Abstract
Background
Triatoma tibiamaculata is a species distributed in ten Brazilian states which has epidemiological importance as it has already been found infecting household areas. The taxonomy of this triatomine has been quite unstable: it was initially described as Eutriatoma tibiamaculata. Later, the species was transferred from the genus Eutriatoma to Triatoma. Although included in the genus Triatoma, the phylogenetic position of T. tibiamaculata in relation to other species of this genus has always been uncertain once this triatomine was grouped in all phylogenies with the genus Panstrongylus, rescuing T. tibiamaculata and P. megistus as sister species. Thus, we evaluated the generic status of T. tibiamaculata using phylogenetic and chromosomal analysis.
Methods
Chromosomal (karyotype) and phylogenetic (with mitochondrial and nuclear markers) analyses were performed to assess the relationship between T. tibiamaculata and Panstrongylus spp.
Results
The chromosomal and phylogenetic relationship of T. tibiamaculata and Panstrongylus spp. confirms the transfer of the species to Panstrongylus with the new combination: Panstrongylus tibiamaculatus.
Conclusions
Based on chromosomal and phylogenetic characteristics, we state that P. tibiamaculatus comb. nov. belongs to the genus Panstrongylus and that the morphological features shared with Triatoma spp. represent homoplasies.
Graphical Abstract

Similar content being viewed by others
Background
The members of the subfamily Triatominae (Hemiptera, Reduviidae) are hematophagous insects of great epidemiological importance as they act as vectors of the protozoan Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida, Trypanosomatidae), the etiological agent of Chagas disease [1]. Chagas disease is a neglected disease that affects about 8 million people and puts another approximately 25 million at risk of infection [1]. The main way to minimize the incidence of new cases is based on the control of vector populations [1], the studies related to these insects being of extreme importance for public health once they can generate results to help vector control programs in the prophylaxis of Chagas disease.
Systematics has contributed to the correct identification of triatomines and consequently to the surveillance activities of vector control programs [2, 3]. However, in the face of evolutionary events (cryptic speciation and phenotypic plasticity [4]) and associated taxonomic problems, in most cases, with classical taxonomy [5, 6] (based on the morphological characterization of the species [3, 6]), > 190 synonymizations have occurred in the Triatominae subfamily [7]. This highlights the importance of integrative taxonomy for the description of new species [6], as performed by Dorn et al. [8], Lima-Cordón et al. [9] and Alevi et al. [10].
Currently, 157 species are described in the subfamily Triatominae (with 154 extant species and three fossil species), grouped into 18 genera and 5 tribes [6,7,8,9,10,11,12]. In Brazil, > 60 species are distributed among the following genera: Alberprosenia Martínez & Carcavallo, 1977, Belminus Stål, 1859, Microtriatoma Prosen & Martínez, 1952, Parabelminus Lent, 1943, Cavernicola Barber, 1937, Psammolestes Bergroth, 1911, Rhodnius Stål, 1859, Eratyrus Stål, 1859, Panstrongylus Berg, 1879, and Triatoma Laporte, 1832 [7]. Rhodnius, Triatoma and Panstrongylus are the most important from an epidemiological point of view [13].
The genera Rhodnius and Triatoma have been considered paraphyletic [13]. Panstrongylus was initially considered monophyletic based on morphological data [2]; however, Marcilla et al. [14], using the internal transcribed spacer 2 (ITS-2) nuclear marker, suggested that Panstrongylus was polyphyletic. Later, several phylogenetic analyses indicated this genus is paraphyletic once species of Panstrongylus are grouped with species of Nesotriatoma Usinger, 1944, and T. tibiamaculata (Pinto, 1926) [13, 15,16,17].
Triatoma tibiamaculata is distributed in ten Brazilian states [7] and has epidemiological importance as it has already been found infecting household areas [18] and colonizing peridomiciliar environments [19]. The taxonomy of this triatomine was quite unstable because Pinto [20], based only on morphological characteristics, initially described this species in the genus Eutriatoma Pinto, 1926, highlighting that it had intermediate characteristics between Rhodnius and Triatoma. Later, the species was transferred from the genus Eutriatoma to Triatoma [21, 22].
Although grouped in Triatoma, the phylogenetic position of T. tibiamaculata in relation to the other species of this genus has always been uncertain once this triatomine was grouped in all phylogenies with the genus Panstrongylus [13, 15,16,17], rescuing T. tibiamaculata and P. megistus (Burmeister, 1835) as sister species [13, 16, 17]. Based on this, Gardim et al. [16] suggested a review of the generic status of T. tibiamaculata, highlighting that this species possibly belongs to Panstrongylus.
Thus, we evaluated the generic status of T. tibiamaculata through phylogenetic and chromosomal analysis.
Methods
Type of material examined
Eutriatoma tibiamaculata Pinto, 1926, syntype. Determined: Pinto, C. 1926, Collected: Travassos, L. 16.XII.1926., Location: Angra dos Reis, Rio de Janeiro, Brazil, deposited in the Entomological Collection of the Instituto Oswaldo Cruz (CEIOC), Rio de Janeiro, Brazil.
Molecular analysis
For molecular analysis, the genomic DNA of five specimens of P. lignarius (Walker, 1873) (from Porto Velho, Rondônia, Brazil), P. lutzi (Neiva & Pinto, 1923) (from Irecê, Bahia, Brazil) and T. tibiamaculata (from Mogi Guaçu, São Paulo, Brazil) was extracted from gonads using the DNeasy Blood and Tissue kit (QIAGEN®). Amplification of the fragments was performed by polymerase chain reaction (PCR), using primers targeting cytochrome b (cytb) and internal transcribed spacer 1 (ITS-1), as described in the literature [23, 24]. The amplified PCR products were visualized by electrophoresis in 1% agarose gel and later purified using the GFX PCR DNA & Gel Band Kit (GE Healthcare and Life Technology®) according to the manufacturer's instructions. Subsequently, this material was submitted for direct sequencing on an ABI 3730 DNA Analyzer (Life Technologies) sequencer from the Research Center on the Human Genome and Stem Cells, University of São Paulo (USP), Brazil.
The gene sequences obtained were grouped with sequences of several molecular markers for 17 taxa available in GenBank (Table 1), which were aligned in the MEGA X program [25] using the Muscle method [26]. For the alignment of ITS-1 and ITS-2, the sequences of the brasiliensis subcomplex species are only available concatenated (Table 1); thus, the sequences for the other species had been previously concatenated and then aligned with species of the Brasiliensis subcomplex (representatives of the Triatoma genus of the Brasiliensis subcomplex were used in the phylogeny because T. tibiamaculata was initially considered in this subcomplex based on morphological data and geographic distribution [16]).
The alignments were concatenated by name using the Seaview4 program [27], resulting in an alignment with 7993 nucleotides, which was converted in Mesquite 3.2 [28]. Data were partitioned for each molecular marker, and the best model for each one (lowest Akaike information criterion value) was determined in the jModeltest 2 program [29] (Table 2). For the phylogenetic reconstruction by Bayesian inference, the data were submitted to MrBayes 3.2 [30] in an analysis with 100 million generations. Trees were sampled every 1000 generations in two independent runs (each with four Markov chains) and burn-in adjusted to 25%. Tracer v. 1.7 [31] was used to verify the stabilization (ESS values > 200) of the sampled trees, and the generated phylogenetic tree was visualized and edited in the FigTree v.1.4.4 program [32].
Cytogenetic analysis
Triatoma tibiamaculata (from Mogi Guaçu, São Paulo, Brazil), P. megistus (from Araraquara, São Paulo, Brazil), P. lignarius (from Porto Velho, Rondônia, Brazil) and P. lutzi (from Irecê, Bahia, Brazil) males were dissected; the testes were removed and stored in methanol:acetic acid solution (3:1). Slides were prepared by the cell crushing technique (as described by Alevi et al. [33]), and cytogenetic analyses were applied to confirm the karyotype of the species using the lacto-acetic orcein technique [33, 34]. The slides were examined using Jenaval light microscopy (Zeiss) coupled to a digital camera and the Axio Vision LE 4.8 image analyzer system, with a 1000-fold increase.
Results
Phylogenetic analysis
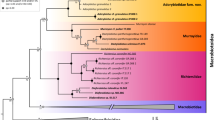
Phylogenetic reconstruction with cytb and ITS-1 combined with several mitochondrial and nuclear DNA sequences was deposited in GenBank (16S, 18S, 28S, COI, COII, ITS-2 and 12S) rescued T. tibiamaculata with Panstrongylus spp. (Fig. 1) in a clade distinct from Triatoma spp., demonstrating that T. tibiamaculata is a species of Panstrongylus.
Chromosomal analysis
The confirmation of the karyotype of the species T. tibiamaculata, P. megistus, P. lignarius and P. lutzi, when combined with literature data [35,36,37,38,39,40], demonstrates that, except for P. megistus and P. lutzi, T. tibiamaculata and all other species of Panstrongylus have the same diploid chromosome set (2n = 23 chromosomes) (Table 3). In addition, based on FISH data in the literature, T. tibiamaculata and all species of Panstrongylus present markings in a pair of autosomes [41,42,43] (Table 3), confirming that T. tibiamaculata is a species of Panstrongylus.
Generic transfer
Kingdom Animalia Linnaeus, 1758, Phylum Arthropoda von Siebold, 1848, Class Insecta Linnaeus, 1758, Order Hemiptera Linnaeus, 1758, Suborder Heteroptera Latreille, 1810, Family Reduviidae Latreille, 1807, Subfamily Triatominae Jeannel, 1919, Tribe Triatomini Jeannel, 1919, Genus Panstrongylus Berg, 1879, Species Panstrongylus tibiamaculatus (Pinto, 1926) comb. nov. (Fig. 2).
Eutriatoma tibiamaculata Pinto, 1926 (p. 134, Figs. C–E [20]).
Triatoma (Eutriatoma) tibia-maculata (Lima, 1940) (p. 199, Fig. 383 [22]).
Triatoma tibiamaculata (Pinto, 1926) (p. 902, Fig. 2 [21]).
Panstrongylus: the genus name comes from the Greek “pan” means whole, and “strongylus” means round, plump, burly, a reference to the insect’s robust, rounded body [44].
tibiamaculatus: the specific epithet comes from the Latin “tibia” and “maculatus,” and the combination means stained tibias, a reference to the insect's tibiae being totally "stained" in orange [44].
The change of the specific epithet “tibiamaculata” to “tibiamaculatus” was carried out based on Art. 31.2 of the International Code of Zoological Nomenclature (ICZN) [45] since “Panstrongylus” is masculine—because (i) the ending '-us' usually indicates masculine words; (ii) the ICZN requires that the specific epithet be of the same grammatical gender as the generic epithet, for example, the species of the genus Panstrongylus are all male, as P. geniculatus (Latreille, 1811), P. lignarius and P. rufotuberculatus (Champion, 1899), and so is the genus; (iii) the Portuguese versions of Latin words retain the grammatical gender: if the term “strongyl” is masculine, so is Panstrongylus [46]—and “tibiamaculatus” is a latinized adjective.
Discussion
The chromosomal and phylogenetic relationship of Panstrongylus tibiamaculatus comb. nov. and Panstrongylus spp. confirms the change of generic status to this species. Thus, the genus Panstrongylus includes 16 species now, namely, P. chinai (Del Ponte, 1929), P. diasi Pinto & Lent, 1946, P. geniculatus, P. guentheri Berg, 1879, P. hispaniolae Poinar, 2013 (fossil species), P. howardi (Neiva, 1911), P. humeralis (Usinger, 1939), P. lenti Galvão & Palma, 1968, P. lignarius, P. lutzi, P. martinezorum Ayala, 2009, P. megistus, P. mitarakaensis Bérenger & Blanchet, 2007, P. rufotuberculatus, P. tibiamaculatus comb. nov. and P. tupynambai Lent, 1942 [3].
As already mentioned, since 2002, phylogenetic studies have shown the relationship between P. tibiamaculatus comb. nov. and Panstrongylus spp. (more specifically, P. megistus) [13, 15,16,17] demonstrating that these taxa share common ancestry. Justi et al. [17], based on phylogenetic reconstruction associated with geological events, suggested that the ancestral population that gave rise to P. tibiamaculatus comb. nov. and P. megistus was distributed along the former connection between the Amazon Forest and the Atlantic Forest and, later, with the climate changes caused by the Andean uplift that resulted in the disappearance of this connection, a vicariance event that resulted in the speciation of P. tibiamaculatus comb. nov. and P. megistus.
Considering the phylogenetic relationship between P. tibiamaculatus comb. nov. and Panstrongylus spp. (more specifically, P. megistus) [13, 15,16,17], Monteiro et al. [5] highlight that these species probably descend from a common ancestor that colonized the moist Atlantic forests of eastern Brazil south of parallel 7S. The authors signaled that P. megistus is widespread across the Atlantic forests but also occurs in gallery forests throughout the drier Cerrado and stretches into the semiarid Caatinga, the Chaco and parts of the Pantanal and Uruguayan savannahs. On the other hand, Monteiro et al. [5] pointed out that P. tibiamaculatus comb. nov. is associated with palms and bromeliads along a narrow strip of coastal Brazil including the Pernambuco, Bahia and Serra do Mar coastal moist forests.
Gardim et al. [16] evaluated ecoepidemiological issues related to P. tibiamaculatus comb. nov. and P. megistus. The authors also emphasized that the close relationship between P. megistus and P. tibiamaculatus comb. nov. may help to explain the recent finding of the latter species invading human domiciles in downtown Salvador, Bahia State, Brazil.
Justi et al. [17] grouped the species of Panstrongylus into two groups: geniculatus and megistus. However, more recently Monteiro et al. [5] considered four groups: P. rufotuberculatus, P. lignarius, P. geniculatus and P. megistus. Our results also retrieved four groups, namely, P. rufotuberculatus (composed of P. chinai, P. rufotuberculatus and P. howardi), P. lignarius (composed of P. lignarius), P. geniculatus (composed of P. geniculatus, P. lutzi and P. tupynambai) and P. megistus (composed of P. megistus and P. tibiamaculatus comb. nov.).
Although P. tibiamaculatus comb. nov. has morphological characteristics that approximate it to Triatoma spp. (which led to the misclassification of the species in this genus), the most prominent morphological feature that distinguishes the genus Panstrongylus from other triatomines is the short head, with antennae close to the eyes [3]. The geometric morphometric of head, for example, is a tool that discriminated Panstrongylus and Triatoma based on the position of the antennal insertion relative to the eyes [47]. Justi et al. [12] highlighted that the morphological divergences observed between P. tibiamaculatus comb. nov. and the other Panstrongylus may be due to morphological convergence with Triatoma spp., because variations in the size of the eyes of Panstrongylus spp. have already been reported in the literature [48], and these variations influence the distances between the antennas and the eyes.
Some morphological similarities between P. tibiamaculatus comb. nov. and the species in the brasiliensis subcomplex led Schofield and Galvão [49] to group these species in this complex. However, based on chromosomal divergences, Alevi et al. [33] proposed the exclusion of the species from this complex. From a karyosystematic point of view, while P. tibiamaculatus comb. nov. has 2n = 23 chromosomes (which approximates it to most species of Panstrongylus), all South American Triatoma species have 2n = 22 (species of the Brasiliensis, Infestans, Maculata, Pseudomaculata, Rubrovaria and Sordida subcomplexes) or 24 chromosomes (Vitticeps subcomplex species) [50]. Based on the ancestral karyotype of Triatominae (2n = 22) [51], Alevi et al. [52] suggested that during the divergence of the common ancestor of Panstrongylus there was a fission in sex chromosome X, which resulted in the karyotype 2n = 23 (karyotype shared by P. chinai, P. geniculatus, P. howardi, P. lignarius, P. rufotuberculatus, P. tibiamaculatus comb. nov. and P. tupynambai). However, the authors suggested that during the karyotypic evolution of Panstrongylus, two possible punctual events occurred: fusion in a pair of autosomes in P. megistus and fission in the sex chromosome X in P. lutzi. The karyotypes of P. megistus and P. lutzi (2n = 21 and 2n = 24, respectively) were observed only in five species of Triatoma (T. nitida Usinger, 1939, T. eratyrusiformis Del Ponte, 1929, T. melanocephala Neiva & Pinto, 1923, T. vitticeps (Stål, 1859) and T. breyeri Del Ponte, 1929 [52]), suggesting that these evolutionary events occurred independently during the chromosomal evolution of triatomines.
In addition, P. tibiamaculatus comb. nov. and all other Panstrongylus species (regardless of the number of chromosomes) have 45S rDNA probes restricted to a pair of autosomes [41,42,43]. Pita et al. [53] suggest that the chromosomal position of 45S rDNA is variable in Triatominae, although it is conserved among closely related species (such as P. tibiamaculatus comb. nov. and Panstrongylus spp.). In addition to the genetic relationships observed between P. tibiamaculatus comb. nov. and Panstrongylus spp., morphological similarities between fifth-instar female nymphs of P. megistus and P. tibiamaculatus comb. nov. (more specifically in the structures of the eighth ventral segment as well as between setae) were observed [54]. Furthermore, Nascimento et al. [55] also observed similarities between spermathecae morphology from P. lignarius, P. megistus and P. tibiamaculatus comb. nov., and Mello et al. [56] recorded a relationship between exocorial cells in eggs of P. tibiamaculatus comb. nov. with Panstrongylus.
Conclusion
Thus, based on chromosomal and phylogenetic characteristics, we state that P. tibiamaculatus comb. nov. belongs to the genus Panstrongylus and that the morphological features shared with Triatoma spp. represent homoplasies.
Availability of data and materials
GenBank accession numbers of sequences generated in this study: P. tibiamaculatus ITS-1 (ON262109), P. lutzi ITS-1 (ON262110) and P. lignarius cytb (ON262111).
References
World Health Organization. Chagas disease (American trypanosomiasis). http://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). 2022. Accessed 04 May 2022.
Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera: Reduviidae) and their significance as vector of Chagas disease. Bull Am Mus Nat Hist. 1979;163:123–520.
Galvão C. Taxonomia dos vetores da doença de Chagas: da forma à molécula, quase três séculos de história. In: Oliveira J, Alevi KCC, Camargo LMA, Meneguetti DUO, editors. Atualidades em medicina tropical no Brasil: vetores. Rio Branco: Strictu Sensu Editora; 2020. p. 9–37.
Abad-Franch F, Pavan G, Jaramillo-O N, Palomeque S, Dale C, Chaverra D, et al. Rhodnius barretti, a new species of Triatominae (Hemiptera: Reduviidae) from western Amazonia. Mem Inst Osw Cruz. 2013;108:92–9.
Monteiro FA, Weirauch C, Felix F, Lazoski C, Abad-Franch F. Evolution, systematics, and biogeography of the Triatominae vectors of Chagas disease. Adv Parasitol. 2018;99:265–344.
Alevi KCC, de Oliveira J, Rocha DS, Galvão C. Trends in taxonomy of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae): from Linnaean to integrative taxonomy. Pathogens. 2021;10:1627.
Galvão C, Carcavallo R, Rocha DS, Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1–36.
Dorn PL, Justi AS, Dale C, Stevens L, Galvão C, Cordon RL, et al. Description of Triatoma mopan sp. n. (Hemiptera, Reduviidae, Triatominae) from a cave in Belize. Zookeys. 2018;775:69–95.
Lima-Cordon RA, Monroy MC, Stevens L, Rodas A, Rodas GA, Dorni PL, et al. Description of Triatoma huehuetenanguensis sp. n., a potential Chagas disease vector (Hemiptera, Reduviidae, Triatominae). Zookeys. 2019;820:51–70.
Alevi KCC, Oliveira J, Garcia ACC, Cristal DC, Delgado LMG, Bittinelli IF, et al. Triatoma rosai sp. nov (Hemiptera, Triatominae): a new species of Argentinian Chagas disease vector described based on integrative taxonomy. Insects. 2020;11:830.
Zhao Y, Galvão C, Cai W. Rhodnius micki, a new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys. 2021;1012:71–93.
Dale C, Justi SA, Galvão C. Belminus santosmalletae (Hemiptera: Heteroptera: Reduviidae): new species from Panama, with an updated key for Belminus Stål, 1859 species. Insects. 2021;12:686.
Justi SA, Russo CAM, dos Mallet JR, Obara MT, Galvão C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasit Vect. 2014;7:149.
Marcilla A, Bargues MD, Abad-Franch F, Panzera F, Carcavallo RU, Noireau F, et al. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi. Infect Genet Evol. 2002;1:225–35.
Hypša V, Tietz D, Zrzavý J, Rego RO, Galvão C, Jurberg J. Phylogeny and biogeography of Triatominae (Hemiptera, Reduviidae): molecular evidence of a new world origin of the asiatic clade. Mol Phylogenet Evol. 2002;23:447–57.
Gardim S, Almeida CE, Takiya DM, Oliveira J, Araújo RF, Cicarelli RMB, et al. Multiple mitochondrial genes of some sylvatic Brazilian Triatoma: nonmonophyly of the T. brasiliensis subcomplex and the need for a generic revision in the Triatomini. Infect Genet Evol. 2014;23:74–9.
Justi SA, Galvão C, Schrago CG. Geological changes of the Americas and their influence on the diversification of the Neotropical kissing bugs (Hemiptera: Reduviidae: Triatominae). PLoS Negl Trop Dis. 2016;10:4.
Steindel M, Pacheco KL, Scholl D, Soares M, Moraes MH, Eger I, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60:25–32.
Santana KSO, Bavia ME, Ribeiro-Jr GJS, Santos CGS, Guimarães ICS, Silva MMN, et al. Presence of Triatoma tibiamaculata (Pinto) nymphs in peridomicilies, in Salvador. Bahia Rev Patol Trop. 2013;42:455–8.
Pinto C. Hypopygio dos Triatomídeos (Hemipteros-Heteropteros-Hematophagos) e do gênero Apiomerus. Bol Biol São Paulo. 1926;2:27–33.
Del Ponte E. Catálogo descriptivo de los géneros Triatoma Lap., Rhodnius Stål, e Eratyrus Stål. Rev Inst Bacteriol Depart Nac Hig. 1930;5:855–937.
Lima AC. Insetos do Brasil. 2° Tomo. Capítulo XXII. Hemípteros. Rio de Janeiro: Escola Nacional de Agronomia; 1940.
Monteiro FA, Perez R, Panzera F, Dujardin JP, Galvão C, Rocha D, et al. Mitochondrial DNA variation of Triatoma infestans populations and its implication on the specific status of T. melanosoma. Mem Inst Oswaldo Cruz. 1999;94:229–38.
Tartarotti E, Ceron CR. Ribosomal DNA ITS-1 intergenic spacer polymorphism in triatomines (Triatominae, Heteroptera). Biochem Genet. 2005;43:365–73.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Ac Res. 2014;32:1792–7.
Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–4.
Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis—version 3.2. 2017. http://www.mesquiteproject.org. Acessed 20 Oct 2021.
Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772.
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, et al. MRBAYES 3.2: efficient Bayesian phylogenetic inference and model selection across a large model space. Syst Biol. 2012;61:539–42.
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67:901–4.
Rambaut A. FigTree–tree figure drawing tool version v.1.4.4. Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, 2018. http://tree.bio.ed.ac.uk/software/figtree/. Acessed 20 Oct 2021.
Alevi KCC, Mendonça PP, Pereira NP, Rosa JA, Azeredo-Oliveira MTV. Karyotype of Triatoma melanocephala Neiva & Pinto (1923). Does this species fit in the Brasiliensis subcomplex? Infect Genet Evol. 2012;12:1652–3.
De Vaio ES, Grucci B, Castagnino AM, Franca ME, Martinez ME. Meiotic differences between three triatomine species (Hemiptera:Reduviidae). Genetica. 1985;67:185–91.
Crossa RP, Hernandez M, Caraccio MN, Rose V, Valente SA, Costa Valente V, et al. Chromosomal evolution trends of the genus Panstrongylus (Hemiptera, Reduviidae), vectors of Chagas disease. Infect Genet Evol. 2002;2:47–56.
Panzera F, Pérez R, Panzera Y, Ferrandis I, Ferreiro MJ, Calleros L. Cytogenetics and genome evolution in the subfamily Triatominae (Hemiptera, Reduviidae). Cytogenet Genome Res. 2010;128:77–87.
Santos SM, Pompolo SG, Gonçalves TCM, Freitas SPC, Rangel EF, Santos-Mallet JRS. New sex-determination system in the genus (Hemiptera: Reduviidae) revealed by chromosomal analysis of Panstrongylus lutzi. Parasit Vect. 2016;9:295.
Alevi KCC, Imperador HL, Fonseca EOL, Santos CGS, Azeredo-Oliveira MTV, Rosa JA, et al. Karyosystematic and karyotype evolution of Panstrongylus lutzi (Neiva & Pinto, 1923) (Hemiptera, Triatominae). Braz J Biol. 2017;78:180–2.
Schreiber G, Pellegrino J. Eteropicnosi di autosomi come possible meccanismo di speciazione. Sci Genet. 1950;3:215–26.
Panzera F, Scvortzoff E, Pérez R, Panzera Y, Hornos S, Cestau R, et al. Cytogenetics of Triatomines. In: Carcavallo RU, Galíndez-Girón I, Jurberg J, Lent H, editors., et al., Atlas of chagas disease vectors in the Americas. Rio de Janeiro: Editora Fiocruz; 1998. p. 621–64.
Panzera Y, Pita S, Ferreiro MJ, Ferrandis I, Lages C, Pérez R, et al. High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera). Cytogenet Genome Res. 2012;138:56–67.
Pita S, Lorite P, Cuadrado A, Panzera Y, Oliveira J, Alevi KCC, et al. High chromosomal mobility of ribosomal clusters in holocentric chromosomes of Triatominae, vectors of Chagas disease (Hemiptera-Reduviidae). Med Vet Entomol. 2022;36:66–80.
Panzera F, Pita S, Lorite, P. Chromosome structure and evolution of triatominae: a review. In: Guarneri AA, Lorenzo MG, editors. Triatominae: the biology of chagas disease vectors. 2021. doi: https://doi.org/10.1007/978-3-030-64548-9
Galvão C. Vetores da doença de Chagas no Brasil. Curitiba: Sociedade Brasileira de Zoologia; 2014.
The International Code of Zoological Nomenclature. 1999. https://www.iczn.org/. Accessed 30 Jan 2022.
Rogolon RG. A Pronúncia do Latim Científico. 2nd ed. Viçosa: Editora UFV; 2019.
Oliveira J, Marcet PL, Takiya DM, Mendonça VJ, Belintani T, Bargues MD, et al. Combined phylogenetic and morphometric information to delimitand unify the Triatoma brasiliensis species complex and the Brasiliensis subcomplex. Act Trop. 2017;170:140–8.
Patterson JS, Barbosa SE, Feliciangeli MD. On the genus Panstrongylus Berg 1879: evolution, ecology and epidemiological significance. Acta Trop. 2009;110:187–99.
Schofield CJ, Galvão C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009;110:88–100.
Reis Y, Alevi KCC. Revisão cariotípica dos vetores da doença de Chagas. In: Oliveira J, Alevi KCC, Camargo LMA, Meneguetti DUO, editores. Atualidades em Medicina Tropical na América do Sul: Vetores. Rio Branco: Strictu Sensu Editora; 2021. p. 70–79.
Ueshima N. Cytotaxonomy of the triatominae (Reduviidae: Hemiptera). Chromosoma. 1966;18:97–122.
Alevi KCC, Oliveira J, Rosa JA, Azeredo-Oliveira MTV. Karyotype evolution of Chagas disease vectors (Hemiptera, Triatominae). Am J Trop Med Hyg. 2018;99:87–9.
Pita S, Lorite P, Nattero J, Galvão C, Alevi KCC, Teves SC, et al. New arrangements on several species subcomplexes of Triatoma genus based on the chromosomal position of ribosomal genes (Hemiptera—Triatominae). Infect Genet Evol. 2016;43:225–31.
Rosa JA, Barata JMS, Barelli N. Spiracles of 5th instar nymphs in six species of Triatominae (Hemiptera, Reduviidae) using scanning electron microscopy. Mem Inst Oswaldo Cruz. 1992;87:301–2.
Nascimento JD, Ribeiro AR, Almeida LA, Oliveira J, Mendonça VJ, Cilense M, et al. Morphology of the spermathecae of twelve species of Triatominae (Heteroptera, Reduviidae) vectors of Chagas disease. Acta Trop. 2017;76:440–5.
Mello F, Jurberg J, Grazia J. Morphological study of the eggs and nymphs of Triatoma dimidiata (Latreille, 1811) observed by light and scanning electron microscopy (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 2009;104:1072–82.
Acknowledgements
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil)-Finance Code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for financial support.
Funding
The study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil)-Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
Author information
Authors and Affiliations
Contributions
ISB: Conceptualization, Methodology, Investigation, Writing—Original Draft Preparation and Writing—Review & Editing, JO: Conceptualization, Methodology, Investigation, Data Curation and Writing—Review & Editing, AR: Methodology, Investigation and Data Curation, FFM: Methodology, Investigation and Data Curation, YVR: Methodology, Investigation and Data Curation, ABBO: Methodology, Investigation and Data Curation, RDV: Methodology, Investigation and Data Curation, GM: Methodology, Investigation and Data Curation, AJCG: Methodology, Investigation and Data Curation, LPP: Methodology, Investigation and Data Curation, ISM: Methodology, Investigation and Data Curation, CG: Conceptualization, Writing—Review & Editing, and Funding acquisition, MTVAO: Conceptualization, Funding acquisition and Writing—Review & Editing, JAR: Conceptualization, Resources and Writing—Review & Editing, KCCA: Conceptualization, Methodology, Investigation, Writing—Original Draft Preparation and Writing—Review & Editing, Supervision, Project administration and Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bittinelli, I.F., de Oliveira, J., dos Reis, Y.V. et al. Do not judge a book by its cover: would Triatoma tibiamaculata (Pinto, 1926) belong to Triatoma Laporte, 1832, or to Panstrongylus Berg, 1879, with misleading homoplasies?. Parasites Vectors 15, 184 (2022). https://doi.org/10.1186/s13071-022-05314-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05314-7