Abstract
Background
Triatomines are blood-sucking insects capable of transmitting Trypanosoma cruzi, the parasite that causes Chagas disease in humans. Vectorial transmission entails an infected triatomine feeding on a vertebrate host, release of triatomine infective dejections, and host infection by the entry of parasites through mucous membranes, skin abrasions, or the biting site; therefore, transmission to humans is related to the triatomine–human contact. In this cross-sectional study, we evaluated whether humans were detected in the diet of three sylvatic triatomine species (Mepraia parapatrica, Mepraia spinolai, and Triatoma infestans) present in the semiarid–Mediterranean ecosystem of Chile.
Methods
We used triatomines collected from 32 sites across 1100 km, with an overall T. cruzi infection frequency of 47.1% (N = 4287 total specimens) by conventional PCR or qPCR. First, we amplified the vertebrate cytochrome b gene (cytb) from all DNA samples obtained from triatomine intestinal contents. Then, we sequenced cytb-positive PCR products in pools of 10–20 triatomines each, grouped by site. The filtered sequences were grouped into amplicon sequence variants (ASVs) with a minimum abundance of 100 reads. ASVs were identified by selecting the best BLASTn match against the NCBI nucleotide database.
Results
Overall, 16 mammal (including human), 14 bird, and seven reptile species were identified in the diet of sylvatic triatomines. Humans were part of the diet of all analyzed triatomine species, and it was detected in 19 sites representing 12.19% of the sequences.
Conclusions
Sylvatic triatomine species from Chile feed on a variety of vertebrate species; many of them are detected here for the first time in their diet. Our results highlight that the sylvatic triatomine–human contact is noteworthy. Education must be enforced for local inhabitants, workers, and tourists arriving in endemic areas to avoid or minimize the risk of exposure to Chagas disease vectors.
Graphical Abstract

Similar content being viewed by others
Background
The study of the dietary composition in sylvatic vectors of zoonotic pathogens is particularly relevant for public health because it provides indirect evidence about the host species involved in the maintenance of endemic vector-borne infections [1,2,3]. Triatomines (Hemiptera: Reduviidae: Triatominae) are hematophagous vector species, commonly known as kissing bugs, capable of transmitting Trypanosoma cruzi (Kinetoplastea: Trypanosomatidae), the parasite that causes Chagas disease [4]. The transmission cycle involves feeding of an infected triatomine on a vertebrate host species, the release of its infective dejections, and the posterior parasitic infection of the host’s mucous membranes, skin abrasions, or the biting site [5]. In addition, oral transmission has been described as an important route of infection, by accidental consumption of insects or their feces in humans, by entomophagy in insectivorous mammals, or as a defensive measure against the triatomine bite in other mammal species [6]. Therefore, transmission to humans is related to the triatomine–human contact frequency [7, 8].
In north-central Chile, there are four described triatomine species, all reported as infected with T. cruzi: the sylvatic diurnal species Mepraia spinolai, Mepraia gajardoi, and Mepraia parapatrica, and the nocturnal species Triatoma infestans [9,10,11,12]. Additionally, 25 mammal species (14 native and 11 exotic) and four lizard species have also been described infected with T. cruzi [12,13,14,15]. The main sylvatic vector of T. cruzi (between ~ 26.5° and 34° S) is M. spinolai [16, 17], an abundant species whose preferred microhabitats include rocky outcrops, rock piles, burrows, and bromeliads, but it has also been found near human settlements and inside rural houses [18,19,20,21,22]. Along its geographical distribution, M. spinolai populations show T. cruzi infection prevalence ranging from 1.3% to 99.0% [22]. However, prevalence and population abundance vary temporally and spatially depending on the local ecological characteristics of the prospected sites (e.g., vertebrate composition) and climatic features [17, 22,23,24]. Mepraia spinolai feeds mostly on native mammals and birds [25,26,27,28,29]. The native rodents Octodon degus and Phyllotis darwini have been found infected by T. cruzi and they are described as the most important feeding sources of M. spinolai [26,27,28,29,30, 17]. However, inside houses, humans are the most frequently detected feeding source, followed by domestic animals [31]. Most previous studies have assessed the feeding profile of M. spinolai in a few specific localities, targeting specific host species in the search process and, therefore, describing only a part of the whole spectrum of vertebrate species included as prey throughout its distribution.
Mepraia gajardoi and M. parapatrica inhabit coastal and insular areas between 18° and 26° S [16, 32, 33]. Both species present low population abundance and can be found under rocks and associated with fishermen’s dwellings. Their T. cruzi infection prevalence ranges from 5.8 to 71.4%, depending on the prospected location [10, 11, 32, 34, 35]. A serological study showed that on the Pan de Azúcar island (26°09′ S, 70°39′ W), specimens of M. parapatrica had fed on marine birds (78%), sea mammals (15%), and reptiles (7%) [36], but not on humans. Moreover, a recent DNA-based detection study carried out on the same island and nearby coastal locations, showed that 61.3% of the blood meal sources corresponded to two lizard species (Microlophus atacamensis and Garthia gaudichaudii), 35.5% to three mammal species (the rodents Mus musculus and Abrothrix olivaceus, and human), and 3.2% to one bird species (the vulture Cathartes aura) [35]. These results highlight the importance of testing for the participation of humans in the kissing bugs’ diet in other localities where these species are occurring, especially near human settlements.
Triatoma infestans used to be associated with the domestic cycle of transmission of T. cruzi, due to the widespread colonies inside houses across the country, where it fed on humans and domestic animals. Currently, this species has been controlled by a sustained campaign of residual insecticide spraying in human dwellings, and mainly flying adults are reported invading houses within the endemic area of Chagas disease [37]. Domiciliary findings of this species show between 20 and 70% of T. cruzi infection [38]. The origin of these individuals is supposed to be sylvatic foci, and they have been reported in bromeliads and rock piles [19, 20, 39]. Infection by T. cruzi in these sylvatic specimens varies, with reports ranging from 25 to 41% [12, 19, 20]. Despite the relevance of studying blood-feeding sources of sylvatic T. infestans, there is no published information describing their diet in Chilean foci.
Trypanosoma cruzi infection has been detected mainly in people from rural and suburban areas of Chile, between 18°30′ and 34°16′ S [40]. Chagas disease prevalence in children and house infestation rates have dropped substantially in the past two decades [41]. Even though Chilean intradomiciliary vector-borne transmission of T. cruzi by T. infestans was declared interrupted in 1999, sylvatic triatomine vectors are still an important problem in rural areas [38, 41, 42], which has maintained the endemicity of the disease in the country. Thus, the description of the whole spectrum of natural hosts should be a priority in public health programs, especially considering the current human infection prevalence of 1.2% by immunoglobulin G (IgG) seropositivity and that ~ 800,000 people are at risk of infection in Chile [43].
Most studies reporting the feeding profile of triatomines have used immunological techniques (e.g., enzyme-linked immunosorbent assay [ELISA]), and only in the last decade DNA-based identification of blood meal sources (e.g., high-resolution melting, Sanger sequencing, next-generation sequencing [NGS]) has been implemented to describe the diet of these vectors [28, 29, 35, 44,45,46,47,48,49]. In the present study, we use DNA-based blood meal source detection, aiming to (i) identify the vertebrate species included in the diet of sylvatic triatomines from most endemic areas of Chile, and (ii) evaluate the frequency of humans in the diet. This information will characterize for the first time the whole spectrum of vertebrate species acting as prey of sylvatic triatomines, provide an indirect triatomine–human contact frequency, and report the potential vertebrate species involved in the transmission cycle and maintenance of T. cruzi in Chile.
Methods
Study sites and triatomine samples
In this cross-sectional study, we assessed the feeding profile of 32 sylvatic triatomine populations, across 1100 km in the arid-semiarid-Mediterranean ecosystems of the Pacific side of southern South America, Chile (23°25′ to 33°26′ S; Fig. 1). Triatomines were collected during summer, from 2014 to 2020. Twenty-one populations were analyzed as part of previous research studies (18 from M. spinolai [N = 2992], two from M. parapatrica [N = 87] and one Mepraia sp. population [N = 38]; sample size per population is shown in Additional file 1: Table S1 [22, 32, 35]), and 11 populations were sampled/analyzed for the present study (10 from M. spinolai [N = 1116] and one from T. infestans [N = 54], sample size per population is shown in Additional file 1: Table S1). In each study site, Mepraia specimens were manually collected for 3–5 days during the daytime (10:00–18:00) by trained researchers using appropriate clothing to avoid triatomine–human contact. Triatoma infestans were collected in a single site, during one night (19:00–09:00), using 85 yeast-baited traps placed underneath bromeliads [12]. Collected specimens were individually stored and frozen at −20 °C until processing.
Sampling procedures were authorized by the Corporación Nacional Forestal (CONAF) from the Atacama and Coquimbo regions (permit numbers: 049/2017, 150/2017, 01/2018, 095/2018). This study was conducted in accordance with the guidelines established by the Institutional Committee for the Care and Use of Animals, University of Chile, Chile (permit number: 17074-FCS-UCH).
DNA extraction of triatomine intestinal content
Triatomine samples collected for the present study were subjected to abdominal extrusion on a clean working area to obtain both intestinal content and intestine samples. An aliquot of a maximum of 25 mg of each sample was mixed with 20 μl of commercial nuclease-free water (Invitrogen™ UltraPure™ Dnase/Rnase-Free Distilled Water).
Whole DNA was isolated from the samples using the DNeasy® Blood & Tissue Kit (QIAGEN, CA, USA). An internal amplification control (IAC) was added to each sample to assess the presence of inhibitors, consisting of 100 pg of Arabidopsis thaliana DNA [50]. The manufacturer's recommendations were followed with a few modifications: the samples were centrifuged for 4 min at 17,000 × g to dry the DNeasy Blood & Tissue Mini spin column, and the final elution volume was 100 μl. Samples were stored at −20 °C until molecular analysis. Triatomine samples from previous studies were processed, as previously described in [22, 32, 35] (Additional file 1: Table S1).
Trypanosoma cruzi infection in sylvatic triatomine populations
Trypanosoma cruzi infection in triatomine samples obtained for this study was tested in a QuantStudio® 3 real-time polymerase chain reaction (PCR) system (Thermo Fisher, USA). All samples were analyzed in duplicate using 0.4 µM of T. cruzi nuclear satellite DNA primers Cruzi 1 and Cruzi 2 [51], 1× HOT FIREPol® EvaGreen® qPCR [quantitative PCR] Mix Plus (Solis BioDyne, Tartu, Estonia), and 5 µl of DNA template in a final volume of 20 µl. DNA from two different T. cruzi strains (TCI-CYC and TCII-CDMC, kindly provided by Dr. Gonzalo Cabrera, Institute of Biomedical Sciences, Faculty of Medicine, University of Chile, Chile) were used as positive control and water instead of DNA as a no-template control. Cycling conditions were 15 min at 95 °C followed by 50 cycles at 95 °C for 15 s, 65 °C for 20 s, and 72 °C for 20 s, finishing with a default melting curve. To detect false negatives, a real-time PCR assay was conducted amplifying IAC DNA using IAC primers with a concentration of 0.4 µM [52] and the same qPCR mix previously described. The PCR conditions were 12 min at 95 °C followed by 40 cycles at 95 °C for 15 s, 64 °C for 15 s, and 72 °C for 15 s, finishing with a default melting curve. Individuals were considered infected when T. cruzi and IAC amplified, and the cycle threshold value (Ct) was lower than 42. In addition, positive samples with a Ct higher than 42 were corroborated by electrophoresis, searching for the expected 166-base-pair (bp) amplicon [51]. For samples from previous studies, T. cruzi infection was detected as previously described in [22, 32, 35], where 121/122 or Cruzi 1/Cruzi 2 primers were used depending on the sampled population (see details in Additional file 1: Table S1).
Vertebrate cytochrome b (cytb) DNA detection in triatomine samples
To assess the feasibility of blood meal detection, we performed a real-time PCR to detect the presence of cytb DNA in the triatomine samples from 30 populations (N = 4200 specimens). For the other two populations (N = 87 specimens), this assay was performed as described in [35]. In Additional file 1, Table S1, the sample size analyzed per population is indicated (see total number of captures). Vertebrate cytb DNA detection was carried out in a QuantStudio® 3 Real-Time PCR System (Thermo Fisher, USA). First, samples were analyzed in duplicate using 0.5 µM of vertebrate cytb gene primers that amplify a 383-bp fragment [53], 1× HOT FIREPol® EvaGreen® qPCR Mix Plus (Solis BioDyne, Tartu, Estonia), and 2 µl of template in a final volume of 20 µl. Each assay included European rabbit (Oryctolagus cuniculus) DNA as positive control, and water instead of DNA as no-template control. Cycling conditions were 15 min at 95 °C followed by 40 cycles at 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 20 s, finishing with a default melting curve. The samples with a Ct value below 34 were considered feasible for blood meal identification by NGS.
Diet detection by NGS and bioinformatics
For 30 populations, we performed NGS using a pool of 10 to 20 blood meal samples of randomly chosen triatomine specimens for each population, in which vertebrate cytb DNA had been previously detected. We included T. cruzi infected and uninfected triatomines in equal proportions, when possible. In the other two populations, NGS was performed as described in [35] (Additional file 1: Table S1). We performed a diversity assay using bTEFAP® Illumina 20 k on cytb of vertebrates, using the same primers described before [53]. The demultiplexed reads were filtered using Sickle 1.33 (https://github.com/ucdavis-bioinformatics/sickle; accessed on 28 October 2021), removing reads shorter than 200 bp or with a quality score lower than Q30. We removed adapter and primer sequences with CutAdapt 1.18 [54]. We then used DADA2 [55] to generate amplicon sequence variants (ASVs). ASVs with less than 100 reads of abundance were removed [56]. Then, each ASV was compared against cytb sequences from the National Center for Biotechnology Information (NCBI) database, with the BLASTn tool, available at https://blast.ncbi.nlm.nih.gov/Blast.cgi. Each ASV was assigned to the species corresponding to the highest blast score.
Data processing and analysis
Blood-feeding sources for each triatomine species were obtained by adding the NGS results from all populations corresponding to that species (i.e., populations from previous studies and those from the present study). Additionally, we added the previously reported NGS diet detection data from two populations of M. parapatrica [35]. The feeding sources were classified into six groups: (i) human, considered independently because of its public health relevance; (ii) human-associated mammals, including livestock (e.g., goats, pigs), pets (e.g., dogs), and synanthropic rodents (e.g., rats, mice); (iii) mammals not associated with humans, which included native mammals, as well as introduced free-ranging mammals (e.g., the European rabbit, European hare); (iv) human-associated bird species; (v) birds not associated with humans; and (vi) reptiles.
Results
Sylvatic triatomine populations and T. cruzi infection
We studied 28 M. spinolai populations (N = 4108 specimens; distance to human settlements: range 59–4574 m and mean ± SD = 746 ± 933 m), two M. parapatrica populations (N = 87 specimens; distance to human settlements: range 45–2143 m and mean ± SD = 1094 ± 1484 m), one Mepraia sp. population (N = 38 specimens; distance to human settlement: 1285 m), and one sylvatic T. infestans population (N = 54 specimens; distance to human settlement: 5 m) (Fig. 1). Given that the Mepraia sp. population was geographically near to the two M. parapatrica populations [33], and all of them were coastal populations from islands or the continent, they were combined for the diet analysis. Trypanosoma cruzi infection frequency ranged from 0.0 to 99.0% for M. spinolai, 20.7 to 36.8% for M. parapatrica, and 55.6% for T. infestans. Details for each population are compiled in Additional file 1: Table S1.
Diet by triatomine species
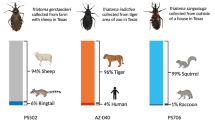
Overall, we obtained between 30,654 and 78,238 reads in the 32 sylvatic triatomine populations. Thirty-seven vertebrate species were identified as feeding sources for all triatomine species combined, including human, human-associated mammals (5), not human-associated mammals (10), human-associated bird (1), not human-associated birds (13), and reptiles (7) (see complete list of ASV assignations in Additional file 2: Table S2). For all populations of M. spinolai, the blood-feeding sources included 36 vertebrate species: human, human-associated mammals (5), not human-associated mammals (10), human-associated bird (1), not human-associated birds (12), and reptiles (7) (Fig. 2a). For M. parapatrica populations, a total of four vertebrate species were detected (human, one mammal, one bird, and one reptile), and no human-associated species was detected (Fig. 2b). Finally, the blood-feeding sources of T. infestans included only three vertebrate species (human and two not human-associated mammals) (Fig. 2c). These three wild triatomine species shared only one blood-feeding source in their diet: the human (Homo sapiens). A complete list of the vertebrate species detected as blood-feeding sources per triatomine species is included in Table 1 and per triatomine population is shown in Additional file 3: Table S3.
When considering the read abundance for each vertebrate group detected as blood-feeding sources for the three species combined, humans represented 12.19%; human-associated mammals, 4.81%; not human-associated mammals, 54.67%; human-associated birds 0.01%, not human-associated birds, 11.52%; and reptiles, 16.81%. When analyzing the feeding sources of each triatomine species separately, the most represented vertebrate group was not human-associated mammals for M. spinolai (59.28%), and human for M. parapatrica and T. infestans (48.46% and 50.69%, respectively) (Fig. 2). For M. spinolai, human DNA represented 6.47%.
The three most represented species in the diet of M. spinolai were the mammals Abrocoma bennettii (18.04%), P. darwini (13.21%), and O. degus (12.82%). Interestingly, species such as moon-toothed degu (O. lunatus), human (H. sapiens), rabbit (O. cuniculus), and three reptiles (Liolaemus fuscus, Gekkota sp., and L. monticola) were also represented in a high proportion (see details in Table 1). For M. parapatrica, the three most important species in the diet were H. sapiens (48.46%), C. aura (34.31%), and Thylamys elegans (17.09%). Finally, in T. infestans, the only three species present in the diet were H. sapiens (50.69%), O. degus (38.01%), and O. cuniculus (11.30%).
Discussion
In the last decade, DNA-based identification of blood meal sources has been implemented to describe the diet of triatomine species. In this study, we used NGS to identify the vertebrate species included in the diet of three sylvatic triatomine species present in the semiarid-Mediterranean ecosystem of Chile and evaluated the frequency of humans as their blood-feeding source.
The blood-feeding sources of M. spinolai included the six established groups, with mammals not associated with humans being the most represented group. Several species of wild mammals are known to play crucial roles in the maintenance of T. cruzi in the Chilean semiarid-Mediterranean ecosystem due to their infection frequencies [12, 17], and this group of vertebrates has been reported as the most frequent and abundant blood meal source in M. spinolai populations, with O. degus and P. darwini as the most common [26,27,28]. In our study, the most represented species was A. bennettii, which had not been previously described as an important blood source; however, this large-sized rodent is closely associated with environments where M. spinolai colonies are present, using the same burrows dug by O. degus [57]. Their larger size and nocturnal habits may compensate their lower densities, providing a stable blood meal source in the semiarid Mediterranean ecosystem [58].
The European rabbit O. cuniculus, a feral invasive species in the Mediterranean ecosystem of Chile [59], represented almost 5% of the diet of M. spinolai, and it deserves special attention. This medium-sized mammal has been previously reported as infected by T. cruzi and a blood-feeding source in the diet of M. spinolai from different populations [26,27,28, 60]. It is reported as a feeding source particularly for fifth-instar nymphs and adults of M. spinolai [29]. Moreover, it has also been proposed as a valuable blood-feeding source, due to a positive relationship between rabbit abundance and population abundance of kissing bugs [22]. This suggests that invasive species may become reservoirs of T. cruzi, if susceptible to this parasite’s infection.
Three reptile species were detected in the diet of M. spinolai. Four lizard species have been recently described as naturally infected by T. cruzi [14], with at least one of them being capable of transmitting the parasite to kissing bugs. Therefore, understanding the role of reptiles in the maintenance and transmission of T. cruzi in the wild cycle is urgent for developing adequate predictive transmission models.
It is important to mention that 11 vertebrate species not found in our samples had been detected by NGS, Sanger sequencing, and/or high-resolution melting in M. spinolai populations as reported in other studies [28, 29, 31]. These species include the lizard Liolaemus pseudolemniscatus, the native birds Mimus thenca and Systellura longirostris, the native rodents Abrothrix longipilis and Oligoryzomys longicaudatus, the fox Lycalopex culpaeus, the domestic cat Felis catus, cattle Bos taurus, horse Equus caballus, donkey Equus asinus, and sheep Ovis aries [28, 29, 31]. It is possible that these species were part of the diet of some of our M. spinolai populations, but due to the random sampling method used to assess the blood meal source by NGS, they were not detected. The presence of domestic animals in one of those studies could be related to their proximity to human settlements; in our study, M. spinolai populations were on average > 740 m from the domicile, so a lower representation of human-associated animals was expected.
In the case of the blood-feeding sources of M. parapatrica, four out of the six established groups were identified. Aside from humans, all species corresponded to animal groups not associated with humans (one mammal, one bird, and one reptile species). In addition to Quiroga et al. [35], only one report [36] studied the diet of M. parapatrica (originally referred to as M. spinolai by [36]), and humans were not part of this kissing bug’s diet. However, other vertebrate species such as the snake Tachymenis peruviana, seabirds, and marine mammals were detected.
Regarding the blood-feeding sources of T. infestans from a sylvatic focus, only a few species were detected, probably as the result of sampling only one population, which has been studied intermittently since its discovery in 2003 [19]. Both synanthropic and wild mammals have been associated with T. infestans Chilean foci [12, 61]. In our study, only three species were found in the diet of T. infestans: humans and two from the group of mammals not associated with humans (O. degus and O. cuniculus). Within the few studies reporting the feeding sources of wild T. infestans populations from Bolivia, rodents were the most detected mammals; bird and reptile species were also represented but to a lesser extent [62].
One of the most relevant epidemiological findings of our study is that the only common species as a feeding source among the three triatomine species was H. sapiens, found in different proportions for each species, but always present in their diet. In M. spinolai, the frequency/proportion of humans in the diet was low when compared against sylvatic triatomine species from other ecosystems of South America [46, 62, 63]. On the other hand, humans represented almost 50% of the blood-feeding sources for M. parapatrica and T. infestans, placing them as the sylvatic triatomine species with the highest representation of humans in their diet compared to previous studies carried out in South America [46, 62, 63]. In Chile, T. infestans was the main vector of T. cruzi inside human dwellings during the twentieth century [40], and human has recently been reported as the most frequent feeding source for domiciliated triatomines from Argentina [8]. According to our results, humans could also be the most relevant feeding source for sylvatic T. infestans in Chile. This high representation of humans in their diet might be the result of T. infestans individuals traveling to nearby human dwellings and returning to their refuges [64]. The presence of humans in the diet of M. parapatrica is probably the result of triatomine invasion of fishermen’s dwellings or tourist’ tents, warning about the epidemiological risk of these sylvatic triatomine bugs in coastal areas of northern Chile [35].
Sylvatic triatomine species from Chile feed on a variety of vertebrate species, and many of them are detected here for the first time in their diet. NGS may also provide updated information on host geographical distribution. The blood-feeding sources detected by NGS included humans as a relevant part of the kissing bugs’ diet. This finding, coupled with the high frequency of T. cruzi infection previously reported in some populations of sylvatic triatomine species in Chile [12, 22, 35] should be cause for concern. The high participation of humans in the diet of M. parapatrica (48.46%) and T. infestans (50.69%) could be an effect of sample size, given by the lower number of sampled populations compared to other reports. On the other hand, the M. spinolai diet is based on a large sample size and human representation was much lower (6.47%). However, because of the wide geographical distribution and high population abundance of M. spinolai, we support the longstanding idea that this species should be monitored, especially considering that constant home invasions are reported to the health services from rural areas [21]. Future studies should attempt individually based triatomine diet analyses, assessing seasonal variations in the diet and evaluating associated anthropic factors to relate them with the presence of humans in the diet of sylvatic triatomines. Moreover, to assess whether there is parasite transmission occurring as a result of these host–triatomine contacts, both human populations living near triatomine foci and other potential hosts—as detected by the feeding sources—should be included in T. cruzi infection studies, to be able to determine whether transmission is zoonotic or enzootic.
Conclusions
Sylvatic triatomine species present in Chile feed on a variety of vertebrate species, including 16 mammals, 14 birds, and seven reptiles. Human is part of the diet of all the analyzed triatomine species, representing 12.19% of the blood-feeding source. Our results based on vertebrate host DNA detection highlight that the sylvatic triatomine–human contact is noteworthy and provide an updated and comprehensive snapshot of the blood-feeding sources of Chilean sylvatic triatomines.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional files. Sequence data available at the NCBI Sequence Read Archive (SRA, https://www.ncbi.nlm.nih.gov/sra), project code PRJNA936617, will be released upon acceptance.
Abbreviations
- BLAST:
-
Basic Local Alignment Search Tool
- cytb :
-
Cytochrome b gene
- Ct :
-
Cycle threshold
- ELISA:
-
Enzyme-linked immunosorbent assay
- IAC:
-
Internal amplification control
- NCBI:
-
National Center for Biotechnology Information
- NGS:
-
Next-generation sequencing
- ASV:
-
Amplicon sequence variants
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative (real-time) polymerase chain reaction
References
Kent RJ. Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol Ecol. 2009;9:4–18. https://doi.org/10.1111/j.1755-0998.2008.02469.x.
Gómez-Díaz E, Figuerola J. New perspectives in tracing vector-borne interaction networks. Trends Parasitol. 2010;26:470–6. https://doi.org/10.1016/j.pt.2010.06.007.
Hylton A, Fitzpatrick DM, Suepaul R, Dobson AP, Charles RA, Peterson JK. Preliminary characterization of triatomine bug blood meals on the island of Trinidad reveals opportunistic feeding behavior on both human and animal hosts. Trop Med Infect Dis. 2020;5:166. https://doi.org/10.3390/tropicalmed5040166.
de Fuentes-Vicente JA, Gutiérrez-Cabrera AE, Flores-Villegas AL, Lowenberger C, Benelli G, Salazar-Schettino PM, et al. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018;183:23–31. https://doi.org/10.1016/j.actatropica.2018.04.008.
Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol. 2001;31:472–81. https://doi.org/10.1016/S0020-7519(01)00153-9.
Telleria J, Tibayrenc M. American trypanosomiasis chagas disease. One hundred years of research. Amsterdam: Elsevier; 2017.
Gürtler RE, Cecere MC, Vázquez-Prokopec GM, Ceballos LA, Gurevitz JM, Fernández MP, et al. Domestic animal hosts strongly influence human-feeding rates of the Chagas disease vector Triatoma infestans in Argentina. PLoS Negl Trop Dis. 2014;8:e2894. https://doi.org/10.1371/journal.pntd.0002894.
Ordóñez-Krasnowski PC, Lanati LA, Gaspe MS, Cardinal MV, Ceballos LA, Gürtler RE. Domestic host availability modifies human-triatomine contact and host shifts of the Chagas disease vector Triatoma infestans in the humid Argentine Chaco. Med Vet Entomol. 2020;34:459–69. https://doi.org/10.1111/mve.12463.
Botto-Mahan C, Sepúlveda M, Vidal M, Acuña-Retamar M, Ortiz S, Solari A. Trypanosoma cruzi infection in the sylvatic kissing bug Mepraia gajardoi from the Chilean Southern Pacific Ocean coast. Acta Trop. 2008;105:166–9. https://doi.org/10.1016/j.actatropica.2007.11.003.
Toledo A, Vergara F, Campos R, Botto-Mahan C, Ortiz S, Coronado X, et al. Trypanosoma cruzi genotypes in Mepraia gajardoi from wild ecotopes in northern Chile. Am J Trop Med Hyg. 2013;88:285–8. https://doi.org/10.4269/ajtmh.2012.12-0227.
Rives-Blanchard N, Torres-Pérez F, Ortiz S, Solari A, Campos-Soto R. Trypanosoma cruzi over the ocean: Insular zones of Chile with presence of infected vector Mepraia species. Acta Trop. 2017;172:229–31. https://doi.org/10.1016/j.actatropica.2017.05.020.
Ihle-Soto C, Costoya E, Correa JP, Bacigalupo A, Cornejo-Villar B, Estadella V, et al. Spatio-temporal characterization of Trypanosoma cruzi infection and discrete typing units infecting hosts and vectors from non-domestic foci of Chile. PLoS Negl Trop Dis. 2019;13:e0007170. https://doi.org/10.1371/journal.pntd.0007170.
Correa JP, Bacigalupo A, Yefi-Quinteros E, Rojo G, Solari S, Cattan PE, et al. Trypanosomatid infections among vertebrates of Chile: a systematic review. Pathogens. 2020;9:661. https://doi.org/10.3390/pathogens9080661.
Botto-Mahan C, Correa JP, Araya-Donoso R, Farías F, Quiroga N, Reyes-Olivares C, et al. Lizards as silent hosts of Trypanosoma cruzi. Emerg Infect Dis. 2022;28:1250–3. https://doi.org/10.3201/eid2806.220079.
Quiroga N, Campos-Soto R, Yañez-Meza A, Rodríguez-San Pedro A, Allendes JL, Bacigalupo A, et al. Trypanosoma cruzi DNA in Desmodus rotundus (common vampire bat) and Histiotus montanus (small big-eared brown bat) from Chile. Acta Trop. 2022;225:106206. https://doi.org/10.1016/j.actatropica.2021.106206.
Garrido R, Bacigalupo A, Peña-Gómez F, Bustamante RO, Cattan PE, Gorla DE, et al. Potential impact of climate change on the geographical distribution of two wild vectors of Chagas disease in Chile: Mepraia spinolai and Mepraia gajardoi. Parasit Vectors. 2019;12:478. https://doi.org/10.1186/s13071-019-3744-9.
Botto-Mahan C, Bacigalupo A, Correa JP, Fontúrbel FE, Cattan PE, Solari A. Prevalence, infected density or individual probability of infection? Assessing vector infection risk in the wild transmission of Chagas disease. Proc R Soc B. 2020;287:20193018. https://doi.org/10.1098/rspb.2019.3018.
Cattan PE, Pinochet A, Botto-Mahan C, Acuña M, Canals M. Abundance of Mepraia spinolai in a periurban zone of Chile. Mem Inst Oswaldo Cruz. 2002;97:285–7. https://doi.org/10.1590/S0074-02762002000300001.
Bacigalupo A, Segura JA, García A, Hidalgo J, Galuppo S, Cattan PE. First finding of Chagas disease vectors associated with wild bushes in the Metropolitan Region of Chile. Rev Med Chile. 2006;134:1230–6. https://doi.org/10.1590/s0074-02762010000500006.
Bacigalupo A, Torres-Perez F, Segovia V, Garcia A, Correa JP, Moreno L, et al. Sylvatic foci of the Chagas disease vector Triatoma infestans in Chile: description of a new focus and challenges for control programs. Mem Inst Oswaldo Cruz. 2010;105:633–41. https://doi.org/10.1590/s0074-02762010000500006.
Frías-Lasserre D, González CR, Valenzuela CR, de Carvalho DB, Oliveira J, Canals M, et al. Wing polymorphism and Trypanosoma cruzi infection in wild, peridomestic, and domestic collections of Mepraia spinolai (Hemiptera: Reduviidae) from Chile. J Med Entomol. 2017;54:1061–6. https://doi.org/10.1093/jme/tjx061.
San Juan E, Araya-Donoso R, Sandoval-Rodríguez A, Yáñez-Meza A, Quiroga N, Botto-Mahan C. Lizards and rabbits may increase Chagas infection risk in the Mediterranean-type ecosystem of South America. Sci Rep. 2020;10:1853. https://doi.org/10.1038/s41598-020-59054-8.
Coronado X, Rozas M, Botto-Mahan C, Ortiz S, Cattan PE, Solari A. Molecular epidemiology of Chagas disease in the wild transmission cycle: the evaluation in the sylvatic vector Mepraia spinolai vector from an endemic area of Chile. Am J Trop Med Hyg. 2009;81:656–9. https://doi.org/10.4269/ajtmh.2009.09-0053.
Oda E, Solari A, Botto-Mahan C. Effects of mammal host diversity and density in the infection level of a sylvatic kissing bug. Med Vet Entomol. 2014;28:384–90. https://doi.org/10.1111/mve.12064.
Rengifo A. Preferencias alimentarias específicas de Mepraia spinolai por vertebrados frecuentes en su hábitat. DVM Undergraduate Thesis, Universidad de Chile, Santiago, Chile; 2000. https://www.bibliotecadigital.uchile.cl/permalink/56UDC_INST/17238n/alma991001496839703936
Canals M, Cruzat L, Molina MC, Ferreira A, Cattan PE. Blood host sources of Mepraia spinolai (Heteroptera: Reduvidae), wild vector of Chagas disease in Chile. J Med Entomol. 2001;38:303–7. https://doi.org/10.1603/0022-2585-38.2.303.
Chacón F, Bacigalupo A, Quiroga JF, Ferreira A, Cattan PE, Ramírez-Toloza G. Feeding profile of Mepraia spinolai, a sylvatic vector of Chagas disease in Chile. Acta Trop. 2016;162:171–3. https://doi.org/10.1016/j.actatropica.2016.06.027.
Saavedra M, Bacigalupo A, Barrera MV, Vergara MJ, Alvarez-Duhart B, Muñoz-San Martín C, et al. Trypanosoma cruzi infection in the wild Chagas disease vector, Mepraia spinolai: Parasitic load, discrete typing units, and blood meal sources. Acta Trop. 2022;229:106365. https://doi.org/10.1016/j.actatropica.2022.106365.
De Bona S, Correa JP, San Juan E, Estay-Olea D, Quiroga N, Bacigalupo A, et al. Opportunistic or selective eaters? Stage-dependent feeding behavior in a wild vector of Chagas disease. Int J Parasitol. 2023;53:55–64. https://doi.org/10.1016/j.ijpara.2022.10.003.
Correa JP, Bacigalupo A, Fontúrbel F, Oda E, Cattan PE, Solari A, et al. Spatial distribution of an infectious disease in a native small mammal community. Sci Nat. 2015;102:51. https://doi.org/10.1007/s00114-015-1304-5.
Gacitúa R. Invasión de Mepraia spinolai a viviendas rurales de la Región de Coquimbo: evaluación de niveles de infección por Trypanosoma cruzi y fuentes de alimentación. DVM Undergraduate Thesis, Universidad de Chile, Santiago, Chile; 2020.
Campos-Soto R, Díaz-Campusano G, Quiroga N, Muñoz-San Martín C, Rives-Blanchard N, Torres-Pérez F. Trypanosoma cruzi-infected triatomines and rodents co-occur in a coastal island of northern Chile. PeerJ. 2020;8:e9967. https://doi.org/10.7717/peerj.9967.
Campos-Soto R, Rodríguez-Valenzuela E, Díaz-Campusano G, Boric-Bargetto D, Zúñiga-Reinoso Á, Cianferoni F, et al. Testing phylogeographic hypotheses in Mepraia (Hemiptera: Reduviidae) suggests a complex spatio-temporal colonization in the coastal Atacama Desert. Insects. 2022;13:419. https://doi.org/10.3390/insects13050419.
González CR, Reyes C, Canals A, Parra A, Muñoz X, Rodríguez K. An entomological and seroepidemiological study of the vectorial-transmission risk of Chagas disease in the coast of northern Chile. Med Vet Entomol. 2015;29:387–92. https://doi.org/10.1111/mve.12131.
Quiroga N, Correa JP, Campos-Soto R, San Juan E, Araya-Donoso R, Díaz-Campusano G, et al. Blood-meal sources and Trypanosoma cruzi infection in coastal and insular triatomine bugs from the Atacama Desert of Chile. Microorganisms. 2022;10:785. https://doi.org/10.3390/microorganisms10040785.
Sagua H, Araya Rojas J, González Cortes J, Neira CI. Mepraia spinolai in the Southeastern Pacific Ocean coast (Chile)—first insular record and feeding pattern on the Pan de Azúcar island. Mem Inst Oswaldo Cruz. 2000;95:167–70. https://doi.org/10.1590/s0074-02762000000200006.
Bacigalupo A, Segovia V, García A, Botto-Mahan C, Ortiz S, Solari A, et al. Differential pattern of infection of sylvatic nymphs and domiciliary adults of Triatoma infestans with Trypanosoma cruzi genotypes in Chile. Am J Trop Med Hyg. 2012;83:473–80. https://doi.org/10.4269/ajtmh.2012.11-0237.
Canals M, González C, Canals L, Canals A, Cáceres D, Alvarado S, et al. What do the numbers tell us about the temporal evolution of Chagas’ disease? Rev Chil Infectol. 2017;2017:120–7. https://doi.org/10.4067/S0716-10182017000200004.
Bacigalupo A, Correa JP, García A, Cattan PE. Focos silvestres de Triatoma infestans en Latinoamérica: análisis y perspectivas para Chile. Parasitol Latinoam. 2015;64:27–35.
Tapia-Garay V, Figueroa DP, Maldonado A, Frías-Lasserre D, González CR, Parra A, et al. Assessing the risk zones of Chagas’ disease in Chile, in a world marked by global climatic change. Mem Inst Oswaldo Cruz. 2018;113:24–9. https://doi.org/10.1590/0074-02760170172.
Salas PR. Epidemiology of Chagas disease: high mortality and incidence rate. Coquimbo Region Rev Chil Infectol. 2020;37:402–12. https://doi.org/10.4067/S0716-10182020000400402.
Canals M, Canals A, Ayala S, Tapia-Garay V, Cáceres D. Eco-epidemiology of Chagas disease in Chile. In: Nissapatorn V, editor. Chagas Disease - basic investigations and challenges. IntechOpen. 2018. https://www.intechopen.com/books/chagas-disease-basic-investigations-and-challenges/eco-epidemiology-of-chagas-disease-in-chile. Accessed 18 Jan 2023.
ENS (Encuesta Nacional de Salud). 2016–2017. Encuesta Nacional de Salud—Chile 2016–2017. Ministerio de Salud-Gobierno de Chile. http://epi.minsal.cl/resultados-encuestas/. Accessed 18 Jan 2023.
Peña VH, Fernández GJ, Gomez-Palacio AM, Mejia-Jaramillo AM, Cantillo O, Triana-Chavez O. High-resolution melting (HRM) of the cytochrome B gene: a powerful approach to identify blood-meal sources in Chagas disease vectors. PloS Negl Trop Dis. 2012;6:e1530. https://doi.org/10.1371/journal.pntd.0001530.
Dumonteil E, Ramirez-Sierra M-J, Pérez-Carrillo S, Teh-Poot C, Herrera C, Gourbière S, et al. Detailed ecological associations of triatomines revealed by metabarcoding and next-generation sequencing: Implications for triatomine behavior and Trypanosoma cruzi transmission cycles. Sci Rep. 2018;8:4140. https://doi.org/10.1038/s41598-018-22455-x.
Arias-Giraldo LM, Muñoz M, Hernández C, Herrera G, Velásquez-Ortiz N, Cantillo- Barraza O, et al. Identification of blood-feeding sources in Panstrongylus, Psammolestes, Rhodnius and Triatoma using amplicon-based next-generation sequencing. Parasit Vectors. 2020;13:434. https://doi.org/10.1186/s13071-020-04310-z.
Lilioso M, Reigada C, Pires-Silva D, Fontes FVHM, Limeira C, Monsalve-Lara J, et al. Dynamics of food sources, ecotypic distribution and Trypanosoma cruzi infection in Triatoma brasiliensis from the northeast of Brazil. PLoS Negl Trop Dis. 2020;14:e0008735. https://doi.org/10.1371/journal.pntd.0008735.
Murillo-Solano C, López-Domínguez J, Gongora R, Rojas-Gulloso A, Usme-Ciro J, Perdomo-Balaguera E, et al. Diversity and interactions among triatomine bugs, their blood feeding sources, gut microbiota and Trypanosoma cruzi in the Sierra Nevada de Santa Marta in Colombia. Sci Rep. 2021;11:12306. https://doi.org/10.1038/s41598-021-91783-2.
Ocaña-Mayorga S, Bustillos JJ, Villacís AG, Pinto CM, Brenière SF, Grijalva MJ. Triatomine feeding profiles and Trypanosoma cruzi infection, implications in domestic and sylvatic transmission cycles in Ecuador. Pathogens. 2021;10:42. https://doi.org/10.3390/pathogens10010042.
Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, et al. Accurate Real-Time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl Trop Dis. 2009;3:e419. https://doi.org/10.1371/journal.pntd.0000419.
Piron M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, et al. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103:195–200. https://doi.org/10.1016/j.actatropica.2007.05.019.
Ramírez JC, Cura CI, da Cruz MO, Lages-Silva E, Juiz N, Velázquez E, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn. 2015;17:605–15. https://doi.org/10.1016/j.jmoldx.2015.04.010.
Boakye DA, Tang J, Truc P, Merriweather A, Unnasch TR. Identification of bloodmeals in haematophagous Diptera by cytochrome B heteroduplex analysis. Med Vet Entomol. 1999;13:282–7. https://doi.org/10.1046/j.1365-2915.1999.00193.x.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;2011:10–2. https://doi.org/10.14806/ej.17.1.200.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. https://doi.org/10.1038/nmeth.3869.
Estrada-Franco JG, Fernández-Santos NA, Adebiyi AA, López-López MdJ, Aguilar-Durán JA, Hernández-Triana LM, et al. Vertebrate-Aedes aegypti and Culex quinquefasciatus (Diptera)-arbovirus transmission networks: Non-human feeding revealed by meta-barcoding and next-generation sequencing. PLoS Negl Trop Dis. 2020;14:e0008867. https://doi.org/10.1371/journal.pntd.0008867
Iriarte A. Mamíferos de Chile. Barcelona: Lynxs Edicions; 2008.
Iriarte JA, Contreras LC, Jaksic FM. A long-term study of a small-mammal assemblage in the Central Chilean Matorral. J Mammal. 1989;70:79–87. https://doi.org/10.2307/1381671.
Camus P, Castro SA, Jaksic FM. European rabbit (Oryctolagus cuniculus L.) in Chile: The human dimension behind a biological invasion. In: Jaksic FM, Castro SA, editors. Biological invasions in the South American Anthropocene: global causes and local impacts. Cham: Springer; 2021. https://doi.org/10.1007/978-3-030-56379-0_8.
Botto-Mahan C, Acuña-Retamar M, Campos R, Cattan P, Solari A. European rabbits (Oryctolagus cuniculus) are naturally infected with different Trypanosoma cruzi genotypes. Am J Trop Med Hyg. 2009;80:944–6. https://doi.org/10.4269/ajtmh.2009.80.944.
Galuppo S, Bacigalupo A, García A, Ortiz S, Coronado X, Cattan PE, et al. Predominance of Trypanosoma cruzi genotypes in two reservoirs infected by sylvatic Triatoma infestans of an endemic area of Chile. Acta Trop. 2009;111:90–3. https://doi.org/10.1016/j.actatropica.2009.02.010.
Buitrago R, Bosseno MF, Depickère S, Waleckx E, Salas R, Aliaga C, et al. Blood meal sources of wild and domestic Triatoma infestans (Hemiptera: Reduviidae) in Bolivia: connectivity between cycles of transmission of Trypanosoma cruzi. Parasit Vectors. 2016;9:214. https://doi.org/10.1186/s13071-016-1499-0.
Pizarro JC, Stevens L. A new method for forensic DNA analysis of the blood meal in Chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca. Bolivia PloS One. 2008;3:e3585. https://doi.org/10.1371/journal.pone.0003585.
Buitrago NLR, Bosseno MF, Waleckx E, Brémond P, Vidaurre P, Zoveda F, et al. Risk of transmission of Trypanosoma cruzi by wild Triatoma infestans (Hemiptera: Reduviidae) in Bolivia supported by the detection of human blood meals. Infect Genet Evol. 2013;19:141–4. https://doi.org/10.1016/j.meegid.2013.07.002.
Acknowledgements
We thank G. Acosta-Jamett, P.P. Álvarez, P. Arroyo, S. de Bona, R. Cares, M. Ehrenfeld, D. Estay-Olea, F. Farías, B. Garrido, C. Lama, R. Montero, T. Palma, N. Peña, O. Rozas, R. Salgado, A. Sandoval-Rodríguez, A. Schuck, and A. Yáñez-Meza, for their invaluable help during field and/or laboratory work. We also thank CONAF for authorizing this study at several National Parks and Reserves across the country, and personnel from the Health Ministry and Secretario Regional Ministerial (SEREMI) from several regions for their support to find sylvatic triatomine colonies.
Funding
This study was funded by the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) [grants no. 1221045, 1170367 (CBM and JPC), 1190392 (AS) and 1140521 (CBM)], VID-UChile ENL01/21, partially by Universidad Viña del Mar project FIIUVM-CTC-2211 (RCS), and ANID-Vinculación Internacional-FOVI1220125 (CBM, AB and ML). RAD and AB were supported by Agencia Nacional de Investigación y Desarrollo (ANID) Programa Becas—Doctorado Becas Chile 2019 [grants no. 72200094 and 72200391, respectively]. The funding sources have no involvement in the study design; the collection, analysis or interpretation of data; the writing of the report; or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
CBM and JPC designed and supervised the study. CBM, ESJ, RAD, JPC, NQ, RCS, and AB collected the samples. NQ and JPC carried out experimental procedures. CBM, ESJ, AB, CSR, RAD, ML, and AS wrote and/or edited the manuscript. ESJ, RAD, and CSR analyzed and interpreted the data. ESJ and CSR prepared the figures. CBM, JPC, RCS, AS, and ML acquired funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol of this study was approved by the Institutional Ethics Committee for the Care and Use of Animals, University of Chile, Chile (permit number: 17074-FCS-UCH).
Consent for publication
All the authors approve the publication of the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Complete information for triatomine populations included in the present study.
Additional file 2:
Table S2. Results of the BLASTn of the ASVs against the NCBI nucleotide database.
Additional file 3: Tables S3.
Complete list of vertebrate species detected as blood-feeding source for each triatomine population after ASV<100-reads filtering.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
San Juan, E., Araya-Donoso, R., Sierra-Rosales, C. et al. Humans as blood-feeding sources in sylvatic triatomines of Chile unveiled by next-generation sequencing. Parasites Vectors 16, 225 (2023). https://doi.org/10.1186/s13071-023-05841-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05841-x