Abstract
Background
Monogenean parasites have never been formally reported on fishes from the Lufira River Basin. In this context, we decided to record the monogenean parasite fauna of three cichlid species found in the Upper Lufira River Basin for the first time by inventorizing their diversity (species composition) and analysing their infection parameters (prevalence, mean intensity and abundance).
Methods
The African cichlid fishes Oreochromis mweruensis, Coptodon rendalli and Serranochromis macrocephalus were selected for the study, given their economic value and their abundance in the Upper Lufira River Basin. Monogeneans were isolated from the gills and stomach, mounted on glass slides with either Hoyer’s medium or ammonium picrate-glycerin for identification under a stereomicroscope, based on morphological analysis of genital and haptoral hard parts. Indices of diversity and infections parameters were calculated.
Results
A total of 13 gill monogenean parasite species (Cichlidogyrus dossoui, C. halli, C. karibae, C. mbirizei, C. papernastrema, C. quaestio, C. sclerosus, C. tiberianus, C. tilapiae, C. zambezensis, Scutogyrus gravivaginus, S. cf. bailloni and Gyrodactylus nyanzae) and one stomach monogenean (Enterogyrus malmbergi) were identified. A species richness (S) of 10 for O. mweruensis, S = 6 for C. rendalli and S = 2 for S. macrocephalus was recorded. Five parasite species were reported to be common amongst O. mweruensis and C. rendalli. According to cichlid species, the most prevalent parasite species was C. halli (prevalence [P] = 80.9%) on O. mweruensis, C. dossoui (P = 92.9%) on C. rendalli and C. karibae and C. zambezensis (both P = 9.1%) on S. macrocephalus. The parasite species with the highest mean intensity (MI) were G. nyanzae (MI = 8.7) on O. mweruensis, C. papernastrema (MI = 17.1) on C. rendalli and C. karibae (MI = 15) on S. macrocephalus. The findings indicate new host ranges for five parasites species (C. quaestio, S. cf. bailloni, E. malmbergi on O. mweruensis, C. halli on C. rendalli and C. karibae on S. macrocephalus) as well as new geographical records for all of them as they are recorded for the first time in the Lufira River Basin.
Conclusions
This study highlighted the richness of monogenean communities in the Upper Lufira River Basin and is a starting point for future helminthological studies, such as on the use of fish parasites as indicators of anthropogenic impacts.
Graphical Abstract

Similar content being viewed by others
Background
The Congo Basin harbours the greatest species richness of fishes across the African continent [1]. The Congo Basin encompasses 3,747,320 km2, with a drainage area that covers most of the Democratic Republic of Congo and parts of some of its bordering countries (Angola, Zambia, Tanzania, Burundi, Rwanda, Central African Republic and Republic of Congo) and a small part of Cameroon [2]. Many different types of habitats are found in the Congo Basin, and these are subdivided into separate drainages: the Upper Congo (called Lualaba), the Middle Congo and the Lower Congo [1, 3, 4]. One of the major tributaries in the Upper Congo drainage is the Lufira River [5], which can also be subdivided into three sections: the Upper Lufira (from the source of the river to Lake Koni), the Middle Lufira (from downstream Lake Koni to the Kyubo Falls) and the Lower Lufira (from downstream the Kyubo Falls to the Kamalondo Depression, at the junction with the Lualaba River) [4, 6]. During the first half of the 20th century two successive dams were built in the Upper Lufira River to provide hydroelectric power, resulting in the creation of two artificial Lakes, Tshangalele (1930) and Koni (1949) [7,8,9]. Lake Tshangalele, located about 35 km east of the town of Likasi, is home to a variety of fish species, and it is also an UNESCO Biosphere Reserve, rich in bird life [10, 11]. Most of the studies on biodiversity undertaken to date in the Lufira River have focussed on vertebrates, such as fishes and birds [12,13,14,15]. Vast and speciose communities, which are often dominated by less sizeable animals such as flatworms or various parasite taxa, remain understudied [16, 17]. In view of the high diversity of potential host species in the tropics, it can be expected that parasitological surveys there would lead to the discovery of many parasite species, including species new to science [18, 19].
The focus of this study was monogenean fish parasites, which due to their diversity, wide distribution, high host specificity and single-host life-cycle are interesting models for studying the extent of parasite biodiversity and the underlying diversification mechanisms [20]. Monogeneans are common parasitic flatworms (Platyhelminthes) that mostly infest fish but sporadically infect aquatic invertebrates, amphibians, reptiles and a single mammalian species (the hippopotamus) [21,22,23,24,25,26]. Parasitic monogeneans present a high risk for aquaculture industries, causing substantial economic losses, and have been associated with reduced growth, morbidity and mortality [27,28,29]. Several monogenean species are reported to have serious economic impacts in the confines of captive or intensive fish farming [30, 31].
The infection sites of monogeneans on fish hosts are typically gills, fins and/or skin [32]; however, very occasionally they are also found in the stomach, urinary bladder, intestine, oral or nasal cavity, eyes and heart [33, 34]. Due to a one-host life-cycle and a close relationship with the respective host species, many monogeneans are specialists, infesting only a single host species (oioxenous specificity), although others are generalists, infesting ≥ 2 host species (stenoxenous specificity) [35,36,37]. Mendlová and Šimková [38] used a more extensive number of categories of host specificity on the basis of the phylogenetic relationships among (cichlid) host species in which parasites can be: (i) strict specialists when infecting only one host species; (ii) intermediate specialists when infecting ≥ 2 congeneric host species; (iii) intermediate generalists when infecting noncongeneric cichlid species belonging to the same tribe; and finally (iv) generalists, when infecting noncongeneric cichlid species of at least two different tribes.
African cichlids (taking also into account the Levant) are known to harbour monogenean parasites belonging to six genera: Enterogyrus Paperna, 1963; Urogyrus Bilong Bilong, Birgi & Euzet, 1994; Onchobdella Paperna, 1968; Scutogyrus Pariselle & Euzet, 1995; Cichlidogyrus Paperna, 1960 (Dactylogyridea) and Gyrodactylus von Nordmann, 1832 (Gyrodactylidea). The latter four are ectoparasitic genera, and among these, Cichlidogyrus is the most species-rich group with more than 131 nominal species described to date [39,40,41]. Its representatives are known to be pathogenic in tilapia aquaculture [42].
The overall aim of this study was to record the monogenean parasite fauna of three cichlid species found in the Upper Lufira River Basin. At the start of this study, these parasites had never been formally reported from this region. The specific objectives include: (i) inventorizing the diversity of gill monogenean communities, and (ii) analysing infection parameters of these monogenean parasites.
Methods
Study area
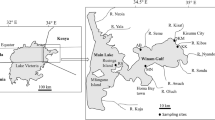
This study was conducted in the Upper Lufira River Basin (altitude: 1114–1160 m a.s.l.) (Fig. 1), which is localized across the mining hinterland area in the west of Haut-Katanga Province (in the south of the former Katanga Province). The climate type is equatorial savanna with a dry winter (type AW6: A, equatorial climate region; W, desert) following the classification of Köppen [43] and a rainy tropical climate with a rainy season extending from November to April [44]. Most precipitation falls from December to March [45]. The Upper Lufira River Basin is characterized by a great diversity of fish species, including members of the families Alestidae, Anabantidae, Amphilidae, Auchenoglanididae, Characidae, Clariidae, Cichlidae, Cyprinidae, Cyprinodontidae, Distichodontidae, Mochokidae, Mormyridae and Schilbeidae [46, 47]. There is organized small-scale fishing for Coptodon rendalli (Boulenger, 1896), Oreochromis mweruensis Trewavas, 1983, Serranochromis macrocephalus Boulenger, 1899, Clarias gariepinus (Burchell, 1822) and Clarias ngamensis (Castelnau, 1861) [48]. Caught fishes are intended for human consumption, for a small part by the local population around the Upper Lufira River Basin and for most part by residents of bigger towns, such as Likasi and Lubumbashi.
Map of sampling sites in the Upper Lufira River Basin: along the Lufira River (Kaboko: 11°4′31.60″ S, 26°55′2.40″ E; Buta: 11°2′21.60″ S, 26°57′23.10″ E) and bordering two stretches of the Lufira River that have been dammed: Lake Tshangalele (Kisunka: 10°50′52.10″ S, 26°57′50.60″ E; Kapolowe Mission: 10°54′59.50″ S, 26°58′17.70″ E; Yuka: 10°56′25.30″ S, 26°58′53.40″ E; Mulandi: 10°57′36.64″ S, 27°6′44.88″ E) and Lake Koni (Koni: 10°43′3.65″ S, 27°17′3.24″ E)
Fish sampling
Three fish species, O. mweruensis, C. rendalli and S. macrocephalus were selected for examination in the study, given their economic value and their abundance in the Upper Lufira River Basin [48]. Fishes were collected using gillnets with a mesh size of 12–20 mm knot to knot or were bought from local fishermen along the shores of the Lufira River, Lake Tshangalele and Lake Koni (Fig. 1) between September 2015 and August 2018. Once obtained, the fishes were kept alive in an aerated tank and transported to a field laboratory where they were identified to the species level following the keys of Lamboj [47]. The fishes were killed by severing the spinal cord just posterior to the cranium, immediately prior to examination, following the method of Olivier et al. [49]. For each fish, total length (TL) and standard length (SL) were measured to the nearest 0.1 cm, and the weight was taken to the nearest 0.1 g.
Parasite sampling
To collect monogenean parasites, we first dissected the fishes and removed the right gill arches by dorso-ventral section. One fish specimen from amongst all of the fish species sampled was randomly dissected and inspected for monogenean parasites in its stomach. Gill arches and the stomach were placed in a Petri dish containing water for examination using an (Optika 4.0.0 stereomicroscope (OPTIKA Srl, Ponteranica, BG, Italy). Parasites were dislodged from the gill filaments using entomological needles and fixed between a slide and coverslip into a drop of either Hoyer’s medium or ammonium picrate-glycerin, according to Nack et al. [50]. After 24 h, the coverslips were sealed with nail varnish. Parasites were deposited in the invertebrate collection of the Royal Museum of Central Africa (RMCA) under accession numbers RMCA_VERMES_43743-44345.
Monogenean community composition, indices of diversity and infection parameters
Morphological identification of the retrieved parasite specimens was conducted based on the sclerotized parts of the haptor, the male copulatory organ and the vagina, using a Motic BA310 microscope (Motic, Speed Fair Co., Ltd., Hong Kong) and a phase-contrast microscope (model BX50; Olympus, Tokyo, Japan). Parasite identification to species level and comparison with known congeners was based on García-Vásquez et al. [51, 52], Přikrylová et al. [53, 54], Gillardin et al. [55], Muterezi Bukinga et al. [56], Pariselle and Euzet [39, 57] and Fannes et al. [58]. Parasite diversity was summarized by the species richness index, Shannon index and Equitability of Pielou [59]. Infection parameters, i.e. prevalence, mean intensity (MI) and mean abundance (MA) were provided following definitions given by Margolis et al. [60] and Bush et al. [61]. Statistical analysis was performed using Past version 3.1 software [62].
Results
Fishes used in this study were of different sizes and weight ranges. For O. mweruensis (n = 47), the mean (± standard deviation) TL and SL were 18.2 ± 4.1 and 14.6 ± 3.2 cm, respectively, and the mean weight was 72.7 ± 38.8 g. For C. rendalli (n = 28), the mean TL and SL were 15.1 ± 2.8 and 12.0 ± 2.4 cm, respectively, and the mean weight was 72.7 ± 38.8 g. For S. macrocephalus (n = 11), the mean TL and SL were 16.9 ± 3.4 and 14.0 ± 2.8 cm, respectively, and the mean weight was 81.9 ± 51.5 g.
Composition and indices of diversity of the Monogenean community in the Upper Lufira River Basin
Specimens representing four genera of monogeneans, Cichlidogyrus, Gyrodactylus, Scutogyrus (on the gills) and Enterogyrus (in the stomach) were collected (Table 1; Additional file 1: Table S1). Among these were 10 known species of Cichlidogyrus, one species of Gyrodactylus, two species of Scutogyrus and one species of Enterogyrus (Figs. 2, 3, 4, 5, and 6). For O. mweruensis, C. rendalli and S. macrocephalus, the parasite diversity indices were, respectively, 10, 6 and 2 for the species richness index; 1.5, 1.2 and 0.6 for the Shannon index; and 0.6, 0.8 and 0.8 for the index of Equitability of Pielou. The distribution of monogeneans per sampling period or per season is shown in Table 2 to provide a picture of the distribution of monogeneans over time.
Photomicrographs of the sclerotized structures of: a the male copulatory organ of Cichlidogyrus halli ex Oreochromis mweruensis from Lake Koni (RMCA_VERMES_44100), b the haptor of C. halli ex O. mweruensis from Lake Koni (RMCA_VERMES_44101), c the male copulatory organ of Cichlidogyrus dossoui ex Coptodon rendalli from Lufira River (RMCA_VERMES_43783), d the vagina of C. dossoui ex C. rendalli from Yuka (RMCA_VERMES_44286), e the male copulatory organ of Cichlidogyrus tiberianus ex C. rendalli from Kapolowe Mission (RMCA_VERMES_44026), f the vagina of C. tiberianus ex C. rendalli from Kapolowe Mission (RMCA_VERMES_43997)
photomicrographs of the sclerotized structures of: a the male copulatory organ of Cichlidogyrus sclerosus ex Oreochromis mweruensis from Buta (RMCA_VERMES_43900), b the haptor of C. sclerosus ex O. mweruensis from Kisunka (RMCA_VERMES_43902), c the male copulatory organ of Cichlidogyrus quaestio ex Coptodon rendalli from Kisunka (RMCA_VERMES_43934), d the haptor of C. quaestio ex C. rendalli from Kisunka (RMCA_VERMES_43934), e the male copulatory organ of Cichlidogyrus tilapiae ex O. mweruensis from Kisunka (RMCA_VERMES_43910), f the haptor of C. tilapiae ex O. mweruensis from Kisunka (RMCA_VERMES_43910)
Photomicrographs of the sclerotized structures of: a the male copulatory organ of Cichlidogyrus mbirizei ex Oreochromis mweruensis from Lufira River (RMCA_VERMES_43753), b the vagina of C. mbirizei ex O. mweruensis from Lufira River (RMCA_VERMES_43766), c the male copulatory organ of Cichlidogyrus papernastrema ex Coptodon rendalli from Lufira River (RMCA_VERMES_43817), d the first pair of marginal hooks of C. papernastrema ex C. rendalli from Lufira River (RMCA_VERMES_43791), e the male copulatory organ of Cichlidogyrus karibae ex Serranochromis macrocephalus from Lufira River (RMCA_VERMES_44341), f the haptor of C. karibae ex S. macrocephalus from Lufira River (RMCA_VERMES_44341)
Photomicrographs of the sclerotized structures of: a the male copulatory organ of Cichlidogyrus zambezensis ex Serranochromis macrocephalus from Lufira River (RMCA_VERMES_44343), b the haptor of C. zambezensis ex S. macrocephalus from Lufira River (RMCA_VERMES_44343), c the male copulatory organ and vagina of Scutogyrus gravivaginus ex Oreochromis mweruensis from Buta (RMCA_VERMES_43896), d the haptor of S. gravivaginus ex O. mweruensis from Buta (RMCA_VERMES_43896), e the vagina of Scutogyrus cf. bailloni ex O. mweruensis from Kapolowe Mission (RMCA_VERMES_43958), f the whole mount of Scutogyrus cf. bailloni ex O. mweruensis from Kapolowe Mission (RMCA_VERMES_43958)
Infection parameters of monogenean parasites in the Upper Lufira River Basin
The prevalence, mean intensity and mean abundance presented here take into account conspecific host individuals grouped without seasonal subdivision as the main objective of the study was to record the monogenean parasite diversity, not the epidemiological variation.
The highest prevalences recorded were 80.9% for Cichlidogyrus halli on O. mweruensis, 92.3% for Cichlidogyrus dossoui on C. rendalli and 9.1% for both Cichlidogyrus zambezensis and Cichlidogyrus karibae on S. macrocephalus. The lowest prevalences recorded were 2.1% for Cichlidogyrus tiberianus, Scutogyrus cf. bailloni on O. mweruensis and 3.8% for Gyrodactylus nyanzae on C. rendalli (Fig. 7).
For G. nyanzae, the highest mean intensity of 8.7 ± 9.9 was recorded from O. mweruensis and the lowest mean intensity of 1 ± 0 was recorded from C. rendalli. Conversely, the mean intensity for Cichlidogyrus papernastrema was 17.1 ± 24 when the latter fish host was examined. From S. macrocephalus, the highest and lowest mean intensities were for C. karibae (MI = 15) and C. zambezensis (MI = 5), respectively (Fig. 8).
Results on the mean abundance revealed that C. halli was the most abundant species on O. mweruensis (MA = 6.4 ± 7.7), C. dossoui was the most abundant species on the gills of C. rendalli (9.7 ± 15.6) and C. karibae was the most abundant species on S. macrocephalus (MA = 1.4 ± 4.5) (Fig. 9).
Discussion
This study was conducted to explore the monogenean parasite fauna of three economically important and abundant cichlid species in the Upper Lufira River Basin, a part of the Upper Congo Basin. During this study we recorded 13 gill and one stomach monogenean species. Parasite species from fish species belonging to the genera Oreochromis Günther, 1889, Coptodon Gervais, 1853 and Serranochromis Regan, 1920 have been previously reported [39, 56, 63]. Although a few studies on monogenean parasites from the Congo Basin have been conducted in the Lake Tanganyika, Bangweulu-Mweru, Upper Lualaba, Kasai, Lower Congo and Pool Malebo Ecoregions (sensu Thieme et al. [64]) [55, 56, 63, 65,66,67,68], the present study is the first to record monogenean parasites in the Lufira River Basin. Based on the results of previous studies and current information, this study extends the known host range of five parasite species. Cichlidogyrus quaestio, S. cf. bailloni and E. malmbergi were recorded for the first time from O. mweruensis; C. halli was recorded for the first time from C. rendalli; and C. karibae was recorded for the first time from S. macrocephalus. Cichlidogyrus karibae was described by Douëllou [69] on Sargochromis codringtonii (Boulenger, 1908) in Lake Kariba (Zambezi Basin, Zimbabwe). Enterogyrus malmbergi was described by Bilong Bilong [70] from the stomach of Oreochromis niloticus (Linnaeus, 1758) in the Sanaga River (Cameroon). Scutogyrus bailloni was formally described by Pariselle and Euzet [57] on Sarotherodon galilaeus (L, 1758) in the Mékrou River (Niger Basin, Niger, West Africa). Since only a single similar parasite specimen was retrieved in the present study on the gills of O. mweruensis, it cannot be assigned to S. bailloni with certainty as the mount was imperfect, although we were able to recognize and identify the principal diagnostic structures (the haptor, the male copulatory organ and the vagina). Verification of its identification with molecular markers is necessary to determine whether this specimen belongs to S. bailloni or to a morphologically similar species currently unknown to science. Nevertheless these (putative in case of S. bailloni) records substantially expand the known geographical distribution of these three monogenean species, as this study is the first time they have been recorded in the Congo Basin.
In terms of species richness, our results are similar to those reported earlier for monogenean gill parasites on these three fish species in the Congo Basin [63, 66, 68]. In the present study, 10 monogenean species were found on O. mweruensis, while Jorissen et al. [63, 66] collected nine parasite species in the Bangweulu-Mweru Ecoregion on O. mweruensis (of which 7 were shared, with the exceptions of Cichlidogyrus mbirizei, C. quaestio and S. cf. bailloni on O. mweruensis from the Lufira River system, and C. cirratus and C. papernastrema on O. mweruensis from the Bangweulu-Mweru Ecoregion). Six monogenean species were found on C. rendalli in the present study, while Jorissen et al. [63, 66] collected five parasite species (all but C. halli corresponding to those found in this study) in the Bangweulu-Mweru Ecoregion. On S. macrocephalus, we found two monogenean species (C. karibae and C. zambezensis), while Jorissen et al. [66] reported only the latter species on S. macrocephalus and its congeners Serranochromis thumbergi (Castenau, 1861), Serranochromis jallae (Boulenger, 1896) and Sargochromis mellandi (Boulenger, 1905).
In terms of infection parameters, on O. mweruensis, one parasite species had a prevalence of > 50% in the Upper Lufira River Basin (C. halli, P = 80.9%) against two monogenean species in the Bangweulu-Mweru Ecoregion reported by Jorissen et al. [66] (P = 57.1% for C. dossoui and S. gravivaginus). On C. rendalli, C. dossoui (P = 92.3%) in the Upper Lufira River Basin, and C. dossoui, C. quaestio and C. tiberianus in the Bangweulu-Mweru Ecoregion have P > 50% following comparison with Jorissen et al. [66]. On S. macrocephalus, no parasite species had a prevalence > 50% in the Upper Lufira River Basin, while C. zambezensis reached a prevalence of 100% in the Bangweulu-Mweru Ecoregion. Regarding the infection intensity (Table 1), the most infected individuals of O. mweruensis in the Upper Lufira River Basin harboured up to 30 specimens of C. halli, followed by 25 specimens of G. nyanzae, against 37 parasite specimens of G. nyanzae and 21 parasite specimens of C. cirratus in Bangweulu-Mweru Ecoregion (reported by Jorissen et al. [66]). The most infected individuals of C. rendalli in the Upper Lufira River Basin harboured up to 84 specimens of C. papernastrema, followed by C. dossoui with 68 monogenean specimens. On the other hand, on the same fish species, in the Bangweulu-Mweru Ecoregion, the monogeneans C. dossoui and C. quaestio reached a lower maximum intensity of infection (29 and 20 specimens, respectively). Finally, on S. macrocephalus in the Upper Lufira, the most infected fish specimens contained up to 15 and 5 parasite specimens of C. karibae and C. zambezensis, respectively, while Jorissen et al. [66] reported up to 21 parasite specimens of C. zambezensis on Serranochromis spp. in the Bangweulu-Mweru Ecoregion. These differences in infection parameters may be due to sample size, season, biogeographical distribution or other environmental parameters, as communities of cichlid-infecting monogeneans have been observed to fluctuate seasonally and between habitat types, and parasite species composition may change between areas and basins [71, 72].
Conclusion
In this study, we recorded the species richness and infection parameters of three cichlid species in the Upper Lufira River Basin that infect the stomach and gills. A total of 13 monogenean species were recovered from O. macrochir, C. rendalli and S. macrocephalus. These findings are the first record of monogeneans in the Lufira River Basin. For future sampling, it will also be interesting to study groups of fish parasites other than monogenean parasites, as well as other fish species or families, to record the diversity of parasites. In many parts of the Congo Basin, there is a lack of baseline data on fish parasites. With this in mind, this study may serve as an important baseline for future studies conducted on fish from the Upper Lufira River Basin as well as the rest of the Congo Basin, enabling the comparison of values to those found in the present study to establish whether there has been a change in parasite composition and parasite load over time. In future studies, molecular analyses may be useful to confirm the morphological identification of parasites and identify phylogeographical patterns.
Availability of data and materials
Slides of monogenean parasites are available in the invertebrate collection of the Royal Museum of Central Africa, Tervuren, Belgium under accession numbers RMCA_VERMES_43743-44345 (Additional file 1: Table S1).
References
Teugels GG, Thieme ML. Biological distinctiveness of African ecoregions: freshwater fish biodiversity in the Congo basin. In: Thieme ML, Abell R, Skelton P, Lenher B, Teugels GG, Dinerstein E, et al., editors. Freshwater ecoregions of Africa and Madagascar. Washington DC: Island Press; 2005. p. 35–70.
Runge J. The Congo River, Central Africa. In: Gupta A, editor. Large rivers: geomorphology and management. Hoboken: Wiley; 2007. p. 293–309.
Roberts TR, Stewart DJ. An ecological and systematic survey of fishes in the rapids of the Lower Zaïre or Congo River. Bull Mus Comp Zool. 1976;147:239–317.
Brummett R, Stiassny M, Harrison I. Background. In: Brooks EGE, Allen DJ, Darwall WRT, editors. The status and distribution of freshwater biodiversity in Central Africa. Gland: IUCN; 2011.
Stiassny MLJ, Brummett RE, Harrison IJ, Monsembula R, Mamonekene V. The status and distribution of freshwater fishes in central Africa. In: Brooks EGE, Allen DJ, Darwall WRT, editors. The status and distribution of freshwater biodiversity in Central Africa. Gland: IUCN; 2011.
Poll M. Poissons recueillis au Katanga par H.J. Bredo. Bull Mus R Hist Nat Belg. 1948;24(21).
Damas H, Magis N, Nassogne A. Contribution à l’étude hydrobiologique des lacs Mwadingusha, Koni & N’zilo. Liege: Université de Liège/Fondation de l’université de Liège pour les recherches scientifiques au Congo et au Ruanda-Urundi; 1959.
Magis N. Nouvelle contribution à l’étude hydrobiologique des lacs de Mwadingusha, Koni et N’zilo. Liege: Université de Liège/Fondation de l’université de Liège pour les recherches scientifiques au Congo et au Ruanda-Urundi; 1961.
Wilmet J. La répartition de la population dans la dépression des rivières Mufuvya et Lufira (Haut-Katanga). Acad Sci Outre-Mer. 1963;14:2.
Doumenge C, Palla F, Scholte P, Hiol Hiol F, Larzillière A. Aires protégées d’Afrique centrale−État 2015. Kinshasa: OFAC; 2015.
Squadrone S, Burioli E, Monaco G, Koya MK, Prearo M, Gennero S, et al. Human exposure to metals due to consumption of fish from an artificial lake basin close to an active mining area in Katanga (D.R. Congo). Sci Total Environ. 2016;568:679–84.
Louette M, Hasson M. Rediscovery of the Lake Lufira Weaver Ploceus ruweti. Bull ABC. 2009;16:168–73.
Craig AJFK, Hasson M, Jordaens K, Breman F, Louette M. Range extension of the Lufira Masked Weaver Ploceus ruweti, endemic to Katanga province, Democratic Republic of Congo. Ostrich. 2011;82:77–8.
Ilunga MK, Abwe E, Decru E, Manda AC, Vreven E. Description of a new small-sized Synodontis species (Siluriformes: Mochokidae) that is important for local subsistence fisheries in the middle Lufira (upper Congo River, DR Congo). J Fish Biol. 2019;96:1–18.
Mulelenu CM, Manda BK, Decru E, Manda AC, The VE, Myers C. (Osteoglossiformes: Mormyridae) of the Lufira basin (Upper Lualaba: DR Congo): a generic reassignment and the description of a new species. J Fish Biol. 1960;2020:1–19.
Fonseca VG, Carvalho GR, Sung W, Johnson HF, Power DM, Neill SP, et al. Second-generation environmental sequencing unmasks marine metazoan biodiversity. Nat Commun. 2010;1:98.
Vanhove MPM, Tessens B, Schoelinck C, Jondelius U, Littlewood DTJ, Artois T, et al. Problematic barcoding in flatworms: a case-study on monogeneans and rhabdocoels (Platyhelminthes). ZooKeys. 2013;365:355–79.
Whittington ID. Diversity “down under”: monogeneans in the antipodes (Australia) with a prediction of monogenean biodiversity worldwide. Int J Parasitol. 1998;28:1481–93.
Vanhove MPM, Snoeks J, Volckaert FAM, Huyse T. First description of monogenean parasites in Lake Tanganyika: the cichlid Simochromis diagramma (Teleostei, Cichlidae) harbours a high diversity of Gyrodactylus species (Platyhelminthes, Monogenea). Parasitology. 2011;138:364–80 (erratum in 138:403).
Pariselle A, Morand S, Deveney MR, Pouyaud L. Parasite species richness of closely related hosts: historical scenario and “genetic” hypothesis. In: Combes C, Jourdan J, editors. Hommage à Louis Euzet—taxonomie, écologie et évolution des métazoaires parasites (Taxonomy, ecology and evolution of metazoan parasites). Perpignan: Les Presses Universitaires de Perpignan; 2003. p. 147 66.
Thurston JP. The larva of Oculotrema hippopotami (Monogenea: Polystomatidae). J Zool. 1968;154:475–80.
Thurston JP. The frequency distribution of Oculotrema hippopotami (Monogenea: Polystomatidae) on Hippopotamus amphibius. J Zool. 1968;154:481–5.
Silan P, Langlais M, Latu G. Dynamique des populations de monogènes, ectoparasites de téléostéens: Stratégies démographiques et implications mathématiques. Ecologie. 1999;30:1.
Silan P, Caltran H, Latu G. Ecologie et dynamique des populations de monogènes, ectoparasites de téléostéens marins: approche et contribution montpelliéraines. In: Combes C, Jourdane J, editors. Hommage à Louis Euzet—taxonomie, écologie et évolution des métazoaires parasites (Taxonomy, ecology and evolution of metazoan parasites). Volume 2. Perpignan: PUP; 2003. p. 212–35.
Whittington ID, Cribb BW, Hamwood TE, Halliday JA. Host-specificity of monogenean (platyhelminth) parasites: a role for anterior adhesive areas? Int J Parasitol. 2000;30:305–20.
Öztürk T, Özer A. Monogenean fish parasites, their host preferences and seasonal distributions in the Lower Kizilirmak Delta (Turkey). Turk J Fish Aquat Sci. 2014;14:367–78.
Shinn AP, Hansen H, Olstad K, Bachmann L, Bakke A. The use of morphometric characters to discriminate specimens of laboratory-reared and wild populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol. 2004;51:239–52.
Paladini G, Longshaw M, Gustinelli A, Shinn AP. Parasitic diseases in aquaculture: their biology, diagnosis and control. In: Austin B, Newaj-Fyzul A, editors. Diagnosis and control of diseases of fish and shellfish. Hoboken: Wiley; 2017. p. 37–107.
Hoai TD. Reproductive strategies of parasitic flatworms (Platyhelminthes, Monogenea): the impact on parasite management in aquaculture. Aquacult Int. 2020;28:421–47. https://doi.org/10.1007/s10499-019-00471-6.
Bakke TA, Harris PD, Cable J. Host specificity dynamics: observations on gyrodactylid monogeneans. Int J Parasitol. 2002;32:281–308.
Pugachev ON, Gerasev PI, Gussev AV, Ergens R, Khotenowsky I. Guide to Monogenoidea of freshwater fish of Palaearctic and Amur regions. 1st ed. Milan: Ledizioni; 2010.
Bagge AM. Factors affecting the development and structure of monogenean communities on Cyprinid fish. PhD thesis. Jyväskylä: University of Jyväskylä; 2005.
Llewellyn J. Amphibdellid (monogenean) parasites of electric rays (Torpedinidae). J Mar Biol Assoc UK. 1960;39:561–89.
Euzet L, Combes C. The selection of habitats among the Monogenea. Int J Parasitol. 1998;28:1645–52.
Jarkovský J, Morand S, Šimková A, Gelnar M. Reproductive barriers between congeneric monogenean parasites (Dactylogyrus: Monogenea): attachment apparatus morphology or copulatory organ incompatibility? Parasitol Res. 2004;92:95–105.
Šimková A, Verneau O, Gelnar M, Morand S. Specificity and specialization of congeneric monogeneans parasitizing cyprinid fish. Evolution. 2006;60:1023–37.
Řehulková E, Mendlova M, Šimková A. Two new species of Cichlidogyrus (Monogenea: Dactylogyridae) parasitizing the gills of African cichlid fishes (Perciformes) from Senegal: morphometric and molecular characterization. Parasitol Res. 2013;112:1399–410.
Mendlová M, Šimková A. Evolution of host specificity in monogeneans parasitizing African cichlid fish. Parasit Vectors. 2014;7:69.
Pariselle A, Euzet L. Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema. 2009;31:849–98.
Řehulková E, Seifertová M, Přikrylová I, Francová K. Monogenea. In: Scholz T, Vanhove MPM, Smit N, Jayasundera Z, Gelnar M, editors. A guide to the parasites of african freshwater fishes, vol. 18. ABC taxa. Brussels: Royal Belgian Institute of Natural Sciences; 2018. p. 185–243.
Cruz-Laufer AJ, Artois T, Smeets K, Pariselle A, Vanhove MPM. The cichlid–Cichlidogyrus network: a blueprint for a model system of parasite evolution. Hydrobiologia. 2021;848:3847–63.
Kabata Z. Parasites and diseases of fish cultured in the tropics. London: Taylor & Francis; 19848.
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z. 2006;15:259–63.
Katemo MB. Evaluation de la contamination de la chaine trophique par les métaux lourds dans le bassin de la Lufira supérieure (Katanga/RD Congo). Lubumbashi: DEA en Biologie Végétale et Environnement; 2009.
SNC-LAVALIN International. Etude sur la restauration des mines de cuivre et de cobalt en République Démocratique du Congo, rapport préliminaire M-6708 (603082). Montreal: NC-LAVALIN International; 2003.
Abwe E. The fish fauna of the Kundelungu National Park (DR Congo): diversity and conservation. PhD thesis. Leuven: Faculty of Science, KU Leuven; 2022.
Lamboj A. The cichlid fishes of Western Africa. Bornheim: Birgit Schmettkamp Verlag; 2004
Goortz A, Margis N, Wilmet J. Les aspects biologiques, humains et économiques de la pêche dans le lac de barrage de la Lufira. Liege: Université de Liège/Fondation de l’université de Liège pour les recherches scientifiques au Congo et au Ruanda-Urundi; 1961.
Olivier PAS, Luus-Powell WJ, Saayman JE. Report on some monogenean and clinostomid infestations of freshwater fish and waterbird hosts in Middle Letaba Dam, Limpopo Province, South Africa. Onderstepoort J Vet Res. 2009;76:187–99.
Nack J, Bitja Nyom AR, Pariselle A, Bilong Bilong CF. New evidence of a lateral transfer of monogenean parasite between distant fish hosts in Lake Ossa, South Cameroon: the case of Quadriacanthus euzeti n. sp. J Helminthol. 2015;90:455–9.
García-Vásquez A, Hansen H, Shinn AP. A revised description of Gyrodactylus cichlidarum Paperna, 1968 (Gyrodactylidae) from the Nile tilapia, Oreochromis niloticus niloticus (Cichlidae), and its synonymy with G. niloticus Cone, Arthur et Bondad-Reantaso, 1995. Folia Parasitol. 2007;54:129–40.
García-Vásquez A, Hansen H, Christison KW, Bron JE, Shinn AP. Description of three new species of Gyrodactylus von Nordmann, 1832 (Monogenea) parasitizing Oreochromis niloticus niloticus (L.) and O. mossambicus (Peters) (Cichlidae). Acta Parasitol. 2011;56:20–33.
Přikrylová I, Matějusová I, Musilová N, Gelnar M. Gyrodactylus species (Monogenea: Gyrodactylidae) on the cichlid fishes of Senegal, with the description of Gyrodactylus ergensi n. sp. from Mango tilapia, Sarotherodon galilaeus L. (Teleostei: Cichilidae). Parasitol Res. 2009;106:1–6.
Přikrylová I, Blazek R, Vanhove MPM. An overview of the Gyrodactylus (Monogenea: Gyrodactylidae) species parasitizing African catfishes, and their morphological and molecular diversity. Parasitol Res. 2012;110:1185–200.
Gillardin C, Vanhove MPM, Pariselle A, Huyse T, Volckaert FAM. Ancyrocephalidae (Monogenea) of Lake Tanganyika: II: description of the first Cichlidogyrus spp. parasites from Tropheini fish hosts (Teleostei, Cichlidae). Parasitol Res. 2012;110:305–13.
Muterezi Bukinga F, Vanhove MPM, Van Steenberge M, Pariselle A. Ancyrocephalidae (Monogenea) of Lake Tanganyika: III: Cichlidogyrus infecting the world’s biggest cichlid and the non-endemic tribes Haplochromini, Oreochromini and Tylochromini (Teleostei, Cichlidae). Parasitol Res. 2012;111:2049–61.
Pariselle A, Euzet L. Scutogyrus gen. n. (Monogenea: Ancyrocephalidae) for Cichlidogyrus longicornis minus Dossou, 1982, C. l. longicornis, and C. l. gravivaginus Paperna and Thurston, 1969, with description of three new species parasitic on African Cichlids. J Helminthol Soc Wash. 1995;62:157–73.
Fannes W, Vanhove MPM, Huyse T. Redescription of Cichlidogyrus tiberianus Paperna, 1960 and C. dossoui Douëllou, 1993 (Monogenea: Ancyrocephalidae), with special reference to the male copulatory organ. Syst Parasitol. 2017;94:133–44.
Shannon CE. The mathematical theory of communication. In: Shannon CE, Weaver W, editors. The mathematical theory of communication. Tenth printing. Urbana: The University of Illinous Press. 1964; p. 29–115.
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA. The use of ecological terms in parasitology (report of an ad hoc committee of the American society of parasitologists). J Parasitol. 1982;68:131–3.
Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–83.
Hammer Ø. PAST. PAleontological STatistics Version 3.12. Reference manual. Oslo: Natural History Museum University of Oslo; 2016.
Jorissen MWP, Huyse T, Pariselle A, Wamuini Lunkayilakio S, Muterezi Bukinga F, Chocha Manda A, et al. Historical museum collections help detect parasite species jumps after tilapia introductions in the Congo basin. Biol Invasions. 2020;22:2825–44. https://doi.org/10.1007/s10530-020-02288-4.
Thieme ML, Abell R, Stiassny MLJ, Skelton P, Lehner B, Teugels GG, et al. Freshwater ecoregions of Africa and Madagascar. A conservation assessment. Washington DC: Island Press; 2005.
Vanhove MPM, Volckaert FAM, Pariselle A. Ancyrocephalidae (Monogenea) of Lake Tanganyika: I: four new species of Cichlidogyrus from Ophthalmotilapia ventralis (Teleostei: Cichlidae), the first record of this parasite family in the basin. Zoologia-Curitiba. 2011;28:253–63.
Jorissen MWP, Pariselle A, Huyse T, Vreven EJ, Snoeks J, Volckaert FAM, et al. Diversity and host specificity of monogenean gill parasites (Platyhelminthes) of cichlid fishes in the Bangweulu-Mweru ecoregion. J Helminthol. 2018;92:417–37.
Jorissen MWP, Pariselle A, Huyse T, Vreven EJ, Snoeks J, Decru E, et al. Six new dactylogyrid species (Platyhelminthes, Monogenea) from the gills of cichlids (Teleostei, Cichliformes) from the Lower Congo basin. Parasite. 2018;25:64.
Geraerts M, Muterezi Bukinga F, Vanhove MPM, Pariselle A, Chocha Manda A, Vreven E, et al. Six new species of Cichlidogyrus Paperna, 1960 (Platyhelminthes: Monogenea) from the gills of cichlids (Teleostei: Cichliformes) from the Lomami River basin (DRC: Middle Congo). Parasit Vectors. 2020;13:187.
Douëllou L. Monogeneans of the genus Cichlidogyrus Paperna, 1960 (Dactylogyridae: Ancyrocephalinae) from cichlid fishes of Lake Kariba (Zimbabwe) with descriptions of five new species. Syst Parasitol. 1993;25:159–85.
Bilong Bilong CF. Enterogyrus malmbergi n. sp. (Monogenea-Ancyrocephalidae) parasite de l’estomac du Cichlidae Tilapia nilotica Linné, 1757 au Sud-Cameroun. Ann Fac Sci Yaoundé Biol-Biochim. 1988;5:51–8.
Akoll P, Fioravanti ML, Konecny R, Schiemet F. Infection dynamics of Cichlidogyrus tilapiae and C. sclerosus (Monogenea, Ancyrocephalinae) in Nile tilapia (Oreochromis niloticus L.) from Uganda. J Helminthol. 2012;86:302–10.
Igeh PC, Gilbert BM, Avenant-Oldewage A. Seasonal variance in water quality, trace metals and infection variables of Cichlidogyrus philander Douëllou, 1993 (Monogenea, Ancyrocephalidae) infecting the gills of Pseudocrenilabrus philander (Weber, 1897) in the Padda Dam, South Africa. Afr J Aquat Sci. 2020;46:1–12.
Acknowledgements
VLIR-UOS is thanked for supporting this study through the South Initiative “Renforcement des capacités locales pour une meilleure évaluation biologique des impacts miniers au Katanga (RD Congo) sur les poissons et leurs milieux aquatiques”. Each member of the local team of the University of Lubumbashi (BEZHU), namely C. Kalombo Kabalika, P. Kiwele Mutambala, B. Katemo Manda, M. Kasongo Ilunga Kayaba and C. Mukweze Mulelenu, is thanked for the help in fish sampling. Members of the international team are thanked; namely I. Přikrylová (University of Limpopo) for her contribution in confirmation of parasite identification; N. Kmentová (Hasselt University) for help with imaging and F.A.M. Volckaert (KU Leuven) and L. Janssens de Bisthoven (Royal Belgian Institute of Natural Sciences) for hosting G.K. Kasembele in their teams during his respective research visits to Belgium. Finally, we thank the Institut Congolais pour la Conservation de la Nature (ICCN) for facilitating and authorizing sampling (Attestations de Recherche 205/2016; 006/2017).
Funding
This research was carried out with the funding support of a VLIR-UOS South Initiative (ZRDC2014MP084). At the time of conducting this investigation, MPM Vanhove was supported by the Belgian Directorate-General for Development Cooperation and Humanitarian Aid (CEBioS program: Capacities for Biodiversity and Sustainable Development), the Belgian Federal Science Policy Office (BR/132/PI/TILAPIA), the Research Foundation—Flanders (FWO-Vlaanderen) (K220314N) and currently by the Special Research Fund of Hasselt University (BOF20TT06). The South African team was supported by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa (Grant No. 101054).
Author information
Authors and Affiliations
Contributions
ACM, JS and MPMV designed and supervised this study. GKK carried out the study (sampled fishes, collected parasites, performed morphological identification of parasites, analysed data and wrote the paper). ACM, EA, EJWMNV contributed to sampling, the collection and identification of fish. FMB, WJLP, WJS, JRS and MPMV helped with the collection and preparation of the gill parasites. AP, MWPJ, MPMV helped with the morphological identification of parasites species. MPMV helped with the writing of the paper, analysis of the data, interpretation and discussion of results and provided scientific background in the field of monogenean research. TH and all the authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Fishes were collected using gillnets or were bought from fishermen. In the absence of relevant animal welfare regulations in the DRC, we used the guidelines and authorization in accordance with the Unité de Recherche en Biodiversité et Exploitation durable des Zones Humides (BEZHU) of the Université de Lubumbashi.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Voucher specimens of monogenean parasites available in the invertebrate collection of the Royal Museum of Central Africa, Tervuren, Belgium under accession numbers RMCA_VERMES_43743-44345.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kasembele, G.K., Manda, A.C., Abwe, E. et al. First record of monogenean fish parasites in the Upper Lufira River Basin (Democratic Republic of Congo): dactylogyrids and gyrodactylids infesting Oreochromis mweruensis, Coptodon rendalli and Serranochromis macrocephalus (Teleostei: Cichlidae). Parasites Vectors 16, 48 (2023). https://doi.org/10.1186/s13071-022-05637-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05637-5