Abstract
Culex quinquefasciatus is a successful invasive species broadly distributed in subtropical regions, including Brazil. It is an extremely annoying mosquito due to its nocturnal biting behavior, in high-density populations and it is a potential bridge between sylvatic arbovirus from birds to man in urban territories. Herein, we present a review concerning the methods of chemical control employed against Cx. quinquefasciatus in Brazil since the 1950’s and insecticide resistance data registered in the literature. As there is no specific national programme for Cx. quinquefasciatus control in Brazil, the selection of insecticide resistance is likely due in part to the well-designed chemical campaigns against Aedes aegypti and the elevated employment of insecticides by households and private companies. There are very few publications about insecticide resistance in Cx. quinquefasciatus from Brazil when compared to Ae. aegypti. Nevertheless, resistance to organophosphates, carbamate, DDT, pyrethroids and biolarvicides has been registered in Cx. quinquefasciatus populations from distinct localities of the country. Concerning physiological mechanisms selected for resistance, distinct patterns of esterases, as well as mutations in the acetylcholinesterase (ace-1) and voltage-gated sodium channel (NaV) genes, have been identified in natural populations. Given environmental changes and socioeconomical issues in the cities, in recent years we have been experiencing an increase in the number of disease cases caused by arboviruses, which may involve Cx. quinquefasciatus participation as a key vector. It is urgent to better understand the efficiency and susceptibility status to insecticides, as well as the genetic background of known resistant mechanisms already present in Cx. quinquefasciatus populations for an effective and rapid chemical control when eventually required.
Similar content being viewed by others
Background
Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) known as the southern house mosquito, is a subtropical mosquito belonging to the complex Culex pipiens, present in the Americas, Australia, Asia, Africa, Middle East and New Zealand, as well as being broadly distributed in Brazil [1, 2]. Amongst the several species in genus Culex registered in Brazil [3], Cx. quinquefasciatus stands out as the most abundant and anthropophilic species [4]. In addition to the considerable discomfort caused by the nocturnal biting behavior, Cx. quinquefasciatus is the main vector of several pathogens, especially including the nematode Wulchereria bancrofti (agent of bancroftian filariasis) and the West Nile virus [5, 6]. As mosquitoes from the complex Cx. pipiens feed both on human and bird blood, they may potentially transport sylvatic arboviruses from migratory birds to man in urban territories [7]. This mosquito is also a potential vector of the arboviruses responsible for the Rift Valley fever [8] and Saint Louis encephalitis [9]. In the recent Brazilian Zika outbreak, samples of Cx. quinquefasciatus from urban environments were detected to be infected with ZIKV, suggesting participation in a new cycle of this emergent arbovirus in some regions [10, 11]. Similarly, its role has also been implied in the transmission of the emergent Mayaro virus in urban centers [12]. Other studies considering systems of infection in the laboratory demonstrated that Cx. quinquefasciatus would also be competent to transmit the protozoan Plasmodium relictum (agent of bird malaria) [13] and Hepatozoon breinli, an intracellular parasite infecting birds, reptiles, amphibians and rodents [14]. An extensive list of viruses, protozoans and nematodes isolated from Cx. quinquefasciatus under natural and laboratory conditions can be found elsewhere [15].
In the absence of effective vaccines available against most of the Culex-transmitted pathogens, the best strategy to avoid transmission relies on the chemical control of the mosquito [16]. At the end of the last century, the World Health Organization (WHO) launched a manual focused on mosquito vector control, including Culex spp., highlighting the necessity of measures to prevent their reproduction and dispersion [17]. Although Culex spp. females preferentially lay their eggs in collections of water, either stagnant or gentle flow, rich in organic matter, Cx. quinquefasciatus is very opportunistic so that any permanent or temporary collection of water may serve as a potential breading site for their larvae [1, 18]. Therefore, vector control planning has to focus on breeding-site elimination or treatment by improving the basic sanitary infrastructure of water supply and waste destination, as well as activities to promote community engagement within an environmental agenda. However, given the accelerated and disorganized process of urbanization in the last decades, especially in the tropical, low-income countries, these tasks are too complex to be fully achieved. Additionally, even in well-developed regions, the density of these mosquitoes may be positively correlated with seasonal high temperatures [19, 20]. In this scenario, chemical larvicides or polystyrene granules can be applied to water collections. Insecticide residual spraying (IRS) in the interior of the houses is generally not effective against Cx. quinquefasciatus given its habit of posing on substrates generally not treated with insecticide, such as cloth, curtains and other suspended fabrics, instead of resting on the walls and ceiling [17].
The first official actions in Brazil specifically targeting Cx. quinquefasciatus based on chemical control were during the years 1951–1955, as a first phase of a governmental campaign to control Bancroftian filariasis [21]. This campaign was coordinated by the National Service of Malaria, opting the use of hexachlorobenzene (BHC), dichlorodiphenyltrichloroethane (DDT) and dieldrin as residual action insecticides [22]. The second phase of this campaign was initiated in 1956, with the creation of the National Department of Rural Endemics (DNERu). By 1960, 120,339 midguts were dissected from female mosquitoes caught in filariasis endemic areas, which recognized Cx. quinquefasciatus (at that time named Cx. pipiens fatigans) as the main vector in the country [21]. In the following decade, Brazilian national campaigns against filariasis were coordinated by the Superintendence of Public Health Campaigns (SUCAM). These campaigns aimed at eradicating or controlling the filariasis transmission in endemic areas by treating committed persons with the chemotherapy diethylcarbamazine, as well as decreasing the density of the mosquito by improving the sanitary infrastructure and applying residual insecticides (BHC and dieldrin) against both larvae and adult stages of Cx. quinquefasciatus [22].
The employment of residual insecticides (BHC, DDT and dieldrin) for controlling adult mosquitoes was incipiently effective. Nevertheless, they became ineffective, their use being suspended [22]. Given the lack of an efficient adulticide together with the high cost of larvicide applications in the breading sites, the chemical treatment was discontinued and the national programmes centralized their actions on the treatment of human cases and health educational programmes [22]. Currently, the Brazilian Ministry of Health (MoH) acquires the insecticides and provides them to the states, which supply the municipalities. In turn, the municipalities have autonomy to complement alternative compounds in their territories, as long as they are approved by the WHO and the Brazilian National Agency of Sanitary Surveillance (ANVISA) [23]. There is no specific national programme for combating Culex, as most of the governmental actions against this mosquito are a side-effect of the well-structured programme for Aedes aegypti control. In this sense, most of the insecticide selection pressure geared toward Cx. quinquefasciatus populations in Brazil is substantially derived from that targeting Ae. aegypti [24].
In the last three decades the main larvicides utilized in Brazil under national scale against Ae. aegypti have been the organophosphate temephos, followed by the IGRs (insect growth regulators class) diflubenzuron, novaluron and more recently, pyriproxyfen. Pyrethroids were adopted as adulticides from 2000 until 2013 when the organophosphate malathion began to be implemented, as the only permissible alternative after reports that pyrethroid resistance in Ae. aegypti was apparent all over the country [23, 25]. Nevertheless, commercial pyrethroids have intensively been sprayed inside the dwellings as well as under thermo-fogging or ultra-low volume in the peri-domicile by private companies. In addition to neurotoxic insecticides, as recommended by the WHO [26], the bacterium Lysinibacillus sphaericus (Lbs), previously known as Bacillus sphaericus (Bs) [27], is also indicated and largely enlisted as a biolarvicide for Culex control [28]. Lysinibacillus sphaericus began to be exploited on a large scale for Culex control in Brazil since 1989, ever since used as a larvicide by the Filariasis Elimination Programme in Recife/PE and on the border of the Pinheiro River in São Paulo [29, 30].
Likewise, as with Aedes and Anopheles mosquitoes, the exacerbated use of insecticides has been selecting resistant Cx. quinquefasciatus populations around the world [31,32,33,34]. Resistance to insecticides is a multi-factorial genetic trait, preceding insecticide exposure. Normally, the frequency of resistant insects in natural populations is very low in an environment free of insecticides, i.e. without a selection pressure. Hence, the continuous application of insecticides favorably selects the resistant individuals, while those susceptible are progressively eliminated, reducing the genetic variability of the target population [35]. Depending on the intensity of the selection pressure over genetically well-structured and isolate populations, resistance may become irreversible due to the lack of susceptible mosquitoes to contribute their genes to the next generations, where migration among other populations is absent or very low [36]. In Brazil, in addition to the chemicals employed in governmental campaigns, the uncontrolled application by households increases during arbovirus outbreaks and also when targeting Culex itself due to its usual high densities and annoying nocturnal biting behavior [37, 38]. As the odor of pyrethroids is less noxious to the people, this class of insecticide is largely preferred [39, 40]. There is evidence that this excessive household use of chemicals is the main factor contributing to pyrethroid resistance selection in Ae. aegypti [41, 42], Cx. quinquefasciatus populations as such being likely to experience a similar phenomenon.
There are four main classes of mechanisms attributed to resistance in a mosquito population: behavioral changes, decrease of cuticular penetration, increase in the metabolic detoxification and alteration in the insecticide target-molecule, these two latter mechanisms being the mostly molecularly elucidated [43,44,45]. An increase in the metabolic detoxification may occur due to an increase in the detoxification power, generally related to the classes of enzymes esterases, glutathione S-transferases (GSTs) and multi-function oxidases P450s, which are able to modify or break up the insecticide molecules before they reach their target. In turn, target-site alterations inhibit the interaction between the insecticide and its action target molecules, rendering the insecticide less effective or even ineffective [46].
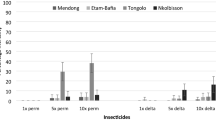
In 2011, the Brazilian MoH launched a surveillance and control methods guide against Cx. quinquefasciatus, recommending the use of neurotoxic (pyrethroids, carbamates or organophosphates) and IGR compounds (juvenile hormones analogues and benzophenil ureas, as chitin synthesis inhibitors) in conjunction with the biolarvicide Lbs [5], very similarly as indicated against Ae. aegypti. The compounds currently indicated by the MoH are: organophosphates and pyrethroids for adult control and spinosyns; bacterium biolarvicides; benzophenilureas; juvenile hormone analogues; and organophosphates against larvae [47]. Nevertheless, there are some reports of Cx. quinquefasciatus populations resistant to some of these compounds in the country (see Fig. 1). A list with insecticide resistance data available in the literature, including susceptibility tests, biochemical and molecular assays, distributed per region and year are provided in Table 1.
Organophosphates and carbamates
First reports of organophosphate resistance in Cx. quinquefasciatus from Brazil came from Rio de Janeiro populations evaluated with chlorpyrifos in 1978 [48] and later in 1994 [49]. Resistance to the larvicide temephos was described in populations from Campinas (São Paulo State) [50], Santa Cruz do Capibaribe (Pernambuco State) and Campo Grande [51] and Naviraí [52] (Mato Grosso do Sul State). In addition, a population from Cuiabá (Mato Grosso State) collected in 2000 was classified as “tolerant”, while Ae. aegypti collected at the same site and year were susceptible to the insecticide [50]. Resistance to the carbamate propoxur was evidenced in Cx. quinquefasciatus collected in the region of Pinheiros River in the center of São Paulo, in 1995 and 1996 [53]. Also, in São Paulo, resistance was detected to both organophosphates malathion and fenitrothion [53, 54] and to malathion (RR50 of 43.81) in Rio de Janeiro [24].
High levels of resistance to malathion were also observed in Cx. quinquefasciatus from other Latin American countries such as Cuba (RR50 of 207.91 and 135.97) and to a lesser extent Venezuela (RR50 of 16.11). The authors considered that the intense employment of the OP themephos, malathion and propoxur and also pyrethroids, for the first dengue outbreaks in the 1980’s, contributed to control Ae. aegypti, however inducing resistance in Cx. quinquefasciatus to OPs and propoxur [55, 56]. In Brazil, resistance to temephos is currently disseminated in Ae. aegypti populations throughout the country which forced the National Dengue Control Programme to replace this chemical by Insect Growth Regulators (IGRs) compounds [25]. This scenario of disseminated resistance to temephos might as well be extended to Cx. quinquefasciatus. For example, more than 40,000 kg of temephos were applied in the city of Santa Cruz do Capibaribe alone against Ae. aegypti during 2007–2010 which was likely the source of pressure that selected resistant Cx. quinquefasciatus in the region [51].
Organophosphates and carbamates both target the acetylcholinesterase enzyme (AchE), causing the accumulation of the neurotransmitter acetylcholine in a synaptic shift, which inhibits the interruption of a nervous impulse and therefore, kills the insect. The metabolic enzyme participation also plays an important role in organophosphate resistance in Culex. For instance, a gene or a set of esterase genes suffered several duplications, causing the increase of their codified enzymes and consequently, more sequestration of the insecticide molecule [57,58,59]. The register of detoxifying enzyme quantification associated with insecticide resistance in Cx. quinquefsciatus from Brazil was noted in populations from Fortaleza [60], São Paulo [53], Santa Cruz do Capibaribe [51] and Rio de Janeiro [24, 49].
A single nucleotide polymorphism (SNP) in the acetylcholinesterase gene (ace-1) with the substitution of a Gly by a Ser in the 119 codon (G119S, ace-1R allele) is a target site mechanism mostly displayed in Culex populations resistant to organophosphates. The same G119S SNP is also found in other insects, including Anopheles mosquitoes [61,62,63]. Other mutations (F290V and F331W) were also described in the Culex ace-1 gene, also possibly related to resistance to organophosphates [64,65,66,67]. The G119S was the only ace-1 mutation found in Cx. quinquefasciatus from Brazil, in the localities of São Paulo [53], Recife [64], Santa Cruz do Capibaribe [51] and Rio de Janeiro [68].
Organoclhorines and pyrethroids
The organochlorine DDT and the pyrethroids act in the voltage-gated sodium channel (NaV) in the neuron membranes, prolonging its open state. This interaction results in a repetitive firing of the nervous impulse, leading the insect to involuntary muscle spasms, exhaustion and death, a phenomenon known as knockdown effect [69]. Organochlorine may also inhibit the gamma-aminobutyric acid (GABA)-gated chloride channel. This is the case of cyclodienes, such as dieldrin which antagonizes the effects of the GABA receptor by preventing chloride ions from entering the neurons, thus inhibiting the return to resting state after an impulse transmission. A classical mutation (A302S) in the GABA receptor induces resistance to dieldrin, thus being referred to as RDL in several insects including Cx. quinquefasciatus [70, 71]. No substitution in the GABA gene has been reported in populations from Brazil. Increase in the action of detoxifying enzymes is usually related to DDT- and pyrethroid resistance, especially GST and monoxygenases P450 classes [72, 73]. However, given the diversity of multiple genes in these classes, it is difficult to find the same specific molecular marker for metabolic resistance across distinct species or even among populations of a same species. In the case of the target-site alterations, however, the same SNPs are found in distinct species, as is the case of the substitution Leu by Phe in the 1014 site of the NaV gene, known as the classical kdr (knockdown resistance) mutation [74].
There is one report of a Cx. quinquefasciatus population from Rio de Janeiro collected in 1994 resistant to DDT [49]. The use of this compound had been discontinued against mosquitoes since 1971 and finally prohibited in Brazil in 1985 [75]. Therefore, this could result from a persisted resistance selected by DDT itself or cross-resistance by the use of other compounds. Resistance to the pyrethoids cyfluthrin and cypermethrin was identified in a population from Campinas collected in 1999, whilst simultaneously collected Ae. aegypti were susceptible [50]. In addition, there was resistance to deltamethrin in a laboratory strain from Divinópolis [76]. Resistance to deltamethrin emerged and spread very rapidly in Ae. aegypti since its introduction by national campaigns against the dengue vector in 2000. One decade later, high levels of resistance to this chemical were acquired throughout the country, especially during dengue epidemic seasons, probably with an important contribution of the use of household insecticide sprays, all pyrethroid-based products [25, 41]. This environment with constant insecticide application near and inside the houses has likewise been selecting resistance to pyrethroids in Cx. quinquefasciatus.
To date, there has only been one report for a kdr mutation in Cx. quinquefasciatus from Brazil in which the classical L1014F was detected, yet under low frequencies (4–7%) in samples from Campo Grande, Rio de Janeiro, Niterói and Belo Horizonte [77]. In these localities, resistance to deltamethrin was apparent in Ae. aegypti probably related to kdr mutations [78]. Similarly, L1014F was detected in Cx. quinquefasciatus from Mexico, indirectly exposed to pyrethroids targeting Ae. aegypti during dengue control campaigns [79]. Other substitutions were found in the same 1014 aminoacid position (L1014S, L1014C) in the Cx. pipiens complex [80]. However, none were ever observed in Cx quinquefasciatus from Brazil, other than L1014F.
Biolarvicides
Bacillus thuringiensis var. israelensis (Bti) and Lysinibacillus sphaericus (Lbs) are bacteria that produce endotoxins that are activated by mosquito larvae intestinal proteases and bind to specific receptors in the intestinal epithelium, causing degeneration and consequently, killing the larvae [81, 82]. The Lbs is more suitable for controlling Cx. quinquefasciatus because it presents higher activity in polluted water when compared to Bti [83]. The cqm1 gene encodes an epithelial protein to which the toxin binds. Some mutations in the cqm1 gene were associated with Lbs resistance. For example, a deletion of 19 nucleotides (cqm1REC) and the substitution of a guanine (G) to an adenine (A) in the codon 1324 (cqm1REC-2), both present in Cx. quinquefasciatus field populations from Recife [84, 85]. Resistance to Lbs was found in a Cx. quinquefasciatus populations from Coque, an urban area of Recife, only two years after its implementation in 1991, reaching a resistance ratio 10-fold higher than the susceptible control [86]. However, this resistance was later reversed with the interruption of the biolarvicide application in that locality [87]. In other areas of Recife city, Lsb continued to be enlisted and several studies have been evaluating the levels of susceptibility to this bioinsecticide in the city as well as identifying new molecular markers [88,89,90]. These studies have shown that the frequency of resistant individuals in Recife city remains at low levels, even with the continued application of the bacterial insecticide, either alone or in combination with other larvicides such as Bti [91,92,93,94]. In a recent study in Colombia, Lbs proved to be efficient against both Cx. quinquefasciatus and Ae. aegypti field populations, suggesting it as an interesting alternative to chemical insecticides [28].
Conclusions
In accordance with the WHO Global Vector Control Response [95], the emergence and spread of vector-transmitted diseases is likely to be intensified in the following years, especially those with the participation of urban mosquitoes such as Culex spp., given their strong adaptation to climate changes and inefficient urban sanitary infrastructures. Therefore, as the application of insecticides is a primarily action against Cx. quinquefasciatus in Brazil, it is urgent to investigate the status of susceptibility/resistance of natural populations to all the chemical compounds available for use. Effective vector control in Brazil is a complex and multifactorial task considering the continental dimensions together with the greatly heterogeneous ecological and demographic aspects [96]. Future successful campaigns based on the use of chemicals have to implement a constant monitoring of insecticide effectiveness, employing integrated methods against all targeted species and considering a plan well adapted to regional peculiarities.
Availability of data and materials
Not applicable.
Abbreviations
- DDT:
-
dichlorodiphenyltrichloroethane
- ace-1 :
-
acethylcholinesterase 1 gene
- AchE:
-
acethylcholinesterase enzyme
- Na V :
-
voltage-gated sodium channel gene
- WHO:
-
World Health Organization
- IRS:
-
insecticide residual spraying
- MoH:
-
Ministry of Health
- IGR:
-
insect growth regulator
- RR:
-
resistance ratio
- OP:
-
organophosphate
- SNP:
-
single nucleotide polymorphism
- Kdr :
-
knockdown resistance
References
Forattini OP. Espécie de Culex (Culex). In: Forattini OP, editor. Culicidologia Médica. São Paulo: Editora Universidade de São Paulo; 2002. p. 693–722.
Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–85.
Tissot AC, da Silva MAN. Lista das espécies de Culicidae (Diptera) depositadas na Coleção de Entomologia Pe. J. S. Moure. Rev Bras Entomol. 2008;52:263–8.
Consoli RAGB. Lourenço de Oliveira R. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro: FIOCRUZ; 1994.
Brasil. Guia de vigilância do Culex quinquefasciatus. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Coordenação Francisco Anilton Alves Araújo, Marcelo Santalucia. 3rd ed. Brasília: Ministério da Saúde; 2011.
Lima-Camara TN. Emerging arboviruses and public health challenges in Brazil. Rev Saude Publica. 2016;50:36.
Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, et al. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–8.
Meegan JN. The Rift Valley fever epizootic in Egypt 1977–78. Description of the epizootic and virological studies. Trans R Soc Trop Med Hyg. 1979;73:618–23.
Monath TP, Tsai TF. St Louis encephalitis: lessons from the last decade. Am J Trop Med Hyg. 1987;37:40S–59S.
Ayres CFJ, Guedes DRD, Paiva MHS, Morais-Sobral MC, Krokovsky L, Machado LC, et al. Zika virus detection, isolation and genome sequencing through Culicidae sampling during the epidemic in Vitória, Espírito Santo, Brazil. Parasit Vectors. 2019;12:220.
Guedes DR, Paiva MH, Donato MM, Barbosa PP, Krokovsky L, Rocha SWDS, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6:e69.
Serra OP, Cardoso BF, Ribeiro ALM, dos Santos FAL, Slhessarenko RD. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2016;111:20–9.
Atkinson CT, Woods KL, Dusek RJ. Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected iiwi (Vestiaria coccinea). Parasitology. 1995;111(Suppl. 1):59–69.
Mackerras MJ. The life history of a Hepatozoon (Sporozoa: Adeleidea) of varanid lizards in Australia. Aust J Zool. 1962;10:35–44.
Bhattacharya S, Basu P. The southern house mosquito, Culex quinquefasciatus: profile of a smart vector. J Entomol Zool Stud. 2016;4:73–81.
WHO. World Health Organization position statement on integrated vector management to control malaria and lymphatic filariasis. Weekly Epidemiological Record. 2011;86:113–28.
Rozendaal JA. Vector control methods for use by individuals and communities. Geneva: World Health Organization; 1997.
Leyva IM, Marquetti CM, Montada DL. Segregation of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) niche under laboratory conditions. Rev Cubana Med Trop. 2012;64:206–11.
Barbosa RMR, Regis LN. Monitoring temporal fluctuations of Culex quinquefasciatus using oviposition traps containing attractant and larvicide in an urban environment in Recife. Brazil. Mem Inst Oswaldo Cruz. 2011;106:451–5.
David MR, Ribeiro GS, Freitas RM. Bionomics of Culex quinquefasciatus within urban areas of Rio de Janeiro, southeastern Brazil. Rev. Saude Publica. 2012;46:858–65.
Medeiros Z, Menezes JA, Cesse EP, Lessa F. Controle da filariose linfática no Brasil, 1951–2000. Epidemiol Serv Saude. 2003;12:77–86.
Rocha EMM, Fontes G. Filariose bancroftiana no Brasil. Rev Saude Publica. 1998;32:98–105.
Bellinato DF, Viana-Medeiros PF, Araujo SC, Martins AJ, Lima JBP, Valle D. Resistance status to the insecticides temephos, deltamethrin, and diflubenzuron in Brazilian Aedes aegypti populations. Biomed Res Int. 2016;2016:8603263.
Coto MM, Lazcano JA, de Fernandez DM, Soca A. Malathion resistance in Aedes aegypti and Culex quinquefasciatus after its use in Aedes aegypti control programs. J Am Mosq Control Assoc. 2000;16:324–30.
Valle D, Bellinato DF, Viana-Medeiros PF, Lima JBP, Martins Junior AJ. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem Inst Oswaldo Cruz. 2019;114:e180544.
Chavasse DC, Yap HH. Chemical methods for the control of vectors and pests of public health importance. Geneva: World Health Organization; 1997.
Ahmed I, Yokota A, Yamazoe A, Fujiwara T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int J Syst Evol Microbiol. 2007;57:1117–25.
Santana-Martinez JC, Silva JJ, Dussan J. Efficacy of Lysinibacillus sphaericus against mixed-cultures of field-collected and laboratory larvae of Aedes aegypti and Culex quinquefasciatus. Bull Entomol Res. 2019;109:111–8.
Regis LN, Silva-Filha MH, de Oliveira CMF, Rios EM, Silva SB, Furtado AF. Integrated control measures against Culex quinquefasciatus, the vector of filariasis in Recife. Mem Inst Oswaldo Cruz. 1995;90:115–9.
Andrade CFS, Campos JC, Cabrini I, Marques Filho CAM, Hibi S. Suscetibilidade de populações de Culex quinquefasciatus Say (Diptera: Culicidae) sujeitas ao controle com Bacillus sphaericus Neide no rio Pinheiros. São Paulo. BioAssay. 2007;2:1–4.
Delannay C, Goindin D, Kellaou K, Ramdini C, Gustave J, Vega-Rúa A. Multiple insecticide resistance in Culex quinquefasciatus populations from Guadeloupe (French West Indies) and associated mechanisms. PLoS One. 2018;13:e0199615.
Nchoutpouen E, Talipouo A, Djiappi-Tchamen B, Djamouko-Djonkam L, Kopya E, Ngadjeu CS, et al. Culex species diversity, susceptibility to insecticides and role as potential vector of lymphatic filariasis in the city of Yaoundé, Cameroon. PLoS Negl Trop Dis. 2019;13:e0007229.
Skovmand O, Sanogo E. Resistance of Culex quinquefasciatus to selected chemical and biological pesticides. Med Res Arch. 2018;6:1–9.
Ukpai OM, Ekedo CM. Insecticide susceptibility status of Culex quinquefasciatus (Diptera: Culicidae) in Umudike, Ikwuano LGA Abia State, Nigeria. Int J Mosq Res. 2019;6:114–8.
WHO. Insecticide resistance and vector control: tenth report of the Expert Committee on Insecticides. Geneva: World Health Organization; 1960.
Crow JF. Genetics of insect resistance to chemicals. Annu Rev Entomol. 1957;2:227–46.
Zylberkan M, Farias A. A invasão dos pernilongos: incidência dos insetos dobra na capital. Revista Veja SP. 2017. https://vejasp.abril.com.br/cidades/infestacao-pernilongo-sao-paulo/ Accessed 07 May 2019.
Garcia GA, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, Lima JB, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis. 2019;13:e0007023.
Diel C, Facchini LA, Dallgnol MM. Inseticidas domésticos: padrão de uso segundo a renda per capita. Rev Saude Publica. 2003;37:83–90.
Oliveira LB, Maria R, Nunes P, Santana M, Rosa A, Mariana N, et al. Profile of the population use of household insecticides against mosquitoes. Semina Cienc Biol Saude. 2015;36:79–92.
Macoris ML, Martins AJ, Andrighetti MTM, Lima JBP, Valle D. Pyrethroid resistance persists after ten years without usage against Aedes aegypti in governmental campaigns: lessons from São Paulo State. Brazil. PLoS Negl Trop Dis. 2018;12:e0006390.
Gray L, Florez SD, Barreiro AM, Valdillo-Sánchez J, González-Olvera G, Lenhart A, et al. Experimental evaluation of the impact of household aerosolized insecticides on pyrethroid resistance Aedes aegypti. Sci Rep. 2018;8:12535.
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91.
Braga IA, Valle D. Aedes aegypti: insecticides, mechanisms of action and resistance. Epidemiol Serv Saude. 2007;16:295–302.
Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–59.
Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4:605–13.
Brasil. Ministério da Saúde. Controle de Vetores: Inseticidas recomendados. 2019. http://portalms.saude.gov.br/vigilancia-em-saude/controle-de-vetores Accessed 02 May 2019.
Curtis CE, Pasteur N. Organophosphate resistance in vector populations of the complex of Culex pipiens L. (Diptera: Culicidae). Bull Entomol Res. 1981;71:153–61.
González T, Bisset JA, Díaz C, Rodríguez MM, Brandolini MB. Insecticide resistance in a Culex quinquefasciatus strain from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 1999;94:121–2.
Campos J, Andrade CFS. Laval susceptibility of Aedes aegypti and Culex quinquefasciatus populations to chemical insecticides. Rev Saude Publica. 2003;37:523–7.
Amorim LB, Helvecio E, de Oliveira CMF, Ayres CFJ. Susceptibility status of Culex quinquefasciatus (Diptera: Culicidae) populations to the chemical insecticide temephos in Pernambuco, Brazil. Pest Manag Sci. 2013;69:1307–14.
Scudeler C, Arruda E, Andrade C, Silva T, Fernandes M, Teixeira T, Cabrini I. Larval susceptibility of two Culex quinquefasciatus populations (Diptera: Culicidae) temephos® in the city of Naviraí, MS. Brazil. Orbital: Electron J Chem. 2015;7:270–4.
Bracco JE, Dalbon M, Marinotti O, Barata JM. Resistance to organophosphorous and carbamates insecticides in a population of Culex quinquefasciatus. Rev Saude Publica. 1997;31:182–3.
Bracco JE, Barata JMS, Marinotti O. Evaluation of insecticide resistance and biochemical mechanisms in a population of Culex quinquefasciatus (Diptera: Culicidae) from São Paulo. Brazil. Mem Inst Oswaldo Cruz. 1999;94:115–20.
Bisset JA, Rodrígues MM, Díaz C, Ortíz E, Marquetti MC, Hemingway J. The mechanisms of organophosphate and carbamate resistance in Culex quinquefasciatus (Diptera: Culicidae) from Cuba. Bull Entom Res. 1990;80:245–50.
Reyes-Lugo M, Neus M. Resistencia del mosquito Culex quinquefasciatus Say 1823 (Diptera: Culicidae) a insecticidas en el estado Zulia. Venezuela. Rev Cient Fac Cien. 2000;10:441–7.
Bourguet D, Pasteur N, Bisset J, Raymond M. Determination of Ace.1 genotypes in single mosquitoes: toward an ecumenical biochemical test. Pestic Biochem Physiol. 1996;55:122–8.
Lenormand T, Guillemaud T, Bourguet D, Raymond M. Appearance and sweep of a gene duplication: adaptive response and potential for new functions in the mosquito Culex pipiens. Evolution. 1998;52:1705–12.
Labbé P, Berthomieu A, Berticat C, Alout H, Raymond M, Lenormand T, Weill M. Independent duplications of the acetylcholinesterase gene conferring insecticide resistance in the mosquito Culex pipiens. Mol Biol Evol. 2007;24:1056–67.
Yébakima A, Yp-Tcha MM, Reiter P, Bisset J, Delay B, Chevillon C, Pasteur N. Detoxifying esterases in Culex pipiens quinquefasciatus from the Caribbean countries. J Am Mosq Control Assoc. 1995;11:363–6.
Alout H, Djogbénou L, Berticat C, Chandre F, Weill M. Comparison of Anopheles gambiae and Culex pipiens acetycholinesterase 1 biochemical properties. Comp Biochem Physiol B Biochem Mol Biol. 2008;150:271–7.
Fournier D, Bride JM, Hoffmann F, Karch F. Acetylcholinesterase: two types of modifications confer resistance to insecticide. J Biol Chem. 1992;267:14270–4.
Fournier D, Mutéro A. Modification of acetylcholinesterase as a mechanism of resistance to insecticides. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1994;108:19–31.
Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, et al. Insecticide resistance in mosquito vectors. Nature. 2003;423:136–7.
Alout H, Berthomieu A, Cui F, Tan Y, Berticat C, Qiao CL, Weill M. Different amino-acid substitutions confer insecticide resistance through acetylcholinesterase 1 insensitivity in Culex vishnui and Culex tritaeniorhynchus (Diptera: Culicidae) from China. J Med Entomol. 2007;44:463–9.
Alout H, Berthomieu A, Hadjivassilis A, Weill M. A new amino-acid substitution in acetylcholinesterase 1 confers insecticide resistance to Culex pipiens mosquitoes from Cyprus. Insect Biochem Mol Biol. 2007;37:41–7.
Wirth MC, Georghiou GP. Organophosphate resistance in Culex pipiens from Cyprus. J Am Mosq Control Assoc. 1996;12:112–8. https://pdfs.semanticscholar.org/ac13/856ce1538aaa0d0e23abfa569a1ee30e3f71.pdf. Acessed 29 Nov 2019
Longo C. Mutações envolvidas com a resistência a inseticidas em populações naturais de Culex quinquefasciatus (Say 1823) do Rio de Janeiro. Monografia. Instituto Federal de Educação, Ciência e Tecnologia do Rio de Janeiro; 2016
Bloomquist JR. Ion channels as targets for insecticides. Annu Rev Entomol. 1996;41:163–90.
Sattelle DB. GABA receptors of insects. Adv Insect Physiol. 1990;22:1–113.
Tantely ML, Tortosa P, Alout H, Berticat C, Berthomieu A, Rutee A, et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Réunion Island. Insect Biochem Mol Biol. 2010;40:317–24.
Terriere LC, Yu SJ. Induction of detoxifying enzymes in insects. J Agric Food Chem. 1974;22:366–73.
Hemingway J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem Mol Biol. 2000;30:1009–15.
Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol Gen Genet. 1996;252:51–60.
D’Amato C, Torres JPM, Malm O. DDT (diclorodifeniltricloroetano): toxicidade e contaminação ambiental: uma revisão. Quim Nova. 2002;25:995–1002.
de Melo AR, Castro DSB, Alves SN. Response of Culex quinquefasciatus larvae to three insecticides. Rev Ins Med Trop Sao Paulo. 2015;57:540.
Steinhagem, FM, Lima, JBP, Valle D, Martins AJ. Kdr mutation in Culex quinquefasciatus populations from Brazil. IN: XLVIII Congress of the Brazilian Society of Tropical Medicine. 2012. https://www.sbmt.org.br/portal/congressos/ Accessed 04 Jun 2019.
Martins AJ, Lima JB, Peixoto AA, Valle D. Frequency of Val1016Ile mutation in the voltage-gated sodium channel gene of Aedes aegypti Brazilian populations. Trop Med Int Health. 2009;14:1351–5.
Ponce G, Sanchez IP, García SM, Torrado JM, Lopez-Monroy B, Flores AE. First report of L1014F kdr mutation in Culex quinquefasciatus in Mexico. Insect Sci. 2016;23:829–34.
Scott JG, Yoshimizu MH, Kasai S. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic Biochem Physiol. 2015;120:68–76.
Charles JF, Nielsen-LeRoux C, Delécluse A. Bacillus sphaericus toxins: molecular biology and mode of action. Ann Rev Entomol. 1996;41:451–72.
Knowles BH, Ellar DJ. Colloid-osmotic lysis is the general mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificity. Biochim Biophys Acta. 1987;924:509–18.
de Barjac H, Sutherland DJ. Bacterial control of mosquitoes & black flies. London: Unwin Hyman Ltd; 1991.
Romão TP, de Melo Chalegre KD, Key S, Ayres CF, de Oliveira CM, de-Melo-Neto OP, Silva-Filha MH. A second independent resistance mechanism to Bacillus sphaericus binary toxin targets its alpha-glucosidase receptor in Culex quinquefasciatus. FEBS J. 2006;273:1556–68.
Menezes HSG, Chalegre KDM, Romão TP, Oliveira CMF, de-Melo-Neto OP, Silva-Filha MHNL. A new allele conferring resistance to Lysinibacillus sphaericus is detected in low frequency in Culex quinquefasciatus field populations. Parasit Vectors. 2016;9:70.
Silva-Filha MH, Regis L, Nielsen-LeRoux C, Charles JF. Low-level resistance to Bacillus sphaericus in a field-treated population of Culex quinquefasciatus (Diptera: Culicidae). J Econ Entomol. 1995;88:525–30.
Silva-Filha MH, Regis L. Reversal of low-level resistance to Bacillus sphaericus in a field population of the southern house mosquito (Diptera: Culicidae) from an urban area of Recife. Brazil. J Econ Entomol. 1997;90:299–303.
Chalegre KDD, Romão TP, Amorim LB, Anastacio DB, de Barros RA, de Oliveira CM, et al. Detection of an allele conferring resistance to Bacillus sphaericus binary toxin in Culex quinquefasciatus populations by molecular screening. Appl Environ Microbiol. 2009;75:1044–9.
Chalegre KD, Romão TP, Tavares DA, Santos EM, Ferreira LM, Oliveira CM, et al. Novel mutations associated with resistance to Bacillus sphaericus in a polymorphic region of the Culex quinquefasciatus cqm1 gene. Appl Environ Microbiol. 2012;78:6321–6.
Rezende TMT, Romão TP, Batista M, Berry C, Adang MJ, Silva-Filha MHNL. Identification of Cry48Aa/Cry49Aa toxin ligands in the midgut of Culex quinquefasciatus larvae. Insect Biochem Mol Biol. 2017;88:63–70.
Silva-Filha MHN, Chalegre KDM, Anastacio DB, Oliveira CMF, Silva SB, Acioli RV, et al. Culex quinquefasciatus field populations subjected to treatment with Bacillus sphaericus did not display high resistance levels. Biol Control. 2008;44:227–34.
Amorim LB, de Barros RA, Chalegre KDD, de Oliveira CMF, Regis LN, Silva-Filha M. Stability of Culex quinquefasciatus resistance to Bacillus sphaericus evaluated by molecular tools. Insect Biochem Mol Biol. 2010;40:311–6.
de Santos EM, de Chalegre KD, de Albuquerque AL, Regis L, Oliveira CMF, Silva-Filha MHNL, et al. Frequency of resistance alleles to Lysinibacillus sphaericus in a Culex quinquefasciatus population treated with a L sphaericus/Bti biolarvicide. Biol Control. 2019;132:95–101.
de Santos EM, Regis LN, Silva-Filha MHNL, Barbosa RMR, de Melo-Santos MAV, Gomes TCS, Oliveira CMF. The effectiveness of a combined bacterial larvicide for mosquito control in an endemic urban area in Brazil. Biol Control. 2018;121:190–8.
WHO. Global Vector Control Response 2017–2030. Geneva: World Health Organization; 2017.
Tauil PL. Perspectives of vector borne diseases control in Brazil. Rev Soc Bras Med Trop. 2006;39:275–7.
Ruas-Neto AL, Silveira SM, Colares ERC. Mosquito control based on larvicides in the state of Rio Grande do Sul, Brazil: choice of the control agent. Cad Saude Públ. 1994;10:222–30.
Acknowledgements
The authors thank Mitchell Raymond Lishon for the language revision of this document.
Funding
This study was partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES) (Finance Code 001), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Processo 423002/2016-3) and the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Processo: E-26/203.177/2016).
Author information
Authors and Affiliations
Contributions
RML compiled the literature search and drafted the review. AJM revised and reviewed the manuscript together with JBPL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lopes, R.P., Lima, J.B.P. & Martins, A.J. Insecticide resistance in Culex quinquefasciatus Say, 1823 in Brazil: a review. Parasites Vectors 12, 591 (2019). https://doi.org/10.1186/s13071-019-3850-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3850-8