Abstract
Background
Environmental conditions in northern Spain allow the development of different arthropods involved in the transmission of significant canine vector-borne pathogens. The aim of the study was to systematically assess seroprevalence rates for Leishmania infantum, Ehrlichia canis, Anaplasma spp., Dirofilaria immitis and Borrelia burgdorferi, and risk factors in dogs from all regions of the north of Spain.
Methods
A total of 556 dogs were included in this study between January 2017 and December 2018, belonging to 30 practices covering all regions in northern Spain (Galicia, Asturias, Cantabria, Basque Country, Navarra, Aragon and Catalonia). All practices were located in the north of every region. Blood samples were analyzed using the 4DX SNAP® test (IDEXX Laboratories, Westbrook, Maine, USA) for the detection of D. immitis antigen and E. canis, B. burgdorferi and Anaplasma spp. antibodies. Leishmania SNAP® test (IDEXX Laboratories) was used for detection of L. infantum antibodies. Associations between prevalence of canine vector-borne pathogens, epidemiological and clinical signs data were statistically analyzed.
Results
The overall prevalence rates were 8.99% for L. infantum, 1.26% for Anaplasma spp., 0.9% for E. canis, 0.72% for B. burgdorferi, and 0.18% for D. immitis. Globally, 11.33% of the dogs included in the study were positive to any tested vector-borne pathogen. Leishmania infantum seroprevalence was the highest and the only one detected in all the regions. Leishmania infantum seropositivity was associated with age > 10 years-old, outdoor access, anemia, fever, dermatological signs, lympadenomegaly, muscular atrophy, ocular signs and renal disease. Ehrlichia canis seropositivity was associated with the summer season and living in urban areas. Apathy, weakness, anorexia, weight loss, anemia, fever and gastrointestinal clinical signs were also associated with E. canis antibody detection. Living in a rural area was also a risk factor for Anaplasma spp. and B. burgdorferi seropositivity.
Conclusions
To our knowledge, this is the first multicenter survey performed in northern Spain assessing different canine vector-borne diseases from all regions. Results show the presence of autochthonous cases of these diseases. The vector-borne pathogens found in this study should be included in the differential diagnosis in dogs from some areas previously considered non-endemic for these pathogens.

Similar content being viewed by others
Background
Canine vector-borne pathogens are a range of microorganisms transmitted by ectoparasites mainly ticks, fleas, mosquitoes and sand flies. The distribution ranges of these agents are increasing worldwide and their epidemiology seems to be constantly evolving due to factors such as climate change, movement of companion animals travelling with their owners, the development of outdoor activities, and environmental changes including the creation of recreational parks with forest fragmentation and the potential increased contact with vectors and sylvatic reservoirs, among others [1, 2].
The north of Spain has been traditionally considered a non-endemic area for some of the vector-borne pathogens usually found in the rest of the country, for example Leishmania infantum, mainly due the specific climate conditions of this area [3, 4]. The north and northwest of Spain constitute one of the biogeographical regions of the country, with a humid Atlantic climate, with mild winters and summers [3, 5]. The regions near the Pyrenees (Navarra, north of Aragon, and northwest of Catalonia) have a Continental climate, with cold winters and mild summers [6]. The northeast of the country is included in the Mediterranean biogeographical region, being also wet due to the influence of the Mediterranean Sea, with mild winters and hot summers [5, 6].
Thus, climatic properties found in northern Spain allow the detection of different arthropod vectors frequently affecting dogs, such as Ixodes spp., Dermacentor spp., Rhipicephalus sanguineus and Phlebotomus perniciosus [6, 7]. Some of the pathogens potentially transmitted by these vectors are remarkable not only from the animal health point of view but also within the framework of human health. Canine vector-borne diseases include parasitic diseases such as babesiosis, dirofilariosis and leishmaniasis, and bacterial diseases such as anaplasmosis, borreliosis and ehrlichiosis.
Leishmaniasis caused by L. infantum is a zoonotic disease with the dog as the main reservoir in Spain, where it is transmitted by phlebotomine sand flies of the genus Phlebotomus. Canine leishmaniasis constitutes a systemic chronic disease characterized by several clinical presentations from subclinical to severe and fatal disease [4]. Clinical signs include lymphadenopathy, dermatitis, alopecia, uveitis, onychogryphosis, lameness, weight loss, cachexia, epistaxis, anemia and proteinuria, among many others [4, 8]. Previous studies have reported low seroprevalence rates for L. infantum in northern Spain (except in some areas of northeast and northwest of the country) [3, 7, 9]. In some autonomous communities (the Basque Country, Navarra or Aragon) seroprevalence studies in dogs are scarce or even absent in owned dogs. Some prevalence studies performed in stray dogs and wild reservoirs [10], human population [11] and sand flies [12] in these areas suggest a potential underestimation of the prevalence in dogs from northern Spain.
Dirofilaria immitis is a nematode transmitted by mosquitoes of the genera Aedes, Anopheles and Culex. This parasitic infection causes a disease known as dirofilariosis or heartworm disease because parasitic adult worms live in the right side of the heart and pulmonary arteries [13, 14]. Dirofilaria immitis has been previously reported and associated with dogs living in the Mediterranean basin (which provides optimum temperature and humidity to viable mosquito population) but is not endemic in northwestern and north-central areas of Spain [9]. The clinical signs associated with dirofilariosis include exercise intolerance, dry chronic cough, weakness, weight loss, epistaxis, cyanosis and pulmonary edema [15].
Canine monocytic ehrlichiosis is a tick-borne bacterial disease transmitted by R. sanguineus with Ehrlichia canis as the causative agent [16]. Previous studies have described a wide distribution in the country and high seroprevalence rates of E. canis in dogs from some areas of northern Spain [9]. Clinical signs for ehrlichiosis include weakness, lethargy, exercise intolerance, fever, anorexia, weight loss, lymphadenomegaly, splenomegaly, hepatomegaly, diarrhea, vomiting, hemorrhage, epistaxis, uveitis, and respiratory and sometimes neurological signs [17].
The species of Anaplasma affecting dogs in Spain are A. phagocytophilum (mainly transmitted by Ixodes ricinus in Europe), the causative agent of canine granulocytic anaplasmosis, which may produce a zoonotic disease [18, 19], and A. platys, the causative agent of thrombocytopenic anaplasmosis and transmitted by R. sanguineus [17]. Infection with Anaplasma spp. can be asymptomatic or cause some unspecific clinical signs. Clinical signs of granulocytic anaplasmosis are fever, lethargy, anorexia, splenomegaly, and sometimes neurological and orthopedic signs [17]. Thrombocytopenic anaplasmosis affects platelets and clinical signs include fever, lethargy, anorexia, weight loss, pale mucous membranes, petechiae, nasal discharge and lymphadenomegaly [17]. Antibodies against Anaplasma spp. have been detected in recent studies throughout the country [9], and Anaplasma spp. have also been detected in ticks collected from dogs in some areas of the north [6]. Anaplasma spp. infect a wide variety of domestic and wild vertebrate hosts [17].
Finally, the spirochete Borrelia burgdorferi also affects a wide variety of hosts including dogs and humans, causing Lyme disease, and is transmitted by I. ricinus [20]. Most infected dogs remain without clinical signs and, when presented, are unspecific. Borreliosis has been associated with hyperthermia, anorexia, lameness, lymphadenopathy and glomerulonephritis [20]. Antibodies against B. burgdorferi have been reported in wild canids [5] and in owned dogs [21] in some areas of Spain.
To the best of our knowledge, these vector-borne pathogens have never been evaluated in some areas in the north of Spain. Thus, the aims of the study were to systematically determine the seroprevalence of selected vector-borne pathogens (Leishmania infantum, Ehrlichia canis, Anaplasma spp., Dirofilaria immitis and Borrelia burgdorferi) in dogs with and without suggestive clinical signs of a vector-borne disease, and the assessment of epidemiological variables as a possible risk factor in all regions of northern Spain.
Methods
Blood samples and questionnaire with epidemiological data
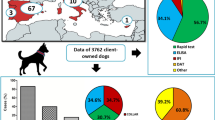
A multicenter study was performed recruiting a total of 556 dog blood samples from 30 private veterinary practices belonging to seven autonomous communities: Galicia (n = 4; 80 dogs); Asturias (n = 4; 79 dogs); Cantabria (n = 5; 91 dogs); the Basque Country (n = 4; 80 dogs); Navarra (n = 4; 72 dogs); northern Aragon (n = 4; 57 dogs); and northern Catalonia (n = 5; 97 dogs). In communities with several provinces, samples were systematically collected from the most northern provinces from every autonomous community. Specifically, samples were collected from A Coruña and Lugo in Galicia, Bizkaia and Gipuzkoa in the Basque Country, Huesca in Aragon and Barcelona, Girona and Lleida in Catalonia (Fig. 1).
Local veterinarians of each area were asked to randomly collect blood samples and complete a questionnaire with clinical and epidemiological data for a maximum of 10 dogs without clinical signs and 10 dogs with clinical signs, suggestive of any vector-borne diseases, including anaplasmosis, borreliosis, dirofilariosis, ehrlichiosis and leishmaniasis.
Clinical signs suggestive for each of these diseases included in the study were defined as follows: fever, anorexia, lethargy, lameness, lymphadenopathy, diarrhea and vomiting, epistaxis, and splenomegaly for anaplasmosis [17]; fever, apathy, weakness, anorexia, weight loss, lymphadenomegaly, splenomegaly, hepatomegaly, epistaxis and neurological signs for ehrlichiosis [17]; weight loss, apathy, exercise intolerance, dyspnea/tachypnea, cough, tachycardia and vena cava syndrome for dirofilariosis [13]; lymphadenomegaly, dermatological signs, ocular signs, weight loss, muscular atrophy, lethargy, anemia, epistaxis, hepatomegaly, splenomegaly, lameness, proteinuria, and fever for leishmaniasis [8]; and lameness, fever, anorexia and proteinuria for borreliosis [20].
Additionally, epidemiological data were recorded from each dog regarding: date of the sample collection; signalment; tick infestation at the time of the physical exam; use of ectoparasiticides; environmental and lifestyle conditions; and travel history.
The only selection criteria for the clinics were to be small animal or mixed practices located in the north of Spain, and to accept to collaborate in the study.
Whole blood was obtained for each dog in EDTA tubes between January 2017 and December 2018 to evaluate the seroprevalence of the different vector-borne pathogens assessed in this study.
Two commercial rapid diagnostic tests, the 4DX SNAP® Test (IDEXX Laboratories, Westbrook, ME, USA) and the Leishmania SNAP® Test (IDEXX Laboratories) were used for D. immitis antigen-detection, and E. canis, B. burgdorferi, Anaplasma spp. and L. infantum antibody detection, in blood samples according to the manufacturer’s instructions. A previous study reported sensitivity and specificity, respectively, using the 4Dx SNAP® Test for antigen detection of D. immitis (99.2%, 100%) and antibody detection of E. canis (96.2%, 100%), B. burgdorferi (98.8%, 100%) and A. phagocytophilum (99.1%, 100%) [14]. On the other hand, the sensitivity and specificity for the Leishmania SNAP® Test were 96.3% and 99.2%, respectively, as per the manufacturer’s instructions (IDEXX Laboratories).
Statistical analysis
Statistical associations between the results obtained from the different serological tests and the epidemiological and clinical data were assessed using Chi-square test or Fisher’s exact test for categorical variables, where appropriate. Confidence interval (95% CI) values were also calculated. Bonferroni-correction for P-values was applied for seroprevalence pairwise comparisons. Odds ratios (OR) were also obtained to assess different risk factors. Student’s t-test was used to compare means of numerical data. The significance level was established at P < 0.05. Statistical analysis was carried out using the software SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Epidemiological data are shown in Table 1. A total of 63 out of the 556 dogs (11.33 ± 1.3%) were seropositive to any vector-borne pathogen tested. The overall seroprevalence for each pathogen was as follows: 8.99 ± 1.2% (50/556) for L. infantum; 1.26 ± 0.5% (7/556) for Anaplasma spp.; 0.9 ± 0.4% (5/556) for E. canis; 0.72 ± 0.4% (4/556) for B. burgdorferi, and 0.18 ± 0.2% (1/556) for D. immitis. Detailed distribution of seropositivity to the different pathogens by region is provided in Fig. 1. Only 3 dogs (0.54 ± 0.3%) were seropositive to two different pathogens (L. infantum + E. canis, L. infantum + Anaplasma spp. and E. canis + Anaplasma spp.). There was no statistically significant association between seropositivity to more than one agent and the different evaluated variables.
Clinical signs were found in more than half of dogs included in the study, 328 (58.99%) out of 556 dogs (Table 2). Specifically, clinical signs suggestive of these vector-borne diseases were found in 283/556 (50.9%) dogs. Among them, 18.02 ± 2.3% (n = 51) were seropositive to any pathogen tested. Individually, 15.19 ± 2.1% (n = 43) were seropositive to L. infantum, 1.77 ± 0.8% (n = 5) to E. canis, 1.06 ± 0.4% (n = 3) to Anaplasma spp., 0.71 ± 0.6% (n = 2) to B. burgdorferi, and 0.35 ± 0.4% (n = 1) to D. immitis. These rates were significantly higher in the subset of dogs with clinical signs in the case of L. infantum (OR: 6.8, 95% CI: 3.00–15.42, P < 0.0001), and in the case of seropositivity for any vector-borne pathogen tested (OR: 4.78, 95% CI: 2.49–9.19, P < 0.0001).
Considering the group without suggestive clinical signs of the same diseases (49.1%, n = 273), the seroprevalence rates were 4.4 ± 1.2% (n = 12) for any pathogen tested. When it was evaluated individually these rates were 2.56 ± 1% (n = 7) for L. infantum, 1.47 ± 0,7% (n = 4) for Anaplasma spp., and 0.73 ± 0.5% (n = 2) for B. burgdorferi.
When evaluating the statistical association between presence of ticks and detection of antibodies against different tick vector-borne agents no significant associations were detected: Anaplasma spp. (OR: 3.14, 95% CI: 0.60–6.54, P = 0.18); E. canis (OR: 1.01, 95% CI: 1.00–1.02, P = 1); and B. burgdorferi (OR: 2.59, 95% CI: 0.26–25.25, P = 0.39). However, the use of ectoparasiticide treatment was statistically associated with the absence of ticks during physical examination of the dogs (χ2 = 27.09, df = 1, P < 0.001).
Leishmania infantum was the most prevalent pathogen and the only one detected in all the autonomous communities included in the present study. The highest seroprevalence (24.56 ± 5.7%) in northern Spain was detected in Aragon. In fact, L. infantum was significantly more prevalent in this community than in all the other communities included in the study, except for Navarra (18.06 ± 4.5%) and Catalonia (13.4 ± 3.5%). Asturias presented the lowest seroprevalence for L. infantum (1.27 ± 1.3%) (Fig. 1; Table 1). Dogs seropositive to L. infantum without travel history, or with travel only to other northern regions of the country, were detected in all the regions included in the study. Dogs seropositive for L. infantum were older (6.97 ± 3.80 years-old) than seronegative dogs (5.37 ± 3.64 years-old) (t(541) = 2.88, P = 0.0042). Specifically, seropositivity for L. infantum was higher in dogs older than 10 years (OR: 2.38, 95% CI: 1.12–5.07, P = 0.0203).
Dogs living outdoors (n = 23, 14.02%) were significantly associated with L. infantum antibody detection (OR: 2.13, 95% CI: 1.18–3.85, P = 0.0101). Fifty-two per cent (26/50) of the positive dogs were living in rural areas (OR: 1.66, 95% CI: 0.93–2.98, P = 0.08).
A total of 32 out of 50 seropositive dogs had suggestive clinical signs of canine leishmaniasis (OR: 7.69, 95% CI: 4.14–14.28, P < 0.0001). Significant associations were detected between L. infantum seropositivity and anemia (OR: 2.04, 95% CI: 1.05–3.95, P = 0.0316), fever (OR: 0.31, 95% CI: 0.09–1.01, P = 0.04), dermatological signs (OR: 4.14, 95% CI: 2.17–7.91, P < 0.0001), lymphadenomegaly (OR: 8.78, 95% CI: 4.49–17.19, P < 0.0001), muscular atrophy (OR: 6.07, 95% CI: 2.83–13.03, P < 0.0001), ocular alterations (OR: 4.00, 95% CI: 1.69–9.49, P = 0.0038), and renal disease (OR: 3.15, 95% CI: 1.21–8.21, P = 0.0266).
The overall prevalence for D. immitis was 0.18 ± 0.2% (Fig. 1). Dirofilaria immitis antigen was detected only in a 3-year-old male German shepherd from Navarra, without travel history (1.39 ± 1.34%). The clinical signs were apathy and jaundice, and the dog was also positive in a previous test for leptospirosis. It was a guard dog that lived in a farm outside and without prophylactic treatments against arthropods.
Antibodies against E. canis were detected in 5 out of 556 dogs (0.9 ± 0.4%). Aragon was the region with the highest prevalence (1.75 ± 1.7%). All dogs from Cantabria and Galicia included in this study were negative (Fig. 1). None of the seropositive dogs had ever travelled out of their region of origin.
Four of the five (80%) canine blood samples positive to E. canis were collected during summer (OR: 14.95, 95% CI: 1.65–135, P = 0.0088).
All the dogs positive to E. canis belonged to the subset of dogs with suggestive signs of vector-borne diseases (OR: 10.8, 95% CI: 0.59–196.3, P = 0.06), and three of them had clinical signs clearly suggestive of ehrlichiosis (OR: 5.22, 95% CI: 0.86–31.59, P = 0.0795).
Regarding clinical signs, significant associations were detected between E. canis antibody detection and apathy (OR: 21.38, 95% CI: 1.18–388.80, P = 0.0047), weakness (OR: 10.6, 95% CI: 1.17–95.56, P = 0.023), anorexia (OR: 42.97, 95% CI: 2.36–782.92, P = 0.0004), weight loss (OR: 7.2, 95% CI: 1.19–43.68, P = 0.046), anemia (OR: 7.48, 95% CI: 1.23–45.42, P = 0.0373), fever (OR: 8, 95% CI: 1.32–48.58, P = 0.0321), and gastrointestinal signs (OR: 15.37, 95% CI: 2.51–94.19, P = 0.0068).
Seropositivity to Anaplasma spp. was 1.26 ± 0.5% (n = 7), Asturias being the area with highest prevalence rate (2.53 ± 1.8%). Anaplasma spp. were not detected in any dog included in the study from Galicia, Navarra and Catalonia (Fig. 1, Table 1). Five out of seven samples were collected in colder months, but no statistically significant differences were found (OR: 2.6, 95% CI: 0.50–13.49, P = 0.2802). Finally, 6 out of the 7 seropositive dogs were living in rural areas (OR: 8.99, 95% CI: 1.07–75.17, P = 0.0199). A total of 3 out of 7 presented clearly suggestive clinical signs of anaplasmosis (OR: 5.3, 95% CI: 1.16–24.22, P = 0.0478).
Antibodies against B. burgdorferi were only detected in Asturias (2.53 ± 1.8%, n = 2), and Catalonia (2.06 ± 1.4%, n = 2). None of the positive dogs had previously travelled out of their area of origin. The four dogs seropositive for B. burgdorferi were living in a rural area (OR: 13.39, 95% CI: 0.71–249.89, P = 0.0268).
When considering all the dogs seropositive to any evaluated vector-borne pathogen in this study, the prevalence rate was 11.33 ± 1.3% (n = 63). A total of 51 out of 63 dogs had clinical signs compatible with vector-borne diseases (OR: 4.78, 95% CI: 2.49–9.19, P < 0.0001) and 35 out of 63 were living in a rural area (OR: 1.98, 95% CI: 1.17–3.37, P = 0.0103).
Fifty-two out of these 63 dogs presented some clinical signs (OR: 3.72, 95% CI: 1.89–7.29, P < 0.0001). Specifically, there was a significant relationship between seropositivity to any pathogen of the study and anorexia (OR:1.74, 95% CI: 0.97–3.11, P = 0.05), weigh loss (OR: 1.88, 95% CI: 1.03–3.44, P = 0.0384), anemia (OR: 2.16, 95% CI: 1.19–3.93, P = 0.01), dermatological signs (OR: 3.18, 95% CI: 1.72–5.89, P = 0.0001), lymphadenomegaly (OR: 6.7, 95% CI: 3.53–12.71, P < 0.0001), muscular atrophy (OR: 4.4, 95% CI: 2.09–9.29, P < 0.0003), and ocular alterations (OR: 2.97, 95% CI: 1.27–6.96, P = 0.0165).
With regard to age, the group of dogs seropositive to any vector-borne pathogen was older (6.70 ± 3.62 years-old) than the seronegative group (5.46 ± 3.66 years-old) (t(541) = 2.69, P = 0.0074). In this sense, the puppy group (2/63, 3.17%) had lower positivity to vector-borne pathogens when compared with the remaining age groups (61/63, 96.82%) (OR: 0.22, 95% CI: 0.05–0.96, P = 0.0277).
Discussion
To the best of our knowledge, this is the first multicenter survey performed assessing different canine vector-borne pathogens in all the autonomous communities of northern Spain, and a total of 11.33 ± 1.3% of dogs were seropositive. Previous studies on other vector-borne pathogens have been performed in this area of Spain, focusing particularly on Babesia spp. and L. infantum [3, 21,22,23,24,25,26,27]. Therefore, this study emerges from the need to provide support to the veterinary practitioners working within the different regions of northern Spain, by identifying potential vector-borne pathogens, clinical signs and risk factors to be considered.
The study shows different prevalence rates in the seven autonomous communities of northern Spain (Fig. 1). These differences could be based on climatic conditions (temperature and humidity), the associated presence of the vectors involved in the life-cycle of the pathogens, and the relationship between dogs and their respective vectors considering a wide range of risk factors [6, 9].
Irrespective of the differences observed in prevalence, a remarkable finding is the presence of autochthonous cases in all the northern regions of Spain, taking into account the detection of seropositive dogs to the evaluated vector-borne pathogens without travel history or without travel history out of the regions included in the study.
The prevalence of L. infantum was found to be considerably variable depending on the area of study (from 24.56 ± 5.7% in northern Aragon to 1.27 ± 1.3% in Asturias). In fact, when comparing the autonomous communities, Aragon, Navarra and Catalonia, were the regions with the highest seroprevalence rates of L. infantum, probably due to the adequate environment that allows sand fly development and completion of the biological life-cycle of the parasite [4].
The north of Spain, especially the central and northwestern regions, has been an area classically considered as non-endemic of canine leishmaniasis [3, 25, 26]. In this respect, previous studies carried out using IFAT in shelter dogs from north Spain (Cantabria, Asturias, Galicia and the Basque Country) found a total seroprevalence of 3% for L. infantum in the Cantabrian coast [25]. Leishmania infantum antibodies were found in all the regions included in this study. The prevalence rates found in Catalonia and Galicia are in concordance with those obtained in a previous multicenter study performed in different areas of Spain [9]. Similarly, the rates found in Catalonia and Pyrenean areas are similar to those found in a recent study in the same area [28]. Furthermore, a study in the Basque Country detected a high prevalence using PCR for L. infantum DNA (28%, 44/156) among wild carnivores, strongly suggesting the presence of the parasite in this area [10]. However, caution is recommended when comparing studies due to potential differences in the diagnostic techniques used, sample size, animal species evaluated, and the origin of the dogs, among other factors [9, 10].
The results presented here reinforce the hypothesis about the global spread of L. infantum in the north of Spain, including more areas than previously considered [3, 26, 29]. This highlights the importance of including L. infantum in the differential diagnosis in areas traditionally considered as non-endemic or peri-endemic for this pathogen, especially in Aragon and Navarra. In this respect, a previous retrospective study of visceral and cutaneous human leishmaniasis prevalence, showed a low rate in northern Spain (0–0.5 cases/100,000 inhabitants) in comparison with other central or southern regions. However, the rate in Navarra (0.5–2.0/100,000 inhabitants) and especially the rates in Aragon and Catalonia (2–3.5/100,000 inhabitants), were considerable higher [11].
The higher seropositivity for L. infantum found in dogs older than 10 years-old could be explained by longer exposure to sand flies over time, and/or immune depletion described in the course of infection, especially affecting elderly dogs. Similar to this study, a bimodal trend has been previously described in canine leishmaniasis [4, 25]. Although some studies showed the predisposition of certain breeds or sex to canine leishmaniasis [30, 31], there was no statistical association in the present study.
Living outdoors represents a risk factor probably due to the increased contact with the vectors (Phlebotomus perniciosus or P. ariasi), which has been detected in previous studies in northern Spain, including some areas previously considered free of leishmaniasis [12, 26, 29].
Canine leishmaniasis is a chameleonic disease that shows up with many possible clinical signs [8], all caused by either the direct action of the parasite or the deposition of circulating immune complexes. Nevertheless, due to the high number of dogs with clinical signs included in this study, clinical signs associated to leishmaniasis were suspected and recognized by most of the practitioners working in the areas of study. It could be explained by the fact that canine leishmaniasis is well known among practitioners despite this wide diversity of clinical signs [9].
Ehrlichia canis seropositivity was also detected in all the evaluated areas, except for Galicia and Cantabria. However, E. canis has previously been detected in the southern area of Galicia [21] and in northern Portugal [15]. The absence of E. canis seropositivity in Galicia and antibodies detected from only one dog from Asturias agrees with a previous study carried out in dogs from the northwest of Spain (Galicia and Asturias), where all 75 dogs tested (IFAT) were seronegative to E. canis [23]. On the other hand, a previous study showed considerably higher seroprevalence for E. canis in Catalonia (n = 5/49; 10.2% in Barcelona) [32]. The overall seroprevalence rate of ehrlichiosis detected in our study (0.9 ± 0.4%) was lower than in previous studies in the same area where E. canis was reported to be the most prevalent rickettsial agent in Spain, with a seroprevalence of 5% [9].
The significant detection of E. canis antibodies during the summer months agrees with the peak of activity found in the area for R. sanguineus from March to July [6] and the subsequent incubation period of the infection (1–3 weeks) [17]. The higher seroprevalence found in dogs living indoors in our study also matches with the higher presence of R. sanguineus detected in indoor-living dogs previously described in Spain [6]. This result could be explained by the endophilic behavior of R. sanguineus, living in anthroponotic areas, in close contact with dogs and humans [33, 34]. Furthermore, ticks were not detected in any dog positive to E. canis at the time of clinical examination, in concordance with the efficacy of ectoparasiticide treatments reported herein. This fact could also be explained for the R. sanguineus host detachment in the different stages of the life-cycle [34, 35], or detection of antibodies that may persist for several months or years reported for E. canis, and also for Anaplasma spp. [17].
Apathy, weakness, anorexia, weight loss, anemia, fever and gastrointestinal signs were significantly associated with E. canis seropositivity. All these clinical signs have been classically described in dogs with E. canis infection [17, 36]. On the other hand, four out of the five dogs positive to E. canis were theoretically protected using ectoparasiticide prevention treatments. This fact could reflect incorrect use of these treatments or an insufficient pattern in their use, highlighting the importance not only of use, but also of correct administration guidelines to prevent this and others tick-borne infections [17].
Anaplasma phagocytophilum and A. platys could not be differentiated with the diagnostic technique used in this study due to test cross-reactivity [14]. Thus, this technique allowed us to detect exposure to the genus level only. Differentiation of these species requires a PCR assay or, with lower sensitivity, blood smear visualization [17]. Starting from this limitation, Anaplasma spp. were the most seroprevalent tick-borne pathogens detected in the study, with Asturias being the area with the highest seroprevalence rate. This prevalence can be expected considering the abundance of the potential vectors (I. ricinus and R. sanguineus) in the area [6]. In contrast, a previous serological study did not find dogs with antibodies against Anaplasma spp. in Asturias [9]. Five out of the seven samples positive for Anaplasma spp. were collected in colder months, although without statistically significant association. Even when the association between seropositivity against Anaplasma spp. and the presence of clinical signs of anaplasmosis could support the possibility of infection in these dogs [17], these results should be cautiously interpreted, taking into account that seropositivity could only reflect exposure to the agent.
Antibodies against B. burgdorferi were only detected in dogs from Asturias and Catalonia, with the overall seroprevalence in northern areas (0.72%) similar to those found in a previous study performed in Spain (0.4%), where there was a similar distribution in the different regions included [9]. In this sense, a similar low seroprevalence rate (0.66%, 3/460) was detected in a previous study of dogs from Mallorca and Catalonia (Barcelona and Tarragona) using the same test [32]. Our findings are in contrast with a previous study performed in southern Galicia (Pontevedra and Ourense) where the seroprevalence by IFAT for B. burgdorferi was 6.26% (30/479) in owned dogs [21]. Differences in the technique and in the studied population could explain the variability in the seroprevalence found in different studies.
Ixodes spp. are the main vectors of borreliosis in Europe and are present in the area of study without clear seasonality for either I. ricinus or I. hexagonus [6], probably explaining the lack of association in our study with the season of sample collection. The detection of B. burgdorferi antibodies in dogs living in rural areas in this study was in concordance with the presence of Ixodes spp. on dogs living in rural areas in these regions of Spain [6]. Contact with vectors and/or potential sylvatic reservoirs could be higher in dogs living in this environment. In this sense, B. burgdorferi antibodies have also been previously detected in wild canids (wolves and foxes) from northern Spain [5]. The lack of association between suspected clinical signs of borreliosis and the detection of antibodies against B. burgdorferi reported herein has been previously shown in northern Spain [37].
The clinical picture in the only dog from Navarra in which D. immitis antigen was detected should be treated with caution, due to the co-infection with Leptospira spp., because the latter could justify most of clinical signs described in this dog.
All dogs with co-infections were seropositive to Anaplasma spp., E. canis or L. infantum, suggesting an immunocompromised status that could facilitate co-infection as previously described [38, 39], or shared vectors and/or routes of transmission. In this sense, E. canis and Anaplasma spp. are commonly found co-infecting dogs [17].
Finally, when considering all the dogs seropositive to any vector-borne pathogen, this study showed a great variation of prevalence among regions, from 26.32 ± 5.8% in northern Aragon to 2.5 ± 1.7% in Galicia. These variations could be due to differences in the environment, vector abundance and/or the interactions between dogs and vectors [6]. Globally, this study showed that living in a rural area represents a risk factor, probably due to the higher exposure to arthropods and wildlife reservoirs of the vector-borne pathogens previously reported in these areas [5, 10]. Similarly, dogs younger than one year-old seem less likely to be infected, probably due to their limited exposure to pathogens; however, they will have an increased opportunity for exposure over time [17, 40, 41].
Real seroprevalence rates could probably be higher than detected herein considering that dogs included in the study were owned dogs, and most of them were protected against ectoparasites. This is in line with the higher previously reported rates in kennel dogs [25], and wild mammals [5] in northern Spain.
Conclusions
To the best of our knowledge, this is the first serological multicenter study focused on selected vector-borne pathogens performed in owned dogs from northern Spain, covering all regions from northeast to northwest. The present study confirms that vector-borne pathogens are prevalent and autochthonous in the areas studied, showing high prevalence rates especially in Navarra, northern Aragon and northern Catalonia. The results presented here highlight the significance of the veterinary control of vector-borne pathogens, and the implementation of effective prophylactic measures against arthropods in the northern areas of Spain. The expansion of canine vector-borne diseases to non-endemic areas and the wide variety of clinical signs presented by the affected dogs is a challenge for practitioners. Surveillance programmes must be established in order to have a better knowledge, management and control of the canine vector-borne pathogens. However, further molecular studies evaluating different pathogens in dogs from these areas are warranted to better understand the epidemiological and clinical situation.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Ab/s:
-
antibody/ies
- Ag/s:
-
antigen/s
- CI:
-
confidence interval
- OR:
-
odds ratio
References
Beugnet F, Marié JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305.
Beugnet F, Chalvet-Monfray K. Impact of climate change in the epidemiology of vector-borne diseases in domestic carnivores. Comp Immunol Microbiol Infect Dis. 2013;36:559–66.
Amusategui I, Sainz A, Aguirre E, Tesouro MA. Seroprevalence of Leishmania infantum in northwestern Spain, an area traditionally considered free of leishmaniasis. Ann N Y Acad of Sci. 2004;1026:154–7.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86.
Sobrino R, Sobrino R, Gortázar C. Seroprevalence of antibodies to Borrelia burgdorferi in wild canids in Spain. Vet Rec. 2008;162:248–9.
Estrada-Peña A, Roura X, Sainz A, Miró G, Solano-Gallego L. Species of ticks and carried pathogens in owned dogs in Spain: results of a one-year national survey. Ticks Tick Borne Dis. 2017;8:443–52.
Ballart C, Alcover M, Portús M, Gállego M. Is leishmaniasis widespread in Spain? First data on canine leishmaniasis in the province of Lleida, Catalonia, northeast Spain. Trans R Soc Trop Med Hyg. 2012;106:134–6.
Paltrinieri S, Solano-Gallego L, Fondati A, Lubas G, Gradoni L, Castagnaro M, et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1184–91.
Miró G, Montoya A, Roura X, Gálvez R, Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: a multicentre study. Parasit Vectors. 2013;6:117.
Del Río L, Chitimia L, Cubas A, Victoriano I, De la Rúa P, Gerrikagoitia X, et al. Evidence for widespread Leishmania infantum infection among wild carnivores in L. infantum periendemic northern Spain. Prev Vet Med. 2014;113:430–5.
Herrador Z, Gherasim A, Jimenez BC, Granados M, San Martín JV, Aparicio P. Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997–2011: raising awareness towards leishmaniasis in non-HIV patients. PLoS Negl Trop Dis. 2016;10:e0004379.
Ballart C, Barón S, Alcover M, Portús M, Gállego M. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in Andorra: first finding of P. perniciosus and wide distribution of P. ariasi. Acta Trop. 2012;122:155–9.
Nelson CT, McCall JW, Rubin SB, Buzhardt LF, Dorion DW, Graham W, et al. Guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in dogs. Vet Parasitol. 2005;133:255–66.
Chandrashekar R, Mainville CA, Beall MJ, O’Connor T, Eberts MD, Alleman AR, et al. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res. 2010;71:1443–50.
Cardoso L, Mendão C, de Carvalho LM. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal - a national serological study. Parasit Vectors. 2012;5:62.
Groves M, Dennis G, Amyx H, Huxsoll D. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res. 1975;36:937–40.
Sainz Á, Roura X, Miró G, Estrada-Peña A, Kohn B, Harrus S, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors. 2015;8:75.
Oteo J, Gil H, Barral M, Pérez A, Jimenez S, Blanco J, et al. Presence of granulocytic ehrlichia in ticks and serological evidence of human infection in La Rioja. Spain. Epidemiol Infect. 2001;127:353–8.
García J, Núñez M, Castro B, Fraile F, López A, Mella M, et al. Human anaplasmosis: the first Spanish case confirmed by PCR. Ann N Y Acad Sci. 2006;1078:545–7.
Littman MP, Gerber B, Goldstein RE, Labato MA, Lappin MR, Moore GE. ACVIM consensus update on Lyme borreliosis in dogs and cats. J Vet Intern Med. 2018;32:887–903.
Amusategui I, Tesouro MA, Kakoma I, Sainz Á. Serological reactivity to Ehrlichia canis, Anaplasma phagocytophilum, Neorickettsia risticii, Borrelia burgdorferi and Rickettsia conorii in dogs from northwestern Spain. Vector Borne Zoonotic Dis. 2008;86:797–804.
Checa R, Fidalgo LE, Montoya A, López AM, Barrera JP, Gálvez R, et al. The role of healthy dog carriers of Babesia microti-like piroplasms. Parasit Vectors. 2019;12:127.
Miró G, Checa R, Paparini A, Ortega N, González-Fraga JL, Gofton A, et al. Theileria annae (syn. Babesia microti-like) infection in dogs in NW Spain detected using direct and indirect diagnostic techniques: clinical report of 75 cases. Parasit Vectors. 2015;8:217.
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336.
Miró G, Checa R, Montoya A, Hernández L, Dado D, Gálvez R. Current situation of Leishmania infantum infection in shelter dogs in northern Spain. Parasit Vectors. 2012;5:60.
Ballart C, Alcover MM, Picado A, Nieto J, Castillejo S, Portús M, et al. First survey on canine leishmaniasis in a non classical area of the disease in Spain (Lleida, Catalonia) based on a veterinary questionnaire and a cross-sectional study. Prev Vet Med. 2013;109:116–27.
René-Martellet M, Lebert I, Chêne J, Massot R, Leon M, Leal A, et al. Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in southern Europe. Parasit Vectors. 2015;8:3.
Velez R, Ballart C, Domenech E, Abras A, Fernández-Arévalo A, Gómez S, et al. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: the example of north-eastern and Pyrenean areas of Spain. Prev Vet Med. 2019;162:67–75.
Lladró S, Picado A, Ballart C, Portús M, Gállego M. Management, prevention and treatment of canine leishmaniosis in north-eastern Spain: an online questionnaire-based survey in the province of Girona with special emphasis on new preventive methods (CaniLeish vaccine and domperidone). Vet Rec. 2016;180:47.
de Vasconcelos TCB, Furtado MC, Belo VS, Morgado FN, Figueiredo FB. Canine susceptibility to visceral leishmaniasis: a systematic review upon genetic aspects, considering breed factors and immunological concepts. Infect Genet Evol. 2019;74:103293.
Miranda S, Roura X, Picado A, Ferrer L, Ramis A. Characterization of sex, age, and breed for a population of canine leishmaniosis diseased dogs. Res Vet Sci. 2008;85:35–8.
Solano-Gallego L, Llull J, Osso M, Hegarty B, Breitschwerdt E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet Res. 2006;37:231–44.
Sobrino R, Millán J, Oleaga Á, Gortázar C, de la Fuente J, Ruiz-Fons F. Ecological preferences of exophilic and endophilic ticks (Acari: Ixodidae) parasitizing wild carnivores in the Iberian Peninsula. Vet Parasitol. 2012;184:248–57.
Gray J, Dantas-Torres F, Estrada-Peña A, Levin M. Systematics and ecology of the brown dog tick. Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171–80.
Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–85.
Harrus S, Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J. 2011;187:292–6.
Solano-Gallego L, Hegarty B, Espada Y, Llull J, Breitschwerdt E. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol. 2006;118:274–7.
Baxarias M, Álvarez-Fernández A, Martínez-Orellana P, Montserrat-Sangrà S, Ordeix L, Rojas A, et al. Does co-infection with vector-borne pathogens play a role in clinical canine leishmaniosis? Parasit Vectors. 2018;11:135.
Attipa C, Solano-Gallego L, Papasouliotis K, Soutter F, Morris D, Helps C, et al. Association between canine leishmaniosis and Ehrlichia canis co-infection: a prospective case-control study. Parasit Vectors. 2018;11:184.
Egenvall A, Bonnett BN, Gunnarsson A, Hedhammar Å, Shoukri M, Bornstein S, et al. Sero-prevalence of granulocytic Ehrlichia spp. and Borrelia burgdorferi sensu lato in Swedish dogs 1991–94. Scand J Infect Dis. 2000;32:19–25.
Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Veterinary Sci. 2011;91:71–6.
Acknowledgements
The authors sincerely thank the support of all the veterinary practices that collaborated in the blood sampling and data collection of this study, including practices from Galicia (Clínica Veterinaria Otter, A Media Lúa Clínica Veterinaria, Centro Veterinario A Marosa, Canido Veterinarios SL., Hospital Veterinario Ramiro Tubio); Asturias (Hospital Veterinario IVET Ribadesella, Clínica Veterinaria Covadonga, Clínica Veterinaria Lindo Pulgoso, Hospital Veterinario Nacho Menes, Hospital Veterinario Buenavista); Cantabria (La Clínica de Pablo, Clínica Veterinaria Ursaria, Clínica Veterinaria Ostende, Centro Veterinario Trasmiera, Hospital Veterinario Cantabria); Basque Country (Clínica Veterinaria Mendiburu, Clínica Veterinaria Abeletxe, Clínica Veterinaria Txingudi, Clínica Veterinaria Zaunka Llodio, Clínica Veterinaria Castaños); Navarra (Clínica Veterinaria Fitovet, Clínica Veterinaria Ansoain, Consulta Veterinaria San Jorge, Clínica Veterinaria San Fermín, Clínica Veterinaria Zelai); northern Aragon (VetMonzon, Centro Veterinario Los Olivos, Clínica Veterinaria Zoo, Centro De Servicios Veterinarios Osca SL, Centro Veterinario Río Veral); and northern Catalonia (Clínica Veterinaria Mon Animal Berga, Clinican Centre Veterinari, Serveis Veterinaris de la Cerdanya, Centre Veterinari la Seu SLP, Hospital Veterinari les Valls). The authors also thank “Departamento de Ayuda a la Investigación, Área de Informática y Comunicaciones”, Complutense University of Madrid for support in statistical analysis.
Funding
The authors would like to acknowledge Boehringer Ingelheim Spain S.A. for the sponsorship of the survey.
Author information
Authors and Affiliations
Contributions
DDR and AS stored the samples and data from all the dogs, analyzed the data, performed statistical analysis and wrote the manuscript. AS, XR, MS and ML planned, designed and supervised the study, analyzed the data and collaborated in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written consent for patient enrolment was obtained for each dog.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Díaz-Regañón, D., Roura, X., Suárez, M.L. et al. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasites Vectors 13, 301 (2020). https://doi.org/10.1186/s13071-020-04172-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04172-5