Abstract
Background
Phlebotomines are a group of insects which include vectors of the Leishmania parasites that cause visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL), diseases primarily affecting populations of low socioeconomic status. VL in Brazil is caused by Leishmania infantum, with transmission mainly attributed to Lutzomyia longipalpis, a species complex of sand fly, and is concentrated mainly in the northeastern part of the country. CL is distributed worldwide and occurs in five regions of Brazil, at a higher incidence in the north and northeast regions, with etiological agents, vectors, reservoirs and epidemiological patterns that differ from VL. The aim of this study was to determine the composition, distribution and ecological relationships of phlebotomine species in an Atlantic Forest conservation unit and nearby residential area in northeastern Brazil.
Methods
Centers for Disease Control and Shannon traps were used for collections, the former at six points inside the forest and in the peridomestic environment of surrounding residences, three times per month for 36 months, and the latter in a forest area, once a month for 3 months. The phlebotomines identified were compared with climate data using simple linear correlation, Pearson’s correlation coefficient and cross-correlation. The estimate of ecological parameters was calculated according to the Shannon-Wiener diversity index, standardized index of species abundance and the dominance index.
Results
A total of 75,499 phlebotomines belonging to 11 species were captured in the CDC traps, the most abundant being Evandromyia walkeri, Psychodopygus wellcomei and Lu. longipalpis. Evandromyia walkeri abundance was most influenced by temperature at collection time and during the months preceding collection and rainfall during the months preceding collection. Psychodopygus wellcomei abundance was most affected by rainfall and relative humidity during the collection month and the month immediately preceding collection time. Lutzomyia longipalpis abundance showed a correlation with temperature and the rainfall during the months preceding collection time. The Shannon trap contained a total of 3914 phlebotomines from these different species. Psychodopygus wellcomei, accounting for 91.93% of the total, was anthropophilic and active mainly at night.
Conclusions
Most of the species collected in the traps were seasonal and exhibited changes in their composition and population dynamics associated with local adaptions. The presence of vectors Ps. wellcomei and Lu. longipalpis underscore the epidemiological importance of these phlebotomines in the conservation unit and surrounding anthropized areas. Neighboring residential areas should be permanently monitored to prevent VL or CL transmission and outbreaks.
Graphical Abstract

Similar content being viewed by others
Background
The leishmaniases are a group of diseases caused by several species of etiologic agents of the genus Leishmania. The two main forms of leishmaniasis are visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL) [1, 2]. Phlebotomines (Diptera: Psychodidae) are small insects, and the hematophagous females of some phlebotomine species are responsible for transmitting protozoan parasites belonging to the genus Leishmania, known vectors of the leishmaniases, resulting in diseases that exhibit a series of clinical manifestations with a potential risk of death. The leishmaniases are considered to be neglected tropical diseases and are related to premature deaths and incapacitating lesions in several countries of the world [3,4,5].
In Brazil, VL is caused by Leishmania infantum, whose main vector is the phlebotomine Lutzomyia longipalpis [1], while CL has different vectors and etiologic agents, the principal vectors being Nyssomyia whitmani, Nyssomyia intermedia, Nyssomyia neivai, Migonemyia migonei and Psychodopygus wellcomei [2, 5,6,7,8]. Leishmania braziliensis is the most widely distributed etiologic agent in the country and is the primary culprit in cases of CL in northeastern Brazil [5].
Although leishmaniases occur in all regions of Brazil, VL cases are concentrated in the northeastern parts, with approximately 45% of all reported cases in 2017 occurring in this region. The northeast and northern regions continue to report a large number of CL cases [9, 10]. Studies show that the presence of settlements in forested areas and environmental degradation are important drivers of the rising number of leishmaniasis cases since they can contribute to the adaptation and expansion of sand fly vectors in areas of human intervention, whether in urban or periurban areas [11,12,13,14].
Our research group has observed changes in the composition and population dynamics of phlebotomines in Atlantic Forest fragments since the 1990s, where Lutzomyia longipalpis is the main phlebotomine species in the area. [15, 16]. The forest harbors wild mammals considered to be parasite reservoirs and is situated in the primary area of VL occurrence in the state and metropolitan region of Natal. In recent years, urbanization has increased in surrounding areas, with increasing numbers of families and their pets, mainly dogs.
The aim of this study was to conduct a long-term study in an endemic area of VL and CL and analyze the abundance, composition and ecological relationships among phlebotomine species in an Atlantic Forest conservation unit under anthropic pressure.
Methods
Study site
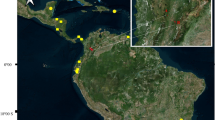
The study was conducted in a fragment of forest located in the Nísia Floresta National Forest (6°05′12.4′′S; 35°11′04.0′′W), an Atlantic Forest conservation unit, and in a nearby residential area in the municipality of Nísia Floresta, Rio Grande do Norte State, northeastern Brazil (Fig. 1). The municipality has reported cases of CL and VL, has a population of 27,260 inhabitants and is located in the metropolitan region of Natal, the state capital [17] (Fig. 1).
Atlantic Forest conservation unit (A) in the municipality of Nísia Floresta, Rio Grande do Norte State, Brazil, where the study took place. U.S. Center of Disease Control traps were placed at three collection points in the native forest area (P1) at a height of 12, 6 and 1 m, respectively, at the forest boundary (P2), in the rural peridomestic environment (P3) and in the peridomestic environment of a residential condominium (P4). The star indicates the Shannon trap collection point in the native forest
The Nísia Floresta National Forest lies in a region of tourism and urban expansion. It covers 168.84 hectares divided into an area of experimental forests where exotic species occur and a preserved area with semi-deciduous forest and coastal tableland.
The fauna in the area exhibits a wide diversity of arthropods, amphibians, reptiles, birds and mammals [18]. Among the mastofauna found are Cerdocyon thous (crab-eating fox), a wild potential reservoir of Leishmania infantum, Didelphis albiventris (white-eared opossum), a wild potential reservoir of L. infantum and parasite host of L. braziliensis, in addition to rodent parasite hosts or potential reservoirs of L. infantum and L. braziliensis [19, 20].
Phlebotomine sampling
Collections with U.S. Center of Disease Control traps
Collections with U.S. Center of Disease Control (CDC) traps occurred in the area inside and surrounding the conservation unit (CU) (Fig. 1), at the following points: (i) three vertical strata at the same site in the conserved forest, denominated point 1 (P1A, P1B, P1C) (6°4′57.45″S/35°11′5.80″W), where the seasonal forest is most conserved; (ii) at the boundary of the seasonal forest, denominated point 2 (P2) (6°5′2.93″S/35°11′17.06″W); (iii) in the rural peridomestic environment, denominated point 3 (P3) (6°4′56.26″S/35°11′13.62″W), including the peridomestic environment with domestic animals (e.g. chickens, dogs and horses); (iv) in a peridomestic environment consisting of a condominium with 263 residents, denominated point 4 (P4) (6°5′11.76″S/35°11′27.05″W), located adjacent to the CU (Fig. 1).
Collections took place three times each month for 36 months, from September 2013 to August 2016, using CDC light traps that operated for 14 consecutive hours, from 5 p.m. to 7 a.m. the following morning, totaling 7776 h of sampling effort. The six traps used were arranged at different heights, as follows: three at P1 (P1A: 12 m; P1B: 6 m; P1C: 1 m), one at P2 (1 m), one at P3 (1 m) and one at P4 (1 m) (Fig. 1).
Collections with Shannon trap
Collections with the Shannon trap [21] took place in the semi-deciduous seasonal forest (Fig. 1) using two 8W white lights to attract the phlebotomines, powered by 6V, 12A batteries. Three collections lasting 24 hours were made, starting at 4 pm and concluding at the same time the next day, in June, August and October 2016, totaling 72 h of sampling. In the study area, the sun rises around 5:15 a.m. and sets about 12 h later. We considered twilight as soft diffused light from the sky when the sun is below the horizon, either from daybreak to sunrise, or from sunset to nightfall.
The captures were made by six collectors (3 pairs), who alternated every 2 h and wore suitable protective clothing. The phlebotomines that landed in the trap or on the collectors were captured with a manual aspirator, stored in plastic vials that were replaced with new vials each hour, taken to the laboratory, mounted and identified.
All phlebotomines captured with CDC and Shannon traps were processed and mounted onto microscopic slides [22]. They were identified using the phylogenetic classification proposed by Galati [23] and stored in the Professor Adalberto Antônio Varela-Freire Entomological Collection of the Federal University of Rio Grande do Norte (preservation code CEAAVF/UFRN/DIP0001).
Data analysis
BioEstat 5.3 (Mamirauá Institute, Tefé, Brazil), a software for (bio)statistical analysis, was used in Pearson’s correlation coefficient and simple linear regression analysis, with the insects representing the dependent variable and rainfall, relative humidity and temperature the independent variables. Meteorological data were obtained from the National Institute of Meteorology (INMET) weather station [24], located 30 km from the CU, which is part of the same mesoregion and displays similar climate characteristics. For all regressions performed, the residuals confirmed the assumption regarding the errors of the linear regression model.
The total number of specimens and the total species at each collection site for both sexes were assessed with analysis of variance using the Kruskal-Wallis and Mann-Whitney tests to compare differences between two means.
Species abundance was compared to mean rainfall, temperature and relative humidity, first assessing the autocorrelation patterns of both time series using multiple regression analysis and then applying the cross-correlation test using the Paleontological Statistics Software Package (PAST 2.17c). Cross-correlation is useful in aligning two time series, one of which is delayed with respect to the other, as its peak occurs at the lag at which the two time series are best correlated [25, 26]. Thus, we used the test to deepen the analysis of the relationships between the meteorological variables and phlebotomine abundance.
The standardized index of species abundance (SISA) [27] was calculated to compare abundance between the species found, with values varying between zero and one, using Microsoft Office Excel 2013 (Microsoft Corp., Redmond, WA, USA). Collection point diversity was analyzed based on species richness and equitability, using the Shannon-Wiener diversity index [28].
Relative species frequency was used to establish a dominance rank (D), according to the categories established by Silveira Neto et al [29], with eudominant > 10%; dominant > 5–10%; subdominant > 2–5%; occasional = 1–2%; and rare < 1%. D% = (i/t) × 100, where i is the number of individuals of a species and t is the total number collected.
Results
Phlebotomine diversity and abundance in forest and anthropized areas
A total of 75,499 phlebotomines belonging to seven genera and 11 species were captured in the CDC light traps. In general, males were more abundant than females; however, there was no significant difference in the male:female ratio (Mann-Whitney U-test: U = 60, Z = 0.0328, P = 0.48).
The most abundant species was Evandromyia walkeri, conributing to 79.7% of the phlebotomines collected (SISA = 1.00), primarily in the trap near the forest floor, where 21,039 Ev. walkeri individuals were captured (SISA = 0.969) (Fig. 2), followed in decreasing order of abundance by Psychodopygus wellcomei (12.7%; SISA = 0.89), Evandromyia evandroi (2.9%; SISA = 0.89) and Lutzomyia longipalpis (3.1%; SISA = 0.87) (Table 1).
Psychodopygus wellcomei was more abundant in the forest (P1C) and boundary areas (P2) (Fig. 2), in addition to occurring in anthropized environments, such as the rural peridomestic (P3) and condominium (P4), albeit not as abundantly as in the forest and boundary areas (Fig. 2).
The highest abundance of Lu. longipalpis occurred in the the rural peridomestic environment (P3) (SISA = 0.831), where it was the second most abundant species. It also occurred in other ecotopes, but at a lower abundance (Fig. 2).
Analysis of the most abundant species in forest and anthropized environments (Fig. 2) revealed that Ev. walkeri and Ps. wellcomei occurred predominantly in the former and Lu. longipalpis primarily in the latter (Table 1).
Vertical and horizontal stratification
The traps with the highest number of phlebotomines were those placed near the ground in forest areas, namely 26,567 collected specimens (35.2%) at P1C and 15,537 (20.6%) at P1B. In an anthropized area, the largest number of phlebotomines occurred in P3, a peridomestic rural area, with 15,434 specimens (20.4%) collected (Table 1).
Evandromyia walkeri was the eudominant species in all ecotypes studied (D% > 10%). Psychodopygus wellcomei was the eudominant species in traps located near the ground (P1B, P1C and P2), dominant species (5% < D > 10%) in tree canopies, subdominant species (2% < D > 5%) and occasional (1% < D > 2%) in traps at P3 and P4, respectively (i.e. in anthropized areas) (Table 2).
Luzomyia longipalpis phlebotomines were rare (D < 1%) in forest environments (P1A, P1B and P1C), occasional at the edge (P2), eudominant in the peridomestic rural area and subdominant in the residential community (P4) (Table 2).
The occurrence of Ev. vandroi was rare in the canopy (P1A) and only occasionally near the ground in a forest area (P1B and P1C). Dominance rose as the distance from this area increased, with this species becoming dominant at the forest edge (P2) and in the peridomestic rural area (P3) and eudominant, along with E. walkeri, in the residential community. The other species were mostly rare, except for Ev. lenti, whose presence was occasional in P2 and subdominant in P3 and P4 (Table 2).
Despite the significant abundance of phlebotomines in all the forest traps, these insects were most active near the ground (P1C) and in the tree canopy. The differences were significant between the traps near the ground and in the canopy (P1A) (Mann-Whitney U-test: U = 23, Z = 2.46, P = 0.0069), and between the midpoint (P1B) and canopy (P1A) (Mann-Whitney U-test: U = 35.5, Z = 1.64, P = 0.0503). No significant difference was found between P1B and P1C (P = 0.1252).
Seasonal and daily phlebotomine activity
The highest abundance of Ev. walkeri was observed in January and February, which is the transition period between the dry and rainy seasons.
Pearson’s correlation test indicated that total phlebotomine abundance was positively correlated only with temperature (r = 0.39, P = 0.019); rainfall (r = − 0.15, P = 0.38) and relative humidity (r = − 0.1158, P = 0.50) were not correlated with insect abundance. The estimate using simple linear regression analysis revealed that for each degree increase in temperature, an average of 888.5 more phlebotomine specimens occurred [Coef. (b) = 888.5, P = 0.019].
In separate analyses of the five main species collected, Ev. walkeri abundance was correlated only with temperature (r = 0.43, P = 0.009), such that for each degree increase, there was an average increase of 904.1 phlebotomines [Coef. (b) = 904.08, P = 0.009]. The species Ev. evandroi and Lu. longipalpis showed no significant correlation between abundance and the meteorological variables (P > 0.05) assessed. Evandromyia lenti abundance was correlated only with temperature (r = 0.3751, P = 0.02); that is, for each degree increase, there was an average of 10.3 more individuals of this species [Coef. (b) = 10.3, P = 0.02]. Psychodopygus wellcomei exhibited a significant correlation between abundance and the variables rainfall (r = 0.47, P = 0.004) and relative humidity (r = 0.53, P = 0.0009); the relation with rainfall was such that for each additional millimeter of rain, there was an average increase of 1.2 individuals of the species [Coef. (b) = 1.23, P = 0.004], while for relative humidity, abundance increased by an average of 73 individuals for each percentage rise in humidity [Coef. (b) = 73.05, P = 0.0009].
Although none of the meteorological variables were correlated with phlebotomine abundance at the moment of collection, it was considered possible that cross-correlation analysis might reveal the influence and dynamics of the time interval that were not observed in the previous analysis. Thus, the total number of phlebotomines from the five most abundant species was analyzed in relation to the rainfall, temperature and relative humidity of previous months. Cross-correlation analysis revealed positive correlations between phlebotomines and rainfall in the insect collection month (Lag 0), and between 1 (Lag-1) and 2 (Lag-2) months before collection. The correlation observed with temperature was negative and significant between 3 and 5 months before collection (Lag-3, Lag-4 and Lag-5). The relative humidity 4 and 5 months before insect collection month (Lag-4, Lag-5) was directly correlated with the number of phlebotomines.
Analysis of the lagged correlation between rainfall and the number of phlebotomines collected revealed significant results between the species Ev. evandroi (Lag-4 and Lag-5), Ev. lenti, Ev. walkeri and Lu. longipalpis and rainfall during 4 and 5 months prior to collection (Lag-4 and Lag-5), while Ps. wellcomei demonstrated a significant positive correlation with rainfall during the collection month (Lag 0) and the previous month (Lag-1) (Table 3).
A significant time lag was also observed when comparing the occurrence of some phlebotomine species with the temperatures of previous months. Evandromyia evandroi showed a significant correlation with the temperatures recorded between 1 and 5 months before collection (Lag-1 to Lag-5). For Ev. lenti, the correlation was significant with the temperature at collection time (Lag 0), and between 1 and 5 months before collection (Lag-1 to Lag-5); for Ev. walkeri, the correlation was significant with temperature at collection time (Lag 0), and at 1, 2 and 5 months before collection (Lag-1, Lag-2 and Lag-5); Lu. longipalpis showed a negative correlation with temperature at 5 months before collection time (Lag-5) and Ps. wellcomei with temperature between 1 and 2 months before collection time(Lag-1 and Lag-2) (Table 4).
The species Ev. evandroi, Ev. walkeri and Lu. longipalpis showed a significant correlation with the 4 and 5-month lag time (Lag-4 and Lag-5). For Ev. lenti, these relations were observed between 3 and 5 months before collection time (Lag-3, Lag-4 and Lag-5), while for Ps. wellcomei, the lagged relations for relative humidity were observed at collection time, and at 1, 4 and 5 months before collection (Lag-1, Lag-4 and Lag-5) (Table 5).
Seasonal species occurrence varied during the 3 collection years. Peak abundance of Ev. walkeri, Ev. lenti and Ev. evandroi was always observed in January and February. The highest occurrence of Ps. wellcomei was observed in the rainy season (May, June and July), revealing marked seasonality during the three years, but this species was essentially absent in the dry season (Fig. 3).
Collections using Shannon traps during three periods of 24 consecutive hours resulted in the capture of 3914 phlebotomines belonging to the species Ps. wellcomei, Ev. walkeri, Ev. evandroi and Lu. longipalpis were collected in the 72-h sampling effort. The most abundant of these was Ps. wellcomei, with 3598 individuals (91.93%) (SISA = 0.92), followed by Ev. walkeri, with 306 individuals (7.82%) (SISA = 0.87). Only eight (0.20%; SISA = 0.47) and two (0.05%; (SISA = 0.44) individuals of Ev. evandroi and Lu. longipalpis, respectively, were captured (Table 6).
The numerical daily Ps. wellcomei occurrence was concentrated in the two twilight periods, as well as early and late at night (Fig. 4). The largest number of specimens were captured in June (89.47% of the total). Similar numbers were recorded in August, the month with the greatest diversity (Table 6), and in October, but the number was much less than during the first capture. The hours of greatest activity varied between the sampling months, with significant differences (Kruskal-Wallis H-test: H = 39.5829, GL = 23, P = 0.0171).
Discussion
Of the 75,499 sand flies belonging to 11 species caught with CDC traps, the most abundant were Ev. walkeri, Ps. wellcomei and Lu. longipalpis. The last two are vectors of the protozoa Leishmania infantum and L braziliensis, etiological agents of CL and VL in Brazil, respectively. A total of 3914 phlebotomines of the species Ps. wellcomei, Ev. walkeri, Ev. evandroi and Lu. longipalpis were captured in the Shannon traps. Analysis of the ecological relationships and behavior of the species revealed that in both collecting systems Ps. wellcomei stands out as dominant species in forest and degraded environments while Lu. longipalpis is present only rarely in the forest environment and is dominant in degraded environments. This result is reflected in the potential risk of transmission in an endemic area, although Ev. walkeri was the most abundant species in the area during the 3 years of the study.
The most abundant species in the CDC trap in all the ecotopes under study was Ev. walkeri, accounting for 79.7% of captures, while Lu. longipalpis accounted for 3.1% of captures. Comparison with an earlier study conducted in an ecologically isolated area in the same municipality, where Ev. walkeri represented only 7.46% of the phlebotomines captured and Lu. longipalpis 64.47% [15], shows a change in species composition, making fine-scale bioecological investigations important. Although Ev. walkeri is not associated with Leishmania transmission in the region, the species was recently found to be naturally infected with L. braziliensis in Acre State, northern Brazil [30]. Of the species collected in our study, Lu. longipalpis is the main vector of L. infantum, causing VL throughout the country, particularly in the northeastern region, with a large number of cases, resulting in death in up to 10% of the total cases [1, 31]. In the present study, Lu. longipalpis was poorly represented, when compared to an earlier study in which it accounted for > 70% of the phlebotomines collected, also in the municipality of Nísia Floresta, but in a peridomestic environment [32]. Changes in the composition and population dynamics of phlebotomines in the study area suggest phlebotomine species succession. All of the species exhibited preferential ground-level behavior, with Lu. longipalpis continuing to be found primarily in degraded or anthropized areas.
Psychodopygus wellcomei was dominant in the June collection, when 92% of all the individuals of this species were captured, a finding related to the rainy season, characterized by relatively higher relative humidity and lower temperatures. Given that the physiological, biochemical and behavioral processes of living beings are influenced by daily and seasonal variations, it is important to underscore the greater occurrence of Ps. wellcomei in the rainy season in Nísia Floresta, which is likely entering into diapause in the dry season, as reported in other studies conducted in the Brazilian Amazon and northeastern Brazil [33,34,35]. With respect to vertical stratification, Ps. wellcomei was observed in the tree canopies, which may be associated with the acquisition of carbohydrates in plant species or the female’s search for a blood meal in arboreal animals, such as birds, Callithrix jacchus (common marmosets) and D. albiventris (opossums), the species found in the area. Different degrees of forest cover may also reflect phlebotomine behavior. A large leaf index area provides suitable conditions for phlebotomine activities, even influencing natural flagellate infection rates [36]. Despite its greater abundance in the preserved forest area (Fig. 2), the presence of this species approximately 20 m from the edge of the conservation unit suggests a selection of new habitats and consequent expansion of the vital area of this species.
The correlation between the climate parameters and the number of phlebotomines reveals the influence of the former over longer time periods on phlebotomine abundance. The lagged correlation between rainfall and the phlebotomines collected showed significant results between the species Ev. evandroi, Ev. lenti, Ev. walkeri and Lu. longipalpis and rainfall during the 4 and 5 previous months, while Ps. wellcomei demonstrated a significant positive correlation with the rainfall of the collection month and the month directly preceding the collection time. Rainfall in the region is irregular, and with a low index, the development peaks of immature individuals likely occur in microhabitats when soil humidity and temperature conditions are favorable, as we observed in the laboratory. Psychodopygus wellcomei shows a close relationship with rainfall, revealing a strong presence in the rainy months over a 3-year study period.
In Rio Grande do Norte, despite the relatively low incidence of CL cases when compared to other Brazilian states, the observations and results obtained are important because Ps. wellcomei has become more abundant in recent years in forest fragments, predominating in forest environments [37] close to or within areas in which people and animals are active during the day. Studies in Amazonia have also shown that infected females are more frequently captured during the day than at night, making transmission by this phlebotomine species more common during the day [34, 38,39,40,41]. Nyssomyia whitmani, which is infrequently observed in the area, was found in the forest and anthropized area. This species requires our attention due to the species of Leishmania found in this phlebotomine and its involvement in CL and VL. With respect to Ev. lenti, there is a record of natural infection in southeastern and northeastern Brazil [42,43,44].
Although it was not possible to perform an analysis of infection, the natural investigation of infection by Leishmania associated with cases of CL is important in all the areas of occurrence of the species. Psychodopygus wellcomei is highly anthrophilic, but its epidemiological importance is restricted to CL cases in the Amazon region; it association with cases of CL has yet to be confirmed in the northeastern region [34, 37,38,39,40]. The Shannon trap 24-h captures reveal a large abundance of Ps. wellcomei, primarily females, which likely approach the traps not only attracted by the light source but also due to high species anthropophilia, being attracted by exhaled odors and CO2, as noted by their attempts to feed on the researchers [37, 45, 46]. Among the species collected, the vectorial capacity or competence of Lu. longipalpis for L. infantum and of Ps. wellcomei and Ny. whitmani for L. braziliensis has been described earlier [34], as has the presence of circulating parasites or detected DNA of some Leishmania species in Ev. walkeri, Ev. evandroi, Ev. lenti, Sc. sordellii and Ev. sallesi [2, 30, 34, 42,43,44,45,46,47,48,49,50,51,52,53] (Table 7). Despite the small sample size used to draw firm conclusions regarding daily phlebotomine activity patterns, it is important to underscore that the daily activity pattern of Ps. wellcomei is similar to that found in another biome, Amazonia, where the species feeds avidly and remains active during the day, primarily in cloudy weather [54]. In our study, Ps. wellcomei females were more active between 5 p.m. and 5 a.m., likely in search of hosts, with a lower occurrence outside of these hours. This information may be useful in preventing CL, since armed with this knowledge, people can avoid the occurrence sites of the vector at peak times or use preventive measures, such as repellent or adequate clothing.
Evandromyia evandroi was more abundant in peridomestic environments and in conserved forest, while Ev. lenti was more abundant at the edge of the forest and in peridomestic environments (Fig. 2), primarily on the rural property, which shows that it is adapted to modified environments. These species are less abundant in the peridomestic area of the residential condominium, likely because it was recently constructed and surrounded by walls that can act as flight barriers, while in the rural setting, the area is open and shaded, with possible food sources, such as fruit trees, chickens, ducks, dogs and horses.
Our observations on phlebotomines in the region reveal that those which occur during the day generally land on the hosts at twilight, remaining throughout the night and disappearing at daybreak, with the exception of female Ps. wellcomei, which remain active during the day, albeit at lower densities than at night, and absent only between 1 and 2 p.m. We found the phlebotomine Ev. walkeri to be active only at twilight and at night, with peak occurrences between 11 p.m. and 3 a.m.. The varying activity times of these insects may be related to competitive strategies for food sources. This competition occurs when different species colonize the same space and may become more intense as food sources are depleted [55, 56].
Finally, in relation to potential vectors, our analyses reveal greater abundance of Ps. wellcomei, a species with vectorial capacity/competence for L. braziliensis, in the conserved forest (Fig. 2), although it also occurs in the peridomestic environment where L. longipalpis, a vector species of L. infantum, is more abundant (Fig. 2). It is notable that it occurs in association with people and domestic animals, which calls attention to the risk of transmission. The expansion of human activities, with environmental impacts and changes in land use, produces new potentially occupiable niches, affects fauna composition and the vector reservoir and parasite behavior, in addition to their interrelationships, which may lead to changes in local leishmaniasis epidemiology.
Conclusions
This species abundance study reveals the predominance of Ev. walkeri in the forest and anthropized areas, followed by Ps. wellcomei, which exhibited the same distribution pattern, predominantly in the rainy season. The structure of the phlebotomine community is influenced by abiotic factors, interactions with plant and animal species and the degree of environmental disturbance. Conditions for L. braziliensis and L. infantum transmission to occur exist in the study area, namely primitive parasite reservoirs, susceptible vertebrate hosts, vectors and environmental conditions favorable to their development, in addition to being a region endemic for VL and CL. The seasonality and daily activity observed for Ps. wellcomei and Lu. longipalpis modulate the relation between the vector and vertebrate host and consequently the risk of infection. As such, these areas need to be protected and surrounding areas containing houses or agricultural activities in urban, periurban or rural settings should be permanently monitored to prevent the onset of VL or CL transmission cycles and outbreaks.
Available data and materials
Data supporting the study are included within the article.
Abbreviations
- CL:
-
Cutaneous leishmaniasis
- CU:
-
Conservation Unit
- INMET:
-
National Institute of Meteorology
- SISA:
-
Standardized index of species abundance
- VL:
-
Visceral leishmaniasis
References
Lainson R, Rangel BF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil—a review. Mem Inst Oswaldo Cruz. 2005;100:811–27.
Diniz MMCSL, Ovallos FG, Gomes CMC, Lavitschka CO, Galati EAB. Host-biting rate and susceptibility of some suspected vectors to Leishmania braziliensis. Parasites Vectors. 2014;7:139.
Martins-Melo FR, Carneiro M, Ramos AN, Heukelbach J, Ribeiro ALP, Werneck GL. The burden of neglected tropicaldDiseases in Brazil, 1990–2016: a subnational analysis from the Global Burden of Disease Study 2016. PLoS Negl Trop Dis. 2018;12:1–24. https://doi.org/10.1371/journal.pntd.0006559.
Okwor I, Uzonna J. Social and economic burden of human leishmaniasis. Am J Trop Med Hyg. 2016;94:489–93.
World Health Organization (WHO). Leishmaniasis situation and trends. https://www.who.int/gho/neglected_diseases/leishmaniasis/en/. Accessed 22 Oct 2019.
Queiroz RG, Vasconcelos IA, Vasconcelos AW, Pessoa FA, Sousa RN, David JR. Cutaneous leishmaniasis in Ceará state in Northeastern Brazil: incrimination of Lutzomyia whitmani (Diptera: psychodidae) as a vector of Leishmania Braziliensis in Baturité municipality. Am J Trop Med Hyg. 1994;50:693–8.
Teodoro U, Alberton D, Kühl JB, Santos ES, Santos DR, Santos AR, et al. Ecologia de Lutzomyia (Nyssomyia) whitmani em área urbana do município de Maringá, Paraná. Rev Saude Publica. 2003;37:651–6.
Azevedo ACR, Rangel EF, Queiroz RG. Lutzomyia migonei (França 1920) naturally infected with peripylarian flagellates in Baturité, a focus of cutaneous leishmaniasis in Ceará State, Brazil. Mem Inst Oswaldo Cruz. 1990;85:479.
Sistema de Informação de Agravos de Notificação (SINAN). Leishmaniose tegumentar e leishmaniose visceral por Município de Residência no Estado do Rio Grande do Norte, Brasil: 2007-2017. 2019. http://sinan.saude.gov.br/sinan. Accessed 12 May 2019.
Ministério da Saúde, Secretaria de Vigilância em Saúde, Sistema de Notificação de Agravos. Casos confirmados de Leishmaniose Visceral e Tegumentar, Brasil, Grandes Regiões e Unidades Federadas. 1990 a 2017. 2018. http://sinan.saude.gov.br/sinan/login/login.jsf. Accessed 12 Apr 2018.
Marzochi MCA, Marzochi KBF. Tegumentary and visceral leishmaniases in Brazil—emerging anthropozoonosis and possibilities for their control. Cad Saude Pública. 1994. https://doi.org/10.1590/S0102-311X1994000800014.
Vale ECS, Furtado T. Leishmaniose tegumentar no Brasil: revisão histórica da origem, expansão e etiologia. An Bras Dermatol. 2005;80:421–8.
Freitas MTS, Ríos-Velasquez CM, Costa CRL, Figueirêdo CAS, Aragão NC, Silva LG, et al. Phenotypic and genotypic variations among three allopatric populations of Lutzomyia umbratilis, main vector of Leishmania guyanensis. Parasites Vectors. 2015;8:448.
Rangel EF, Lainson R, Souza AA, Ready P, Azevedo ACR. Variation between geographical populations of Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) sensu lato (Diptera:Psychodidae:Phlebotominae) in Brazil. Mem Inst Oswaldo Cruz. 1996;91:43–50.
Ximenes MFFM, Castellón EG, Souza MF, Freitas RA, Pearson RD, Wilson ME, et al. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in the State of Rio Grande do Norte, Brazil. J Med Entomol. 2000;37:162–9.
Ximenes MFFM, Castellon EG, Souza MF, Menezes AAL, Queiroz JW, Silva VPM, et al. Effect of abiotic factors on seasonal population dynamics of Lutzomyia longipalpis (Diptera: Psychodidae) in Northeastern Brazil. J Med Entomol. 2006;43:990–5.
Instituto Brasileiro de Geografia e Estatística (IBGE). Estimativa da População 2018, Nísia Floresta, Rio Grande do Norte, Brasil. 2018. https://cidades.ibge.gov.br/brasil/rn/nisia-floresta/panorama. Accessed 12 June 2019.
Ministério do Meio Ambiente (MMA). Plano de Manejo da Floresta Nacional de Nísia Floresta (Volume 1). 2017. http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/Volume_I_Diagnóstico_02ago12.pdf. Accessed 1 Jul 2019.
Lainson R, Shaw JJ, Lins ZC. 1969. Leishmaniasis in Brazil IV. The fox, Cerdocyon thous (L.) as a reservoir of Leishmania donovani in Para state, Brazil. Trans R Soc Trop Med Hyg. 1969;63:741–5.
Roque ALR, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl. 2014;3:251–62.
Shannon RC. Methods for collecting and feeding mosquitoes in jungle yellow fever studies 1. Am J Trop Med Hyg. 1939;19(Suppl. 1):131–40.
Vilela ML, Rangel EF, Lainson R. Métodos de coleta e preservação de flebotomíneos. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Editora Fiocruz; 2003. p. 353–67.
Galati EAB. Morfologia e Taxonomia: Morfologia, terminologia de adultos e de comunicação dos táxons da América. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Editora Fiocruz; 2003. p. 53–175.
National Institute of Meteorology (INMET). Banco de Dados Meteorológicos para Ensino e Pesquisa. 2016. http://inmet.gov.br/portal/index.php?r=bdmep/bdmep. Accessed 12 Dec 2018.
El-Gohary M, McNames J, Ellis T, Goldstein B. Time delay and casuality in biological systems using whitened cross-correlation analysis. In: Conference Proceedings of the IEEE Engineering in Medicine and Biology Society, 2006; 2006, p. 6169–6172. https://doi.org/10.1109/IEMBS.2006.260255
Shumway RH, Stoffer DS. Time series analysis and its applications. New York: Springer; 2011.
Roberts DR, Hsi BP. An index of species abundance for use with mosquito surveillance. Environ Entomol. 1979;8:1007–13.
Magurran AE. Ecological diversity and its measurement. London: Cambridge University Press; 1988.
Silveira Neto S, Nakano O, Barbin D, Nova NAV. Manual de ecologia dos insetos. São Paulo: Ceres; 1976.
Ávila MM, Brilhante AF, De Souza CF, Bevilacqua PD, Galati EAB, Brazil RP. Ecology, feeding and natural infection by Leishmania spp. of phlebotomine sand flies in an area of high incidence of American tegumentary leishmaniasis in the municipality of Rio Branco, Acre, Brazil. Parasites Vectors. 2018;11:1–12.
Jeronimo SMB, Oliveira RM, Mackay S, Costa RM, Sweet J, Nascimento ET, et al. An urban outbreak of visceral leishmaniasis in Natal, Brazil. Trans R Soc Trop Med Hyg. 1994;88:386–8.
Ximenes MDFFDM, Silva VPME, Queiroz PVS De, Rego MM, Cortez AM, Batista LMDM, et al. Flebotomíneos (Diptera: Psychodidae) e leishmanioses no Rio Grande do Norte, Nordeste do Brasil: reflexos do ambiente antrópico. Neotrop Entomol. 2007;36:128–37. https://doi.org/10.1590/s1519-566x2007000100016
Pinheiro MPG, Silva JHT, Inacio CLS, Ximenes MFFM. Anthropophily of Lutzomyia wellcomei (Diptera: Psychodidae) in an Atlantic Forest conservation unit in northeast Brazil. J Med Entomol. 2016;53:1444–8.
Rangel EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: Aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009;104:937–54.
Tachinardi P. Efeitos das variações de temperatura ambiental em ritmos circadianos. Rev da Biol. 2012;9:13–8.
Vasconcelos Dos Santos T, Prévot G, Ginouvès M, Duarte R, Silveira FT, Póvoa MM, et al. Ecological aspects of phlebotomines (Diptera: Psychodidae) and the transmission of American cutaneous leishmaniasis agents in an Amazonian/Guianan bordering area. Parasit Vectors. 2018;11:1–13.
Pinheiro MPG, Silva MMM, Júnior JBS, Silva JHT, Alves ML, Ximenes MFFM. Sand flies (Diptera, Psychodidae, Phlebotominae), vectors of Leishmania protozoa, at an Atlantic Forest Conservation Unit in the municipality of Nísia Floresta, Rio Grande do Norte state, Brazil. Parasites Vectors. 2016;9:83.
Lainson R, Shaw JJ, Ward RD, Fraiha H. Leishmaniasis in Brazil: IX. Considerations on the Leishmania braziliensis complex: Importance of sand flies of the genus Psychodopygus (Mangabeira) in the transmission of L. braziliensis braziliensis in North Brazil. Trans R Soc Trop Med Hyg. 1973;67:184–96.
Wilkes TJ, Ready PD, Lainson R, Killick-Kendrick R. Biting periodicities of nulliparous and parous females of Psychodopygus wellcomei. Trans R Soc Trop Med Hyg. 1984;78:846–7.
Ryan L, Lainson R, Shaw JJ. Leishmaniasis in Brazil. XXIV. Natural flagellate infections of sand flies (Diptera: Psychodidae) in Pará State, with particular reference to the role of Psychodopygus wellcomei as the vector of Leishmania braziliensis braziliensis in the Serra dos Carajás. Trans R Soc Trop Med Hyg. 1987;81:353–9.
Ready PD, Ribeiro AL, Lainson R, de Alencar JE, Shaw JJ. Presence of Psychodopygus wellcomei (Diptera: Psychodidae), a proven vector of Leishmania braziliensis braziliensis, in Ceará State. Mem Inst Oswaldo Cruz. 1983;78:235–6.
Lopes JV, Michalsky EM, Pereira NCL, de Paula AJV, Lara-Silva FO, Silva-Lana R, et al. Entomological Studies in Itaúna, Brazil, an area with visceral leishmaniasis transmission: fauna survey, natural Leishmania infection, and molecular characterization of the species circulating in phlebotomine sand flies (Diptera: Psychodidae). J Med Entomol. 2019;56:1368–76.
Margonari C, Soares RP, Andrade-Filho JD, Xavier DC, Saraiva L, Fonseca AL, et al. Phlebotomine sand flies (Diptera: Psychodidae) and Leishmania infection in Gafanhoto Park, Divinópolis, Brazil. J Med Entomol. 2010;47:1212–9. https://doi.org/10.1603/ME09248.
Rêgo FD, Rugani JMN, Shimabukuro PHF, Tonelli GB, Quaresma PF, Gontijo CMF. Molecular detection of Leishmania in phlebotomine sand flies (Diptera: Psychodidae) from a cutaneous leishmaniasis focus at Xakriabá Indigenous Reserve, Brazil. PLoS One. 2015;10:1–14.
Alexander B. Sampling methods for phlebotomine sand flies. Med Vet Entomol. 2000;14:109–22.
Galati EAB, Nunes VLB, Dorval MEC, Cristaldo G, Rocha HC, Gonçalves-Andrade RM, et al. Attractiveness of black Shannon trap for phlebotomines. Mem Inst Oswaldo Cruz. 2001;96:641–7.
Chagas ECDS, Silva AS, Fé NF, Ferreira LS, Sampaio VDS, Terrazas WCM, et al. Composition of sand fly fauna (Diptera: Psychodidae) and detection of Leishmania DNA (Kinetoplastida: Trypanosomatidae) in different ecotopes from a rural settlement in the central Amazon, Brazil. Parasites Vectors. 2018;11:1–10.
Souza NA, Brazil RP, Araki AS. The current status of the Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) species complex. Mem Inst Oswaldo Cruz. 2017;112:161–74.
Souza AAA, dos Santos TV, Jennings YLL, Ishikawa EAY, Barata I da R, Silva M das GS, et al. Natural Leishmania (Viannia) spp. infections in phlebotomine sand flies (Diptera: Psychodidae) from the Brazilian Amazon region reveal new putative transmission cycles of American cutaneous leishmaniasis. Parasite. 2016;23:22.
Carvalho GML, Brazil RP, Rego FD, Ramos MCNF, Zenobio APLA, Andrade Filho JD. Molecular Detection of Leishmania DNA in wild-caught phlebotomine sand flies (Diptera: Psychodidae) from a cave in the State of Minas Gerais, Brazil. J Med Entomol. 2017;54:196–203.
Pereira-Filho AA, Fonteles RS, Da Conceição Abreu Bandeira M, Moraes JLP, Rebêlo JMM, Melo MN. Molecular identification of Leishmania spp. in Sand Flies (Diptera: Psychodidae: Phlebotominae) in the Lençóis Maranhenses National Park, Brazil. J Med Entomol. 2018;55:989–94.
Guimarães-E-Silva AS, De Oliveira Silva S, Da Silva RCR, Pinheiro VCS, Rebêlo JMM, Melo MN. Leishmania infection and blood food sources of phlebotomines in an area of Brazil endemic for visceral and tegumentary leishmaniasis. PLoS One. 2017;12:1–19.
Moya SL, Giuliani MG, Santini MS, Quintana MG, Salomón OD, Liotta DJ. Leishmania infantum DNA detected in phlebotomine species from Puerto Iguazú City, Misiones province, Argentina. Acta Trop. 2017;172:122–4.
Fraiha H, Shaw JJ, Lainson R. Phlebotominae brasileiros: II Psychodopygus wellcomei, nova espécie antropófila de flebótomo do grupo squamiventris, do Sul do Estado do Pará, Brasil (Diptera, Psychodidae). Mem Inst Oswaldo Cruz. 1971;69:489–500.
Natal D, Gonçalves EFB, Taveira LA. Proliferação de mosquitos (Diptera, Culicidae) em cemitérios e perspectivas de controle. Iesus. 1997;6:103–10.
Volf P, Hostomska J, Rohousova I. Molecular crosstalks in Leishmania-sand fly-host relationships. Parasite. 2008;15:237–43.
Acknowledgments
We thank the Chico Mendes Institute of Biodiversity (ICMBio) and biologist Patrícia Macêdo, head of the Nísia Floresta National Forest, for her permission and support. The National Council of Scientific and Technological Development (CNPq – 552004/2011-1) and the Coordination for the Improvement of Higher Education Personnel (CAPES) for funding. We thank Carlos Eduardo, Ivan de Oliveira and José Hilário for their contribution to the field collections.
Funding
The National Research Council for Scientific and Technological Development (CNPq – 552004/2011-1) and the Coordination for the Improvement of Higher Education Personnel (CAPES) provided funding.
Author information
Authors and Affiliations
Contributions
MPGP and MFFMX were involved to the experimental design. MPGP, CLSI, MMMS and PSFA collected and identified insects and analyzed the data. MPGP, CLSI, MMMS and MFFMX prepared the manuscript. MFFMX coordinated the project and financial resources, provided supervision and assisted with analysis and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Authorized by the Chico Mendes institute for Biodiversity Conservation (ICMBio/SISBIO, protocol number 32738-4)
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pinheiro, M.P.G., Silva-Inacio, C.L., Silva, M.M.d. et al. Potential vectors of Leishmania spp. in an Atlantic Forest conservation unit in northeastern Brazil under anthropic pressure. Parasites Vectors 14, 38 (2021). https://doi.org/10.1186/s13071-020-04523-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04523-2