updated distributions and bionomics. Med Vet Entomol. 2021;35:1–29.
Darsie Jr RF, Ward RA. 1981. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Walter Reed Army Inst of Research Washington.
Kitron U, Pener H. Distribution of mosquitoes (Diptera: Culicidae) in northern Israel: a historical perspective II. Culicine mosquitoes. J Med Entomol. 1986;23:182–7.
Robert V, Günay F, Le Goff G, Boussès P, Sulesco T, Khalin A, et al. Distribution chart for Euro-Mediterranean mosquitoes (western Palaearctic region). J Euro Mosq Control Assoc. 2019;37:1–28.
Zittra C, Waringer J. Species inventory, ecology, and seasonal distribution patterns of Culicidae (Insecta: Diptera) in the National Park Donau-Auen (Lower Austria). Aquat Insects. 2014;36:63–77.
Bartlett-Healy K, Crans W, Gaugler R. Temporal and spatial synchrony of Culex territans (Diptera: Culicidae) with their amphibian hosts. J Med Entomol. 2008;45:1031–8.
Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, et al. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am J Trop Med Hyg. 2008;79:809–15.
Crans WJ. The blood feeding habits of Culex territans Walker. Mosq News. 1970;30:445–7.
Bartlett-Healy K, Crans W, Gaugler R. Vertebrate hosts and phylogenetic relationships of amphibian trypanosomes from a potential invertebrate vector, Culex territans Walker (Diptera: Culicidae). J Parasitol. 2009;95:381–7.
Ferguson LV, Smith TG. Reciprocal trophic interactions and transmission of blood parasites between mosquitoes and frogs. Insects. 2012;3:410–23.
Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, et al. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector-Borne Zoon Dis. 2007;7:365–86.
Reinhold JM, Fazal A, Robert J, McLeod D, Paulson S, Auguste J, et al. Culex territans Walker as a potential vector of ranaviruses. Virginia: Virginia Polytechnic Institute and State University; 2023.
Bienentreu JF, Lesbarrères D. Amphibian disease ecology: are we just scratching the surface? Herpetol. 2020;76:153–66.
Forzán MJ, Heatley J, Russell KE, Horney B. Clinical pathology of amphibians: a review. Vet Clin Pathol. 2017;46:11–33.
Campbell Grant EH, Miller DA, Muths E. A synthesis of evidence of drivers of amphibian declines. Herpetol. 2020;76:101–7.
Voyles J, Johnson LR, Briggs CJ, Cashins SD, Alford RA, Berger L, et al. Temperature alters reproductive life history patterns in Batrachochytrium dendrobatidis, a lethal pathogen associated with the global loss of amphibians. Ecol Evol. 2012;2:2241–9.
Gahl MK, Longcore JE, Houlahan JE. Varying responses of northeastern North American amphibians to the chytrid pathogen Batrachochytrium dendrobatidis. Conserv Biol. 2012;26:135–41.
Tobler U, Schmidt BR. Within- and among-population variation in chytridiomycosis-induced mortality in the toad Alytes obstetricans. PLoS ONE. 2010;5:e10927.
Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycol. 2004;96:9–15.
Brannelly LA, McMahon TA, Hinton M, Lenger D, Richards-Zawacki CL. Batrachochytrium dendrobatidis in natural and farmed Louisiana crayfish populations: prevalence and implications. Dis Aquat Org. 2015;112:229–35.
Kilburn VL, Ibáñez R, Green DM. Reptiles as potential vectors and hosts of the amphibian pathogen Batrachochytrium dendrobatidis in Panama. Dis Aquat Org. 2011;97:127–34.
McMahon TA, Brannelly LA, Chatfield MW, Johnson PT, Joseph MB, McKenzie VJ, et al. Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proc Natl Acad Sci. 2013;110:210–5.
Oficialdegui FJ, Sánchez MI, Monsalve-Carcaño C, Boyero L, Bosch J. The invasive red swamp crayfish (Procambarus clarkii) increases infection of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). Biol Invasions. 2019;21:3221–31.
Pontes M, Augusto-Alves G, Lambertini C, Toledo L. A lizard acting as carrier of the amphibian-killing chytrid Batrachochytrium dendrobatidis in southern Brazil. Acta Herpetol. 2018;13:201–5.
Toledo LF, Ruggeri J, Ferraz L, Campos L, Martins M, Neckel-Oliveira S, et al. Midges not only sucks, but may carry lethal pathogens to wild amphibians. Biotropica. 2021;53:722–5.
Gould J, Valdez JW, Stockwell MP, Clulow S, Mahony MJ. Mosquitoes as a potential vector for the transmission of the amphibian chytrid fungus. Zool Ecol. 2019;29:36–42.
Malassigné S, Valiente Moro C, Luis P. Mosquito mycobiota: an overview of non-entomopathogenic fungal interactions. Pathogens. 2020;9:564.
Tawidian P, Rhodes VL, Michel K. Mosquito-fungus interactions and antifungal immunity. Insect Biochem Mol Biol. 2019;111:103182.
Reinhold JM, Mcleod D, Lahondére C. Some like it cold: a study of the interactions between Culex territans and its amphibian hosts. Virginia: Virginia Polytechnic Institute and State University; 2023.
Silver JB. Mosquito ecology: field sampling methods. 3rd ed. Dordrecht: Springer Netherlands; 2007.
Kitano T, Umetsu K, Tian W, Osawa M. Two universal primer sets for species identification among vertebrates. Int J Legal Med. 2007;121:423–7.
QIAGEN CLC Genomics Workbench 23.0.1 (https://digitalinsights.qiagen.com/).
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Martoff BS. Territoriality in the green frog. Rana clam Ecol. 1953;34:165–74.
Reinhold JM, Chandrasegaran K, Oker H, Crespo JE, Vinauger C, Lahondère C. Species-specificity in thermopreference and CO2-gated heat-seeking in Culex mosquitoes. Insects. 2022;13:92.
Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci. 2011;108:9502–7.
Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org. 2004;60:141–8.
Kerby JL, Schieffer A, Brown JR, Whitfield S. Utilization of fast qPCR techniques to detect the amphibian chytrid fungus: a cheaper and more efficient alternative method. Methods Ecol Evol. 2013;4:162–6.
Watters JL, Davis DR, Yuri T, Siler CD. Concurrent infection of Batrachochytrium dendrobatidis and ranavirus among native amphibians from northeastern Oklahoma, USA. J Aquat Anim Health. 2018;30:291–301.
iNaturalist community. Observations of species list from Mountain Lake Biological Station, USA, observed on/between 6 Jun 2017–31 Aug 2022. https://www.inaturalist.org on 11 May 2023.
Rothermel BB, Walls SC, Mitchell JC, Dodd CK Jr, Irwin LK, Green DE, et al. Widespread occurrence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in the southeastern USA. Dis Aquat Org. 2008;82:3–18.
Wimsatt J, Feldman SH, Heffron M, Hammond M, Ruehling MP, Grayson KL, et al. Detection of pathogenic Batrachochytrium dendrobatidis using water filtration, animal and bait testing. Zoo Biol. 2014;33:577–85.
Hughey MC, Becker MH, Walke JB, Startwort MC, Belden LK. Batrachochytrium dendrobatidis in Virginia amphibians: within and among site variation in infection. Herpetol Rev. 2014;45:428–38.
Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ. Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg’s “10,000 Zoospore Rule.” PLoS ONE. 2011;6:e16708.
Longo AV, Burrowes PA, Joglar RL. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis Aquat Org. 2010;92:253–60.
Talley BL, Muletz CR, Vredenburg VT, Fleischer RC, Lips KR. A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biol Conserv. 2015;182:254–61.
Watters JL, McMillin SL, Marhanka EC, Davis DR, Farkas JK, Kerby JL, et al. Seasonality in Batrachochytrium dendrobatidis detection in amphibians in central Oklahoma, USA. Zoo Wildl Med. 2019;50:492–7.
Julian JT, Gould VA, Glenney GW, Brooks RP. Seasonal infection rates of Batrachochytrium dendrobatidis in populations of northern green frog Lithobates clamitans melanota tadpoles. Dis Aquat Org. 2016;121:97–104.
Gervasi SS, Urbina J, Hua J, Chestnut T, Relyea A, Blaustein RR. Experimental evidence for American bullfrog (Lithobates catesbeianus) susceptibility to chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth. 2013;10:166–71.
Greenspan SE, Calhoun AJ, Longcore JE, Levy MG. Transmission of Batrachochytrium dendrobatidis to wood frogs (Lithobates sylvaticus) via a bullfrog (L. catesbeianus) vector. J Wildl Dis. 2012;48:575–82.
Schloegel LM, Picco AM, Kilpatrick AM, Davies AJ, Hyatt AD, Daszak P. Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol Conserv. 2009;142:1420–6.
Weather Underground. TWC Product and Technology LLC. 2023. https://www.wunderground.com/dashboard/pws/KVANEWPO68/table/2019-11-1/2019-11-1/monthly. Accessed 22 Jan 2023.
Kriger KM, Pereoglou F, Hero JM. Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conserv Biol. 2007;21:1280–90.
Emlen ST. Territoriality in the bullfrog Rana catesbeiana. Copeia. 1968. https://doi.org/10.2307/1441748.
Oduola AO, Awe OO. Behavioural biting preference of Culex quinquefasciatus in human host in Lagos metropolis Nigeria. J Vector Borne Dis. 2006;43:16–20.
Vinauger C, Lahondère C, Wolff GH, Locke LT, Liaw JE, Parrish JZ, et al. Modulation of host learning in Aedes aegypti mosquitoes. Curr Biol. 2018;28:333–44.
Wynne NE, Chandrasegaran K, Fryzlewicz L, Vinauger C. Visual threats reduce blood-feeding and trigger escape responses in Aedes aegypti mosquitoes. Sci Rep. 2022;12:21354.
Acknowledgements
We would like to thank Mountain Lake Biological Station personnel for their support. DSM is grateful for the support received from James Madison University, the University of Virginia, and the North Carolina State Museum of Natural Sciences. The following reagents were obtained from the University of Michigan CZEUM: Batrachochytrium dendrobatidis strain JEL0423. Funding was provided by the Virginia Tech Department of Biochemistry, the Fralin Life Science Institute, the Center for Emerging Zoonotic and Arthropod-borne Pathogens, Sigma Xi Grants in Aid of Research (GIAR), and the National Science Foundation REU site #1950734. The graphical abstract, Fig. 2A, B and Fig. 3A, B were created with Biorender.com.
Funding
This research was funded by the Department of Biochemistry, the Fralin Life Science Institute, the Center for Emerging Zoonotic and Arthropod-borne Pathogens at Virginia Tech, Sigma Xi GIAR, and the National Science Foundation REU site #1950734.
Author information
Authors and Affiliations
Contributions
Conceptualization, CL, JR, and DM; data curation, JR, DM, CL, CDS, KMS, SNS, EH, and MR; formal analysis, JR, EH, and CL; funding acquisition, JR and CL; writing—original draft, JR and CL; writing—review and editing, JR, CDS, KMS and CL All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Anuran sample collection and handling was conducted under Virginia Department of Game and Inland Fisheries collection permit nos. 064750 and 070446 and under approved IACUC protocols (#19-003 and #22-066) at the Virginia Polytechnic Institute and State University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reinhold, J.M., Halbert, E., Roark, M. et al. The role of Culex territans mosquitoes in the transmission of Batrachochytrium dendrobatidis to amphibian hosts. Parasites Vectors 16, 424 (2023). https://doi.org/10.1186/s13071-023-05992-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05992-x