Abstract
Background
Larvicides are typically applied to fixed and findable mosquito breeding sites, such as fish farming ponds used in commercial aquaculture, to kill immature forms and thereby reduce the size of adult malaria vector populations. However, there is little evidence suggesting that larviciding may suppress community-wide malaria transmission outside Africa. Here, we tested whether the biological larvicide VectoMax FG applied at monthly intervals to fish farming ponds can reduce malaria incidence in Amazonian Brazil.
Methods
This study was carried out in Vila Assis Brasil (VAB; population 1700), a peri-urban malaria hotspot in northwestern Brazil with a baseline annual parasite incidence of 553 malaria cases per 1000 inhabitants. The intervention consisted of monthly treatments with 20 kg/ha of VectoMax FG of all water-filled fish ponds in VAB (n ranging between 167 and 170) with a surface area between 20 and 8000 m2, using knapsack power mistblowers. We used single-group interrupted time-series analysis to compare monthly larval density measurements in fish ponds during a 14-month pre-intervention period (September 2017–October 2018), with measurements made during November 2018–October 2019 and shortly after the 12-month intervention (November 2019). We used interrupted time-series analysis with a comparison group to contrast the malaria incidence trends in VAB and nearby nonintervention localities before and during the intervention.
Results
Average larval densities decreased tenfold in treated fish farming ponds, from 0.467 (95% confidence interval [CI], 0.444–0.490) anopheline larvae per dip pre-intervention (September 2017–October 2018) to 0.046 (95% CI, 0.041–0.051) larvae per dip during (November 2018–October 2019) and shortly after the intervention (November 2019). Average malaria incidence rates decreased by 0.08 (95% CI, 0.04–0.11) cases per 100 person-months (P < 0.0001) during the intervention in VAB and remained nearly unchanged in comparison localities. We estimate that the intervention averted 24.5 (95% CI, 6.2–42.8) malaria cases in VAB between January and December 2019.
Conclusions
Regular larviciding is associated with a dramatic decrease in larval density and a modest but significant decrease in community-wide malaria incidence. Larviciding may provide a valuable complementary vector control strategy in commercial aquaculture settings across the Amazon.
Graphical abstract

Similar content being viewed by others
Background
Despite significant progress towards malaria elimination over the past two decades, focal transmission persists in the Americas, where 889,000 cases were estimated to occur in 2019 [1]. The Amazon Basin, extending over nine countries of South America, contributes nearly 90% of the malaria burden on the continent [2].
Malaria transmission in the Amazon is greatest in farming settlements, mining camps, and riverine villages, and typically low in cities and towns [2, 3]. However, infections can also be acquired within and near urban centers across the region [4,5,6,7,8,9], where large populations of the primary local vector, Anopheles darlingi, thrive in natural and artificial water habitats. Human-made tanks or natural water bodies used for commercial aquaculture are increasingly common larval habitats in urban and peri-urban areas in the Amazon [7, 10,11,12,13].
The main malaria transmission pocket in the Amazon Basin of Brazil is the upper Juruá Valley, next to the border with Peru [14]. With < 0.5% of Amazon’s population, the region contributes ~ 8% of the overall country’s malaria burden, estimated at 157,454 cases in 2019 [1]. Malaria transmission in the Juruá Valley extends into urban areas, where large outbreaks have been associated with larval habitats next to human residences, which were originally opened or modified for fish farming [7, 11,12,13].
Larval source management, and particularly the application of biological larvicides, may supplement core interventions such as long-lasting insecticidal net (LLIN) distribution and indoor residual spraying (IRS) with insecticides. Larviciding is well suited to control outdoor feeding (exophagic) and outdoor resting (exophilic) mosquito vectors in densely populated areas with well-delineated, easy to find, and readily accessible breeding sites [15]. Fish farming ponds in urbanized spaces of the Juruá Valley offer a perfect fit for these criteria. First, the biting and resting behavior of An. darlingi has changed over the past decades [16], and this vector now feeds and rests predominantly outdoors (e.g., [17]). Second, it displays more intense human biting activity at dusk and a minor peak at dawn (e.g., [18]). Importantly, LLIN use is unlikely to prevent early-evening biting, except for infants who are more likely to sleep under a bednet at daytime, and IRS has little effect against exophilic and exophagic mosquitoes. Third, fish farming ponds in the region are fixed and findable water bodies that typically remain water-filled during the 5-month dry season. Nevertheless, it remains undetermined whether larviciding can reduce focal malaria transmission in commercial aquaculture settings next to cities and towns across the Amazon.
To address this critical knowledge gap, we assess the impact on malaria prevalence and incidence of community-wide larviciding of fish farming ponds in a peri-urban transmission hotspot in Brazil. We use an environmentally safe biological larvicide with robust residual activity (90–100% reduction in larval density) lasting for 35 days after retreatment of water habitats [19] and negligible effects on non-target populations, such as other invertebrates, fish, and humans. We tested whether larviciding at monthly intervals might reduce larval density in fish farming ponds leading to decreased malaria incidence in the intervention community, compared with untreated communities in the same region.
Methods
Study area
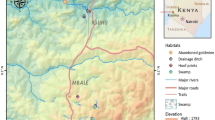
The peri-urban village of Vila Assis Brasil (VAB; 07°35′30ʺS, 72°48′29ʺW), with ~ 1700 permanent residents, is part of the municipality of Cruzeiro do Sul (CZS; population 87,673), upper Juruá Valley, Acre State (Fig. 1). VAB is connected to the municipal seat of CZS, a city with ~ 63,800 inhabitants, by a 17-km paved road. Average monthly rainfall estimates from the Climate Hazards Group Infrared Precipitation with Stations (CHIRPS) data set, which uses modeled satellite-based infrared data (http://chg.geog.ucsb.edu/data/chirps), ranged, during the study period, from 241 to 332 mm in the rainy season (October–April) and from 52 to 138 mm in the dry season (May–September).
Study site (Vila Assis Brasil; VAB) in northwestern Brazil and the location of individual fish farming ponds that were treated with biological larvicides (red dots). The inset shows a map of Brazil, with Acre State highlighted in dark gray and the municipality of Cruzeiro do Sul (where VAB is situated) in red
VAB experiences year-round malaria transmission, with an average annual parasite incidence of 553 cases per 1000 inhabitants between 2016 and 2018. Anopheles darlingi is the primary vector, but larvae of Anopheles albitarsis sensu lato (Galvão and Damasceno) are also abundant in nearby water bodies [12]. A relatively small proportion of the VAB population was covered by core vector control measures at the study baseline; only 58.6% of study participants reportedly slept under an LLIN in the preceding week, and 38.1% of participants’ houses had been sprayed with pyrethroid insecticides within the past 6 months.
Monthly larval density monitoring pre-intervention
Fieldwork was carried out between September 2017 and November 2019. Over a period of 14 months prior to intervention (September 2017 through October 2018), we identified and georeferenced all fish farming ponds in VAB (Fig. 1). The average surface area of aquatic habitats was 1,208.86 m2 (ranging from 20 to 8000 m2). No other natural or artificial water bodies were characterized as major larval habitats (on the basis of both size and larval density) in the area. During that pre-intervention period, we measured the larval density in fish ponds monthly using a standard dipping technique for sampling water bodies [20]; 2–3 dips were taken, using a 500-ml ladle, every 3 m along the edges of the fish pond (total, 20–80 dips per water body). Larvae were classified as first (L1), second (L2), third (L3), or fourth (L4) instar, and then reclassified as early (L1 and L2) or late (L3 and L4) instars. The presence and number of pupae were recorded but not used in the analysis due to the impracticability of morphological differentiation of genera under field conditions. Larval density in each water body was calculated as the average count per dip. At the study outset, in September 2017, 11 of the 144 local fish ponds were empty. Of the 133 water-filled ponds examined, 112 (84.2%) were positive for immature anopheline stages; 131 (98.5%) of them supported active fish farming. Culicine larvae or pupae were found in 107 (80.5%) water-filled ponds at baseline. Additional ponds were excavated during the study period, while others were transiently emptied for fish harvesting or were abandoned. The number of ponds examined for larval density prior to intervention ranged from 127 in March 2018 (10 ponds were empty at the time of the survey) to 165 in October 2018 (four were empty).
Biological larvicide application and post-treatment larval density monitoring
Starting in November 2018 and continuing through October 2019, we carried out monthly larviciding in all fish farming ponds of VAB. The biological larvicide used in this study was VectoMax FG, a granular formulation that combines toxins from Bacillus thuringiensis serovar israelensis (strain AM65-52) and Lysinibacillus (formerly Bacillus) sphaericus 2362 (strain ABTS-1743) in a single microparticle, with a potency of 50 international toxin units. These toxins, once ingested by larvae, lead to lysis of the insect’s gut epithelium. VectoMax FG (Valent BioSciences, Libertyville, IL, USA) at the concentration of 20 kg/ha was applied using 18-l capacity knapsack power mistblowers (Guarany, Itu, Brazil) operating at a walking speed of 0.5 m/s, with a reach of 10 m, covering a surface area of 5 m2/s [19].
Based on the extended residual effect of VectoMax FG applied to fish ponds described in the region [19], habitats were retreated monthly; the only exception was May 2019, when treatment failed due to an unexpected delay in the shipping of larvicides to the field site. As a consequence, there was a 2-month interval between the treatment applied in April 2019 and the next treatment in June 2019. Larval density in each habitat was monitored monthly (including May 2019), 21 days after (re)treatment, exactly as done before the intervention. Note that the last larval density measurement was made in November 2019, exactly 21 days after the last larvicide treatment (in mid-October 2019). The number of water-filled ponds that were treated with biological larvicides and monitored for larval density ranged from 167 to 170 between November 2018 and October 2019. No habitat was treated in November 2019, but all fish ponds were monitored for larval density. VAB is located > 10 km from other villages with similar malaria epidemiology that served as comparison localities, thus preventing spill-over effects on anopheline populations.
Cross-sectional malaria prevalence surveys
Malaria prevalence rates in VAB were measured before and during the intervention. To this end, we surveyed the entire population of VAB in August–September 2018 (prior to larvicide treatment) and in March 2019 and September–October 2019 (during larvicide treatment). During each survey, we collected a finger-prick capillary blood sample from consenting individuals aged ≥ 3 months for malaria diagnosis by microscopy and real-time PCR. At least three visits were made before a house was considered transiently or permanently uninhabited. Because of temporary absences and refusals, the number of study participants screened for malaria parasites during each survey varied between 1185 and 1500 (microscopy) and between 1076 and 1498 (real-time PCR).
For on-site microscopic diagnosis of malaria, thick blood smears were stained with Giemsa and read by a local expert microscopist within 24 h. At least 200 thick smear fields were examined at × 1000 magnification before a slide was declared negative. Infections diagnosed by microscopy on-site were promptly treated as per current malaria treatment guidelines in Brazil [21]. For confirmatory molecular diagnosis, 50-μl aliquots of finger-prick blood samples were used to isolate parasite DNA, using the QIAsymphony investigator kit (Qiagen, Hilden, Germany). Participants with available 50-μl blood aliquots for DNA extraction were screened for malaria parasites using a highly sensitive real-time PCR protocol that targets a genus-specific segment of the mitochondrial cytochrome b (cytb) gene [22], with a detection threshold of ~ 0.5 parasites per μl of blood. Oligonucleotide primer sequences were previously described [22]. Amplifications were run on a QuantStudio 6 Flex real-time PCR system (Applied Biosystems, Foster City, CA, USA) with the following cycling program: 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. No-template negative controls, containing all reagents for amplification except for the DNA template, were run for every PCR microplate.
Malaria surveillance data
We retrieved all notifications of malaria cases diagnosed between September 2017 and December 2019 in CZS that were entered into the electronic malaria notification system of the Ministry of Health of Brazil. Because malaria is a notifiable disease in Brazil and diagnostic testing and treatment are not available outside the network of government-run healthcare facilities, the case notification database is estimated to comprise 99.6% of all laboratory-diagnosed malaria cases countrywide [23]. These data combined with population size estimates obtained during periodic census surveys carried out by the local malaria control program were used to calculate the monthly incidence of malaria in VAB and the contrast group (see below) before and during the larviciding intervention.
For operational malaria control purposes, CZS is divided into smaller geographic units, or “localities,” with shared ecoepidemiological characteristics [24]. Seven localities in CZS were chosen for inclusion in the nonintervention contrast group using the criteria and approach described under Data analysis.
Data analysis
We used a quasi-experimental design to compare larval density and malaria incidence rates before and during the intervention in VAB while controlling for confounding from secular trends and other contextual changes over time [25]. We define intervention as the repeated application of larvicides, at monthly intervals, from November 2018 through October 2019. We used single-group interrupted time-series analysis (ITSA), with the variables T (time since the start of the study), X (dummy variable representing the intervention), and XT (an interaction term), to measure the change in average larval density after introducing the intervention. The first regression line was fitted to both pre- and post-intervention larval density data, with the slope corresponding to the secular trend, while a second line was fitted to capture the deviation of the post-intervention data from the first line (change in level and/or trend). Calculations were made with the itsa command in STATA version 14.1 (Stata, College Station, TX, USA), which uses an ordinary least-squares regression model with Newey–West standard errors to adjust for autocorrelation of residuals over time (events closer together in a time series tend to be more similar than events further apart in time) [26]. This analysis was also adjusted for rainfall 15 days prior to larval sampling, which was positively correlated with larval density measured before and during the intervention (r = 0.799, P < 0.001). To this end, we added an independent variable (precipitation in mm) to the standard ITSA procedure as described [26]. Statistical significance was defined at the 5% level (two-tailed tests) and 95% confidence intervals (CI) were estimated whenever appropriate.
We also used ITSA with a comparison group to test whether the decrease in malaria incidence was greater in VAB than in nonintervention localities starting in January 2019. We defined a 2-month lag period before any effect of larviciding on malaria incidence rates in VAB was expected (November–December 2018) because: (a) infectious adult mosquitoes that were already present in the area would not be affected by larviciding and might continue transmitting malaria, and (b) following infectious mosquito bites, individuals take at least 2 weeks to develop malaria symptoms and seek treatment. ITSA with a nonintervention group, compared with single-group ITSA, included four additional variables: Z (dummy variable to denote the treatment assignment, whether intervention or control) and the interaction terms XT, ZT, and ZXT. Comparison localities in CZS had to meet three selection criteria: (i) no significant difference in malaria incidence rate, compared with VAB, prior to intervention, (ii) no significant difference in the trend of malaria incidence over time, compared with VAB, prior to the intervention, with significance here defined at the 10% level [26], and (iii) distance > 10 km from VAB to avoid spill-over effects of the intervention. Seven rural localities, with a combined population of 3,740 residents, were chosen to form a comparator group: Boca do Moa, Igarapé Preto, São José, Santa Bárbara, Santa Rosa, Santa Luzia (BR-364), and Boca da Alemanha. Because no microscopy- or PCR-based malaria prevalence data were available for localities other than VAB, no attempt was made to evaluate the impact of larviciding on malaria prevalence using ITSA with a nonintervention group.
Results
Reduced density of anopheline larvae in fish ponds during larvicide application
The average larval density during the 14 months of pre-intervention monitoring of fish farming ponds, from September 2017 through October 2018, was 0.467 (95% CI, 0.444–0.490) anopheline larvae per dip. Larval density decreased to an average of 0.046 (95% CI, 0.041–0.051) larvae per dip from November 2018 through November 2019, following larvicide treatment from November 2018 through April 2019 and from June 2019 through October 2019 (Fig. 2). We fitted regression lines to pre- and post-intervention larval density data adjusted for rainfall 15 days prior to larval sampling (Fig. 3). The ITSA approach estimated the starting level of larval density at 0.690 larvae per dip, with a significant monthly decrease prior to the intervention by 0.033 (95% CI, 0.007–0.059, P = 0.016) larvae per dip, while adjusting for monthly rainfall. There was a further drop in larval density by 0.231 (95% CI, 0.010–0.452, P = 0.042) larvae per dip shortly after the intervention (November 2018), which characterizes a change in level [26], with sustained low larval densities until November 2019 (Fig. 3). No treatment was carried out in May 2019, leading to a transient rise in larval density (Fig. 2), but ITSA results remain unchanged if this sampling point was omitted (data not shown).
Monitoring of anopheline larval density in fish farming ponds of Vila Assis Brasil (VAB), northwestern Brazil, from September 2017 through November 2019. Monthly averages of larvae per dip are shown for early instar (L1 and L2; dark green) and late instar larvae (L3 and L4; light green) collected in fish farming ponds in VAB (number of ponds ranging between 127 and 165 before the intervention and between 167 and 170 during and shortly after the intervention). Vertical arrows indicate the timing of larvicide treatment (from October 2018 through April 2019 and from June 2019 through October 2019). Larval density measurements were carried out 21 days after each larvicide application during the intervention. The light blue area chart indicates the monthly rainfall (mm)
Interrupted time-series regression analysis of average monthly larval densities in Vila Assis Brasil before (September 2017–October 2018), during (November 2018–October 2019), and shortly after larviciding (November 2019). The solid line shows the trend based on least-squares linear regression fitted to empirical data (black dots) adjusted for a time-dependent variable (rainfall estimates 15 days prior to larval sampling) and for autocorrelation of residuals over time. The dashed vertical line indicates the time of the first larvicide application. No larvicide treatment was applied in May 2019 (see the main text for details)
Malaria prevalence before and during larvicide application
During the pre-intervention prevalence survey in September–October 2018, 1500 permanent residents in VAB were examined by microscopy. Of them, 40 (2.67%; 95% CI, 1.94–3.65%) had malaria parasites detected (red diamond in Fig. 4). There were 33 infections with Plasmodium vivax, six with P. falciparum, and one mixed-species infection with P. vivax and P. falciparum. Prevalence rates dropped substantially in the following surveys. In March 2019, only eight of 1416 individuals examined (0.56%; 95% CI, 0.26–1.15%) had malaria parasites detected by microscopy (4.8-fold decrease in prevalence), with seven instances of P. vivax infection and only one P. falciparum infection. Finally, 1185 individuals were examined in September–October 2019, and only two (0.17%, 95% CI, 0.03–0.68%) were positive, both for P. vivax (15.6-fold decrease in prevalence compared with the baseline).
Malaria incidence and prevalence in Vila Assis Brasil (intervention site) and nearby larvicide-untreated localities in northwestern Brazil. The monthly incidence of microscopy-confirmed clinical malaria (P. vivax and P. falciparum infections combined) in Vila Assis Brasil (VAB) and comparison localities between September 2017 and December 2019 is shown as gray (VAB) and black lines (seven comparison localities in the municipality of Cruzeiro do Sul). Prevalence rates measured by microscopy and PCR in VAB before (September 2018) and during (March 2019 and September 2019) the intervention are indicated by red and green diamonds, respectively. Vertical arrows indicate the timing of larvicide treatment, and the light blue area chart indicates the monthly rainfall (mm)
The proportions of positive genus-specific PCR results per survey are shown as green diamonds in Fig. 4. Of 1498 individuals tested in September–October 2018, 169 (11.28%; 95% CI, 9.72–12.99%) were positive. The proportion of PCR-positive individuals remained nearly unchanged in March 2019: of 1374 individuals tested, 147 (10.69%; 95% CI; 9.11–12.45%) were positive. However, we found a more substantial decline in PCR positivity in September–October 2019. At the end of the intervention, 1076 individuals were tested for malaria parasites by PCR, and 55 (5.11%; 95% CI, 3.87–6.60%) were positive (2.2-fold decrease in prevalence compared with the baseline). The proportion of malarial infections that were subpatent (i.e., missed by microscopy but detected by PCR) increased substantially during and shortly after the intervention, compared with the baseline survey. Accordingly, PCR detected 4.2-fold more infections than microscopy in the first survey, 19.1-fold in the second survey, and 30.1-fold more infections in the third survey.
Malaria incidence before and during larvicide application
We compared the monthly incidence of malaria in VAB and comparison localities before and during the intervention using routinely collected morbidity data (Fig. 4). As expected, given the selection criteria applied, malaria rates dropped at similar rates in VAB and the comparison group from September 2017 through December 2018, with average incidence rates of 3.27 cases per 100 person-months (95% CI, 1.06–7.47) in VAB and 4.27 cases per 100 person-months (95% CI, 2.30–8.50) in the seven comparison localities altogether. Between January 2019 (2 months after the first larvicide application) and December 2019 (2 months after the last larvicide application), malaria incidence continued to decline in VAB, but not in the comparison localities (Fig. 4). The average incidence for the 12-month intervention period was 0.70 cases per 100 person-months (95% CI, 0.23–1.59) in VAB and 1.41 cases per 100 person-months (95% CI, 0.69–2.14) in the comparison group. No drop or change in incidence level was observed shortly after introducing the intervention (Fig. 5). Using ITSA, we estimate that malaria incidence declined by 0.08 (95% CI, 0.04–0.11) malaria cases per 100 person-months (P < 0.0001) between January and December 2019 in VAB, but remained nearly unchanged in comparison localities (nonsignificant increase by 0.04 malaria case per 100 person-months, P = 0.3031) (Fig. 5). The average difference in malaria incidence was 0.12 (95% CI, 0.03–0.21) cases per 100 person-months in VAB relative to the comparison group (P = 0.0080). This difference translates to 24.5 (95% CI, 6.2–42.8) microscopy-positive clinical malaria cases averted between January and December 2019 among the 1700 VAB residents.
Interrupted time-series regression analysis of malaria incidence in Vila Assis Brasil (black dots and solid lines) and comparison localities (open dots and dashed lines) before and after the larviciding intervention. The least-squares linear regression model fitted to empirical data (dots) is adjusted for autocorrelation of residuals over time. The vertical dashed line separates pre- and post-intervention incidence measurements. The intervention started in November 2018, but we include only incidence estimates obtained from January 2019 in the “post-intervention period,” as we expect a 2-month delay in the effect of larval density reduction on malaria transmission (see “Methods”)
Discussion
Larviciding aims to reduce the population size of malaria vectors by killing the aquatic immature forms, so that fewer will develop into adults and transmit the parasite. Surprisingly few studies have examined whether chemical or biological larviciding can reduce malaria transmission across endemic settings with different primary vectors [27]. We have previously shown that 20 kg/ha of VectoMax FG has a substantial residual effect against An. darlingi larvae and pupae, lasting for at least 4 weeks, when applied to natural or human-made fish ponds [19]. Here, we investigated, for the first time, the impact of biological larviciding on community-wide malaria incidence in the Amazon. We show a tenfold reduction in average larval densities in treated fish farming ponds, compared to pretreatment averages, with a modest but significant decrease in malaria incidence rate associated with monthly VectoMax FG application, compared with non-intervention localities.
Our findings have major public health implications for malaria control and elimination across the Amazon. Malaria transmission associated with commercial aquaculture is increasingly common in the region [10, 11, 28, 29]. Indeed, in the mid-2000s, shortly after the Acre State government launched the Program for the Development of Fish Farming in the Juruá Valley, Brazil, explosive malaria outbreaks were recorded across the region [11, 29]. Fish farming ponds within and near urban centers pose a major risk of malaria transmission in densely populated areas in the Amazon [7, 11, 29]. Importantly, larviciding may be more effective to suppress outdoor biting and resting mosquitoes, such as the primary Amazonian malaria vector An. darlingi [2, 17], than core vector control interventions that target endophilic and endophagic anophelines, such as LLIN distribution and IRS [30].
Cluster-randomized controlled trials (cRCTs) are ideally suited for the evaluation of community-wide interventions. However, only a single published cRDT, carried out in Sri Lanka nearly 20 years ago, has evaluated the effectiveness of (chemical) larviciding for malaria control [31]. This cRDT, included in a recent systematic Cochrane review [27], found a nearly fivefold decrease in malaria incidence in intervention villages (5 cases per 100 person-years) compared with control villages (23 cases per 100 person-years). Larviciding is rarely assessed using cRCTs, in part because of the high cost and the complex logistics of these studies, with substantial risk of intervention spill-over affecting nearby control communities [30]. Conversely, ITSA with comparison group(s) has been increasingly used to measure the effectiveness of community interventions [32, 33] and may provide a cost-effective alternative to cRCTs in the evaluation of larviciding and other malaria control interventions.
Our study has some limitations. First, in contrast with cRDTs, we did not have a true control arm. Of note, malaria incidence rates have decreased in both VAB and the comparison localities during the 14-month pre-intervention period, but the rate of decline changed during the intervention (Fig. 4), highlighting the critical need for an appropriate comparison group in our ITSA approach. Second, we may have underestimated malaria incidence rates in VAB and comparison localities. We retrieved malaria morbidity data from an electronic case notification database that is assumed to comprise nearly all clinical malaria episodes diagnosed by microscopy and treated in CZS [23], but transient or chronic submicroscopic parasitemias that do not develop into clinically apparent infections detectable by conventional microscopy remain mostly undetected. Importantly, the proportion of malarial infections that were missed by microscopy but diagnosed by a more sensitive molecular method increased substantially during the intervention. Third, we measured malaria prevalence rates by using microscopy and molecular diagnostic methods in VAB but not in the comparison localities. Therefore, we cannot determine to what extent the sharp decline in the prevalence of patent infections in consecutive cross-sectional surveys in VAB, with a much less marked change in the prevalence of subpatent infections, results from the intervention or merely reflects an overall trend in malaria transmission in the region. Finally, we have not systematically characterized the anopheline fauna in VAB and comparison localities, where fish farming ponds are much less abundant, but available data show a significant variation in species abundance and composition in the anopheline communities from localities separated by 10–30 km across Juruá Valley [18].
In conclusion, our results suggest that the periodic application of biological larvicides with extended activity represents a valuable complementary malaria vector control strategy in commercial aquaculture settings of the upper Juruá Valley, northwestern Brazil. Further studies are warranted to examine the generalizability of these findings across the Amazon, especially in areas where larval habitats are present in widely different sizes and anopheline species other than An. darlingi contribute significantly to malaria transmission.
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The data sets used are available at the University of São Paulo data repository (https://uspdigital.usp.br/repositorio/). Researchers who are interested in potential collaboration should contact the corresponding author (muferrei@usp.br).
Abbreviations
- CI:
-
Confidence interval
- cRCT:
-
Cluster-randomized controlled trial
- CZS:
-
Cruzeiro do Sul
- IRS:
-
Indoor residual spraying
- ITSA:
-
Interrupted time-series analysis
- LLIN:
-
Long-lasting insecticidal net
- VAB:
-
Vila Assis Brasil
References
World Health Organization. World Malaria Report 2020. Geneva: World Health Organization; 2020. Available at: https://www.who.int/publications/i/item/9789240015791. Accessed 16 June 2021.
Ferreira MU, Castro MC. Malaria situation in Latin America and the Caribbean: residual and resurgent transmission and challenges for control and elimination. Methods Mol Biol. 2019;2013:57–70.
Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S, Lacerda MVG. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J. 2017;16:273.
Camargo LM, dal Colletto GM, Ferreira MU, Gurgel SM, Escobar AL, Marques A, Krieger H, Camargo EP, da Silva LH. Hypoendemic malaria in Rondonia (Brazil, western Amazon region): seasonal variation and risk groups in an urban locality. Am J Trop Med Hyg. 1996;55:32–8.
Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, Roncal N, et al. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27.
Tada MS, Marques RP, Mesquita E, Dalla Martha RC, Rodrigues JA, Costa JD, et al. Urban malaria in the Brazilian Western Amazon Region I: high prevalence of asymptomatic carriers in an urban riverside district is associated with a high level of clinical malaria. Mem Inst Oswaldo Cruz. 2007;102:263–9.
Costa KM, de Almeida WA, Magalhães IB, Montoya R, Moura MS, de Lacerda MV. Malaria in Cruzeiro do Sul (Western Brazilian Amazon): analysis of the historical series from 1998 to 2008 [in Portuguese]. Rev Panam Salud Publica. 2010;28:353–60.
Almeida ACG, Kuehn A, Castro AJM, Vitor-Silva S, Figueiredo EFG, Brasil LW, et al. High proportions of asymptomatic and submicroscopic Plasmodium vivax infections in a peri-urban area of low transmission in the Brazilian Amazon. Parasit Vectors. 2018;11:194.
Salla LC, Rodrigues PT, Corder RM, Johansen IC, Ladeia-Andrade S, Ferreira MU. Molecular evidence of sustained urban malaria transmission in Amazonian Brazil, 2014–2015. Epidemiol Infect. 2020;148:e47.
Maheu-Giroux M, Casapía M, Sotto-Calle VE, Ford LB, Buckeridge DL, Coomes OT, et al. Risk of malaria transmission from fish ponds in the Peruvian Amazon. Acta Trop. 2010;115:112–8.
Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mâncio Lima County, Brazil. Emerg Infect Dis. 2010;16:1108–15.
dos Reis IC, Codeço CT, Degener CM, Keppeler EC, Muniz MM, de Oliveira FG, et al. Contribution of fish farming ponds to the production of immature Anopheles spp. in a malaria-endemic Amazonian town. Malar J. 2015;14:452.
Reis IC, Honório NA, Barros FS, Barcellos C, Kitron U, Camara DC, Pereira GR, Keppeler EC, da Silva-Nunes M, Codeço CT. Epidemic and endemic malaria transmission related to fish farming ponds in the Amazon frontier. PLoS ONE. 2015;10:e0137521.
Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J. 2016;15:284.
World Health Organization. Guidelines for Malaria Vector Control. Geneva, World Health Organization; 2019. Available at: https://apps.who.int/iris/handle/10665/310862. Accessed 16 June 2021.
Hiwat H, Bretas G. Ecology of Anopheles darlingi Root with respect to vector importance: a review. Parasit Vectors. 2011;4:177.
Gil LH, Alves FP, Zieler H, Salcedo JM, Durlacher RR, Cunha RP, Tada MS, Camargo LM, Camargo EP, Pereira-da-Silva LH. Seasonal malaria transmission and variation of anopheline density in two distinct endemic areas in Brazilian Amazonia. J Med Entomol. 2003;40:636–41.
Martins LMO, David MR, Maciel-de-Freitas R, Silva-do-Nascimento TF. Diversity of Anopheles mosquitoes from four landscapes in the highest endemic region of malaria transmission in Brazil. J Vector Ecol. 2018;43:235–44.
Fontoura PS, da Costa AS, Ribeiro FS, Ferreira MS, Castro MC, Ferreira MU. Field efficacy of VectoMax FG and VectoLex CG biological larvicides for malaria vector control in northwestern Brazil. J Med Entomol. 2020;57:942–6.
World Health Organization. Entomological Field Techniques for Malaria Control. Geneva: World Health Organization; 1992. Available at: https://apps.who.int/iris/handle/10665/38693. Accessed 15 June 2017.
Ministry of Health of Brazil. Practical Guidelines for Malaria Therapy. Ministry of Health of Brazil, Brasília, Brazil; 2020 [In Portuguese]. Available at: http://bvsms.saude.gov.br/bvs/publicacoes/guia_pratico_malaria.pdf. Accessed 16 June 2021.
Putaporntip C, Buppan P, Jongwutiwes S. Improved performance with saliva and urine as alternative DNA sources for malaria diagnosis by mitochondrial DNA-based PCR assays. Clin Microbiol Infect. 2011;17:1484–91.
Daher A, Silva JCAL, Stevens A, Marchesini P, Fontes CJ, Ter Kuile FO, Lalloo DG. Evaluation of Plasmodium vivax malaria recurrence in Brazil. Malar J. 2019;18:18.
World Health Organization. A Framework for Malaria Elimination. Geneva, World Health Organization; 2017. Available at: https://www.who.int/malaria/publications/atoz/9789241511988/en/. Accessed 16 June 2021.
Ashton RA, Bennett A, Yukich J, Bhattarai A, Keating J, Eisele TP. Methodological considerations for use of routine health information system data to evaluate malaria program impact in an era of declining malaria transmission. Am J Trop Med Hyg. 2017;97(3Suppl):46–57.
Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15:480–500.
Choi L, Majambere S, Wilson AL. Larviciding to prevent malaria transmission. Cochrane Database Syst Rev. 2019;8:CD012736.
Maheu-Giroux M. An eco-epidemiological study of fish farming and malaria occurrence in the Peruvian Amazon. MSc thesis. Montreal: McGill University; 2009. Available at: https://escholarship.mcgill.ca/downloads/9s161717m?locale=en. Accessed 16 June 2021.
Johansen IC, Ferreira MU. Unintended consequences of ‘development’ in the Amazon: Commercial aquaculture and malaria in Mâncio Lima, Brazil. In: Ioris AAR, editor. Environment and development: challenges, policies and practices. London: Palgrave Macmillan; 2021. p. 387–410.
Zhou G, Lo E, Githeko AK, Afrane YA, Yan G. Long-lasting microbial larvicides for controlling insecticide resistant and outdoor transmitting vectors: a cost-effective supplement for malaria interventions. Infect Dis Poverty. 2020;9:162.
Yapabandara AM, Curtis CF, Wickramasinghe MB, Fernando WP. Control of malaria vectors with the insect growth regulator pyriproxyfen in a gem-mining area in Sri Lanka. Acta Trop. 2001;80:265–76.
Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci. 2000;1:31–49.
Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–55.
Acknowledgements
We thank Amanda Sampaio for fieldwork; Maria José Menezes and Amanda N. Campos for their excellent administrative support; Dr. Anderson Rocha for data management; Dr. José Bento P. Lima and Prof. Maria Anice Sallum for helpful suggestions; and Hélio Cameli and Muana Araújo for their invaluable logistical support.
Funding
Financial support was provided by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (Grant 2016/18740-9 to M.U.F.) and the David Rockefeller Center for Latin American Studies, Harvard University (to M.C.C.). P.S.F. (Grant 2016/25617-9) and P.T.R. (Grant 2018/03902-9) received FAPESP post-doctoral fellowships, and M.U.F. receives a senior research scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. Valent BioSciences supplied the biological larvicides and one mistblower. Dr. Peter DeChant (formerly at Valent BioSciences) provided technical expertise in field trial implementation. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
PSF, MCC, and MUF conceived and designed the study. PSF, MFS, ASC, FSR, MSF, and SLA carried out fieldwork. JT and PTR did molecular diagnosis of malaria. PSF, MCC, and MUF analyzed the data. MUF wrote the first manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the Institute of Biomedical Sciences, University of São Paulo, Brazil, and by the National Committee of Ethics in Research of the Ministry of Health of Brazil (CAAE number 64767416.6.0000.5467). Written informed consent and assent were obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fontoura, P.S., Silva, M.F., da Costa, A.S. et al. Monthly biological larviciding associated with a tenfold decrease in larval density in fish farming ponds and reduced community-wide malaria incidence in northwestern Brazil. Parasites Vectors 14, 445 (2021). https://doi.org/10.1186/s13071-021-04964-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04964-3