Abstract
Background
The identification of Trypanosoma cruzi and blood-meal sources in synanthropic triatomines is important to assess the potential risk of Chagas disease transmission. We identified T. cruzi infection and blood-meal sources of triatomines caught in and around houses in the state of Bahia, northeastern Brazil, and mapped the occurrence of infected triatomines that fed on humans and domestic animals.
Methods
Triatominae bugs were manually captured by trained agents from the Epidemiologic Surveillance team of Bahia State Health Service between 2013 and 2014. We applied conventional PCR to detect T. cruzi and blood-meal sources (dog, cat, human and bird) in a randomized sample of triatomines. We mapped triatomine distribution and analyzed vector hotspots with kernel density spatial analysis.
Results
In total, 5906 triatomines comprising 15 species were collected from 127 out of 417 municipalities in Bahia. The molecular analyses of 695 triatomines revealed a ~10% T. cruzi infection rate, which was highest in the T. brasiliensis species complex. Most bugs were found to have fed on birds (74.2%), and other blood-meal sources included dogs (6%), cats (0.6%) and humans (1%). Trypanosoma cruzi-infected triatomines that fed on humans were detected inside houses. Spatial analysis showed a wide distribution of T. cruzi-infected triatomines throughout Bahia; triatomines that fed on dogs, humans, and cats were observed mainly in the northeast region.
Conclusions
Synanthropic triatomines have a wide distribution and maintain the potential risk of T. cruzi transmission to humans and domestic animals in Bahia. Ten species were recorded inside houses, mainly Triatoma sordida, T. pseudomaculata, and the T. brasiliensis species complex. Molecular and spatial analysis are useful to reveal T. cruzi infection and blood-meal sources in synanthropic triatomines, identifying areas with ongoing threat for parasite transmission and improving entomological surveillance strategies.
Similar content being viewed by others
Background
Chagas disease is the most frequent cause of heart failure in rural populations in vector-endemic countries in Latin America [1, 2]. It is an infection caused by Trypanosoma cruzi (Chagas, 1909), a protozoan transmitted by blood-feeding bugs [3]. No vaccines or effective antiparasitic treatments are available to cure Chagas cardiomyopathy, so vector surveillance and control are the main strategies to prevent human infection in areas with vectorial transmission [4].
In Brazil, the control of Chagas disease vectors was implemented systematically between 1975 and 1983 when the main vector, Triatoma infestans (Klug, 1834), infested domiciles in 12 states. In 1991, Brazil integrated an international consortium to reduce vectorial transmission through insecticide spraying [4, 5]. The systematic actions of chemical treatment were effective; in 2006, the World Health Organization (WHO) certified Brazil as free of T. cruzi transmission by T. infestans. However, some recent outbreaks have been associated with the oral transmission, mainly due to açai palm juice consumption and other T. cruzi-contaminated food in the Brazilian Amazon, where Rhodnius species are frequent [6]. Moreover, new cases of vector-borne Chagas disease transmitted by either sylvatic vectors invading houses or domestic/peridomestic populations [e.g. T. brasiliensis, T. pseudomaculata, T. sordida, Panstrongylus megistus (Burmeister, 1835)] are being recorded in Brazil [7, 8]. Epidemiological data show 2.2 deaths per 100,000 inhabitants in 2017 in Brazil and the highest value was recorded at Goiás State (22.4 deaths per 100,000 inhabitants). Between 2007 and 2016, 35 Brazilian municipalities accounted for 85% of confirmed cases in the Notification Disease Information System (SINAN). Of these 35 municipalities, 99.5% are located in the Amazon region and 87% in the State of Pará (Additional file 1: Figure S1). Most of the new confirmed acute Chagas disease cases notified to the Brazilian Ministry of Health were classified as oral transmission [8]. The presence of ~60 species of native vectors in Brazil [9] in a wide endemic area of Chagas disease with different transmission scenarios [10] and a progressive reduction of the human and financial resources needed to sustain the continuity of the control actions, highlight the need for updated studies about surveillance of triatomines in Brazilian states.
Endemic areas for T. cruzi transmission in the state of Bahia were described a few years after the discovery of Chagas disease and were mainly associated with P. megistus [11] and T. infestans [12]. More recently, outbreaks of T. cruzi transmission associated with T. sordida showed the potential role of this species to transmit T. cruzi to humans [13, 14]. In addition, residual foci of T. infestans were found in Bahia [15] and one acute case of Chagas disease was confirmed in 2018 (Additional file 1: Table S1).
In Bahia, 26 triatomine species have been registered [9, 16]; most are strictly associated with the wild environment or peridomiciles, but others are detected inside houses where they feed on domestic animals and humans [15, 17,18,19,20,21]. The identification of T. cruzi infection and blood-meal sources in synanthropic triatomines is important to assess the potential risk of Chagas disease transmission in human dwellings. Here, we identified T. cruzi infection and blood-meal sources of triatomines caught at different environments in Bahia, northeast Brazil, and mapped the occurrence of infected triatomines that fed on humans and domestic animals between 2013 and 2014.
Methods
Study area
The state of Bahia has 417 municipalities, and it is situated in the northeast region of Brazil (Fig. 1). In the western region of Bahia, the Cerrado is the main biome, with relatively high precipitation between 300 and 800 mm and a tropical climate. A tropical climate of altitude is present in the region of Chapada Diamantina; however, in the semiarid region, where the Caatinga biome predominates, rainfall indices are very low, between 100 and 300 mm, and there are long dry periods. On the marine coast, annual rainfall can exceed 1500 mm, and the main biome is Atlantic Forest.
Insect collection
Geographical information system (GIS) data and triatomine bugs were obtained by trained agents from the Epidemiologic Surveillance team of Bahia State Health Service (SESAB) and IGM/FIOCRUZ-BA. Triatomines were captured monthly between 2013–2014. The collections were carried out as established by the National Programme for the Control of Chagas disease [22] in the localities with a prior history of infestation by triatomines (Fig. 2 ) and were part of the regular activity of the technicians of the health programs of Bahia health surveillance system. The inspections of the internal and external walls of the residence and inside rooms of the house unit and its annexes were carried out following the standardized inspection protocol of Brazil ministry of health [23].
Health agents performed exhaustive sampling and captured all the specimens found inside houses and at the peridomestic environment (kennel, cattery, corral, etc.). Additionally, triatomines were collected from the wild environment (away from human settlements) to serve as negative controls in the analyses of blood-meal sources, as DNA of humans and domestic animals (cats, dogs, and humans) was not expected in wild bugs. Metal forceps and flashlights were used to survey crevices and nonluminous sites. We selected the sampled municipalities according to epidemiological priority criteria and the capacity of SESAB at each municipality to carry out the program activities. The teams carried out collections based on the surveillance strategy of the Chagas Disease Control Programme of SESAB.
Triatomines were identified using specific identification keys [24]. Then, we photographed dorsally and ventrally, and dissected the bugs, separating head and wings, legs, and abdomen. Samples were stored in 70% alcohol + 5% glycerin at 5 °C and labeled with a QR code. We carried out a blind identification process at LACEN-BA and FIOCRUZ-BA, independently, and the teams discussed conflicting identifications individually. In addition, voucher specimens of triatomines were deposited into the SESAB entomological collection, as a reference of triatomine vectors, and all images taken of the insects are available for consultation.
Molecular procedures
Triatomines that were dead/dry or stored incorrectly and first- and second-stage nymphs were not included in the molecular analyses to increase the efficacy of DNA extraction with a DNAzol commercial kit (DNAzol; Gibco BRL/Life Technologies, Gaithersburg, MD, EUA). We dissected triatomines in a biological safety cabinet to avoid contamination of the sample with human DNA. We macerated abdomen samples with TissueLyser L-Beader (Loccus, São Paulo, Brazil), plastic disposable maceration pistils and metal beads. Each sample was kept in a 2 ml autoclaved conical tube with a screw cap, and 1 ml of DNAzol and five autoclaved stainless-steel metal beads were added following the DNAzol standard protocol.
After purification, we quantified the DNA with a NanoDrop™ Spectrophotometer and the samples were set to a concentration of ~100 ng/µl. Conventional [25] and multiplex [26, 27] PCRs were performed with specific primers to detect T. cruzi based on mini-exon genes and the cytochrome c oxidase subunit 2 (cox2) gene.
To confirm that good-quality DNA was present in the samples, we amplified a 127-bp fragment of the ITS2 nuclear rDNA marker [28]. For the amplification of molecular targets, a commercial kit with Qiagen PCR Master Mix (QIAamp, Qiagen, Hilden, Germany) was used in a Mastercycler Gradient thermocycler (Eppendorf, Foster City, California, USA). The PCR conditions and primers are described in Additional file 1: Tables S2–S4.
Samples of T. cruzi cultures were obtained from the Experimental Chagas Disease Laboratory (LACEI/CPqGM) and used for positive controls. The DNA samples of dogs (Canis lupus familiaris L.), birds (Gallus gallus L.) and cats (Felis catus L.) were obtained from the blood of healthy animals from the laboratory. The human blood sample was obtained from researchers of the team (GR and CGSS). All the samples used as controls had the DNA purity evaluated with a NanodropTM spectrophotometer and adjusted to a concentration of ~100 ng/µl. Then, the samples were aliquoted and kept at − 70 °C until use.
During the standardization of the PCR, amplified products of the PCR (10 μl) were separated by electrophoresis in an agarose gel, stained with SYBR Safe (Invitrogen, CA, USA), visualized with blue light and photographed with a Photo-documenter MultiDoc-it (UVP, Imaging Systems, Upland, CA, USA). Images were analyzed with UVP GelStudio™ (VisionWorks, CA, USA) software. Before standardization, we analyzed PCR results by capillary electrophoresis in an Applied Biosystems ABI-3500 DNA sequencer [29]. The generated electropherograms were analyzed with GeneScan analysis software version 3.1.
Statistical analysis and mapping procedures
Trypanosoma cruzi infection and blood-meal frequencies were compared between triatomine species and habitats (intra-, peridomestic, wild environment) by the Chi-square or Fisherʼs exact tests using the StartCalc tool in EpiInfo™. For statistical analyses, 95% confidence intervals (CI) and P-values (< 0.05) were evaluated. Records of triatomines in Bahia were referenced to geographical coordinates using a GPS. When there was no information on the specific GIS coordinates, we calculated the municipality centroid using ArcGIS/ArcMap 10.5 software which was also used to map triatomine spatial distribution.
We analyzed vector hotspots with kernel density spatial analysis. In order to determine if the spatial pattern of the data is either clustered, dispersed or random, the spatial autocorrelation was evaluated by global Moranʼs index (I), z-score and P-value statistics interpretation in the Spatial Autocorrelation tool [58]. To determine the appropriate distance threshold or radius to elaborate kernel density analysis, we used the Incremental Spatial Autocorrelation tool [58]. We used the vector measures spatial autocorrelation for a series of distance increments and reports, for each distance increment, the associated Moranʼs index, expected index, variance, z-score, and P-value. Peaks in z-scores reflect distances where the spatial processes promoting clustering are most pronounced. Hotspots were represented by the Kernel Density tool [58]. The layers (.shp) used during this study were obtained from the IBGE website (https://downloads.ibge.gov.br/).
Results
In total, we collected 5906 triatomines belonging to 15 species from 127 of 417 municipalities in Bahia. Most of them (n = 4640) were collected in 823 household units (intra- and peridomestic environments), especially in peridomestic areas (90%). The distributions of sampled triatomines by species, collection environments and municipalities are shown in Table 1.
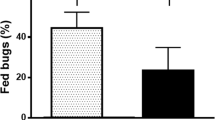
We collected 610 specimens of 10 species inside domiciles in 55 municipalities. We detected colonies of T. sordida, T. pseudomaculata and T. juazeirensis in houses mainly in municipalities in the Caatinga biome. We collected 4030 specimens of 13 species in peridomiciles of 97 municipalities. Triatoma sordida was the most captured and widely distributed species in Bahia State, followed by T. pseudomaculata (Table 1). We detected a colony of T. infestans with more than 400 specimens inside a chicken coop, five meters from a household. We also captured 484 triatomines of four species in the wild environment in four municipalities.
We selected 696 triatomines for molecular evaluation of T. cruzi infection and blood-meal analysis (Table 1). A total of 99.85% (n = 695) showed specific amplification for triatomine DNA with an ITS2 nuclear rDNA marker, indicating the DNA integrity of the samples. All molecular targets evaluated have shown a spatial pattern expressed as clustered and showed an appropriate radius of 1.86 km (T. cruzi), 2.11 km (human), 1.98 km (dog), 1.77 km (cat), 1.98 km (bird) (Fig. 3). Almost 10% (n = 68) of the triatomines were infected with T. cruzi (95% CI: 7.5–12.1%), and the infection rate was highest in the T. brasiliensis species complex (Table 2). The proportion of T. cruzi-infected triatomines was higher in the wild environment (χ2 = 134, df = 1, P < 0.001). Infected triatomines were detected in 25 municipalities, mainly in the Caatinga biome. The kernel spatial analysis showed higher density areas of T. cruzi-infected triatomines in the northeast and central Bahia (Fig. 3b).
Maps showing the distribution of triatomines by Trypanosoma cruzi infection and blood-meal sources in the State of Bahia, Brazil, between 2013–2014. The grey lines and numbers show the limits of the biomes in Bahia State. a Distribution of all triatomines used in molecular detection. b T. cruzi-infected triatomines. c–f Spatial distribution of triatomines fed on humans, dogs, cats, and birds. The heat gradient represents areas with the highest density of vectors by kernel density with a radius of 2.4 km
We found most bugs fed on birds (74.2%), other blood-meal sources were dogs (6%), cats (0.6%) and humans (1%) (Table 3). Triatomines that fed on birds were detected widely in Bahia State (Fig. 3), while those that fed on humans, dogs and cats were mainly detected in the northeast region, near the State of Pernambuco (Fig. 3). There was no significant difference in the frequencies of triatomines fed on cats and humans between intra and peridomestic environments (P > 0.05) but we detected a higher frequency of bugs that fed on dogs inside houses (χ2 = 4.07, df = 1, P = 0.04). The frequency of triatomines that fed on birds was practically the same as in the wild (86.30%) and peridomestic (86.57%) environments (χ2 = 0.013, df = 1, P = 0.9076) and statistically higher in the peridomestic environment than in the domestic environment (χ2 = 8.0, df = 1, P = 0.004).
We detected T. cruzi-infected triatomines fed on humans, dogs, and cats inside houses and triatomines fed on dogs and humans in peridomestic habitats. All infected triatomines detected in the wild environment contained bird DNA.
Discussion
The most salient findings about T. cruzi infection and blood-meal sources in synanthropic triatomines in Bahia were: (i) T. cruzi-infected triatomine bugs fed on human blood; (ii) T. cruzi-infected triatomines were widespread, but bugs that fed on dogs, humans, and cats were observed mainly in the northeast region; and (iii) most bugs fed on birds. These results show that triatomine bugs maintain the presence of T. cruzi in wild and domestic environments in the State of Bahia, Brazil.
We found 15 of the 26 recorded triatomine species in the State of Bahia during our two-year study. This result highlights the diversity of triatomines in this region [9] referring to Bahia as the Brazilian state with the highest number of triatomine species in Brazil. Ten species were recorded inside houses in sampled municipalities, mainly Triatoma sordida, T. pseudomaculata, and the T. brasiliensis species complex. The results differ from those observed before systematic Chagas disease vector control was carried out between 1975 and 1983 when P. megistus and T. infestans were the most captured species inside houses [5].
We observed house-invading P. megistus in few residences, and T. infestans occurred in one municipality in our study (Novo Horizonte); T. infestans were also recorded in other two municipalities in Bahia in the last years (Ibipeba and Tremedal) [15, 30, 31]. These results show the success in controlling domestic triatomines with the virtual elimination of T. infestans in Bahia municipalities [32, 33].
Our data show that T. sordida and T. pseudomaculata are the most frequently captured species in the State of Bahia, as already reported [9, 21, 34] which demonstrates their ability to colonize synanthropic environments. Other studies showed that domestic infestation with T. pseudomaculata increases when houses are located near preserved forests with natural ecotopes such as bird nests, tree hollows and palms [34]. Our results showed a wide distribution of T. sordida in the State of Bahia. A study of synanthropic triatomines in the southwest of Bahia between 2008 and 2013 [21] also showed that T. sordida was the most frequently captured species and presented the highest percentage of infection with T. cruzi. Although most specimens were captured in peridomestic habitats, as expected [9], we detected the presence of colonies and infected specimens inside houses revealing a potential risk for vectorial transmission. The other possible scenario is the risk of T. cruzi oral outbreaks mediated by T. sordida specimens inside houses, as already recorded in Bahia State [27].
Our results also revealed the high frequency of the T. brasiliensis species complex in Bahia. Costa et al. [35] showed high domestic infestation and infection rates for T. brasiliensis in Bahia between 1993 and 1999 when compared with other states of the northeast region. We detected T. brasiliensis brasiliensis in 20 municipalities, mainly in the northeast region of the state. To the best of our knowledge, our results show the first record of T. brasiliensis brasiliensis in the Bahia State. The most recent data recorded only T. juazeirensis, T. melanica, T. lenti, T. bahiensis, T. sherlocki and T. petrocchiae in Bahia [36]. Therefore, our results expand the knowledge of the geographical distribution of T. brasiliensis brasiliensis in northeastern Brazil. Triatoma juazeirensis is a recently described species [37] that was commonly misidentified as T. brasiliensis. Our results add new information about the behavior of this species revealing a higher number of triatomines in houses than in peridomestic habitats. We also revealed a high infection rate of T. sherlocki in a domestic environment in Bahia. Colonies of T. sherlocki were already found in houses of Bahia with T. cruzi infection of ~11% [38], revealing a domiciliation process and the potential risk for vectorial transmission to humans.
Trypanosoma cruzi has been detected in vectors in all regions of Bahia. We found high infection rates for T. sherlocki and T. tibiamaculata as already observed by Almeida et al. [38] and Ribeiro et al. [20], respectively. Trypanosoma cruzi infections in triatomines based on parasite morphology after optical microscopy are underestimated [39]. Consequently, the risk of T. cruzi transmission should be higher than the entomological-parasitological routine surveillance suggests [40, 41]. For example, test-specific naïve indices of T. cruzi infection in triatomines varied from 17.8%, considering only optical microscopy results, to 41.5%, considering PCR results (23.1% positive by conventional PCR and 41.3–41.4% by qPCR) [41]. Our results revealed a triatomine infection rate of approximately 10% by conventional PCR, suggesting that the triatomine infection in Bahia may be even higher than that observed in our study. These infection rates also vary according to the species and development stage of the sampled specimens. For example, T. cruzi infection rates observed for T. tibiamaculata ranged between 50–65% [20].
The infection rates of T. cruzi observed in our study were similar to those obtained in recent studies carried out in Pernambuco [42], Mato Grosso do Sul [43, 44] Ceará [45, 46], the Rio Grande do Norte [47, 48] and Bahia [19, 20]. Infection rates were high for T. brasiliensis species complex, especially for T. sherlocki (43.1%). Almeida et al. [47] detected T. cruzi in 52–71% of T. brasiliensis captured in Rio Grande do Norte, a higher percentage than that observed in our study in Bahia (15.8%) that could be explained by different blood-feeding habitats. The T. sherlocki infection rate observed in our study was four times higher than that reported by Almeida et al. [38]. Most of T. sherlocki specimens fed on avian blood, but the high level of infection rates of T. cruzi indicate an eclectic feeding behavior of T. sherlocki. The infection with T. cruzi detected in Ps. tertius, a species commonly associated with furnariid birds could also be explained by opportunistic feeding on mammals that eventually are found in furnariid nests. The results suggest a previous feeding of Ps. tertius with infected mammal blood.
Trypanosoma cruzi infection rates in T. sordida were generally less than 5% based on parasitological methods; however, Minuzzi-Souza et al. [41] estimated rates of 35% based on qPCR, a more sensitive evaluation method, which reinforces the relevance of this species as a potential T. cruzi vector. Triatoma infestans was not found to be infected by T. cruzi, as all the specimens were collected from a single colony into a chicken coop near the household unit; this is an unusual situation for this species, as it is considered to be exotic and domestic in Bahia.
The most frequent blood-meal source detected in triatomines in Bahia was birds (74%), similar to other studies [20, 49]. Birds are an important link in the domiciliation process of triatomines because they are common blood-meal sources in the peridomestic habitat due to be an important source of human food, through the raising of chickens, usually in the peridomicile of households [50,51,52]. Birds were also the main food source for T. brasiliensis species complex, contrasting with other studies highlighting the importance of rodents as feeding sources for T. brasiliensis in the Rio Grande do Norte [47, 48] and Ceará [53]. Human, dog and cat DNA, at 1%, 6%, and 0.6%, respectively, were observed less frequently. It is important to point out that previous studies have shown the key role of domestic animals in maintaining T. cruzi circulation within human dwellings [54, 55]. We found a higher frequency of triatomines fed on dogs inside houses highlighting the role of dogs as a potential source of T. cruzi in domestic transmission cycles, as already discussed [56], showing that dogs can sleep in places that are more accessible to the bugs, increasing the probability of infecting an initially uninfected bug.
The species found with human DNA were T. brasiliensis, T. juazeirensis, and T. pseudomaculata, and several other studies have revealed that these species are capable of transmitting T. cruzi to humans in the domestic environment [34, 35, 37, 48, 53, 57]. Regarding the spatial distribution of T. cruzi and blood-meal sources, we observed clusters of infected triatomines that fed on humans and domestic animals in the municipalities of Curaçá and Irecê, located in the northeast region of Bahia. Simulations of vulnerability to T. cruzi vector-borne transmission in Brazil based on the most prevalent species also have indicated the northeast region of Bahia as having high vulnerability to the vector-borne transmission of T. cruzi; vulnerable municipalities exhibited a higher occurrence of synanthropic triatomines, lower socioeconomic levels (high percentage of properties in rural areas with individuals living in extreme poverty), and more extensive anthropized areas (percentage of deforested area in the municipality) [10].
Conclusions
Triatomines remain widely distributed in Bahia, with relevant T. cruzi infections and feeding on human and domestic animals inside houses, mainly in the northeast region of Bahia, thus maintaining the potential risk of T. cruzi transmission to humans. Ten species were recorded inside houses, mainly Triatoma sordida, T. pseudomaculata, and the T. brasiliensis species complex. Molecular and spatial analysis are useful to reveal T. cruzi infection and blood-meal sources in synanthropic triatomines, identifying areas with an ongoing threat for parasite transmission and improving entomological surveillance strategies.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional file. The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- PCR:
-
polymerase chain reaction
- SESAB:
-
Epidemiologic Surveillance team of Bahia State Health Service
- DNA:
-
deoxyribonucleic acid
- ITS2:
-
internal transcribed spacer 2
- GPS:
-
Global Position System
- GIS:
-
Geographical Information System
- qPCR:
-
quantitative polymerase chain reaction
- SINAN:
-
Notification Disease Information System
References
Stanaway JD, Roth G. The burden of Chagas disease: estimates and challenges. Glob Heart. 2015;10:139–44.
Hotez PJ, Dumonteil E, Woc-Colburn L, Serpa JA, Bezek S, Edwards MS, Hallmark CJ, et al. Chagas disease: “the new HIV/AIDS of the Americas”. PLoS Negl Trop Dis. 2012;6:e1498.
Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagasʼ disease. Bull Am Mus Nat His. 1979;163:123–520.
Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–12.
Silveira AC. Entomological survey (1975–1983). Rev Soc Bras Med Trop. 2011;44(Suppl. 2):26–32.
Santana RAG, Guerra M, Sousa DR, Couceiro K, Ortiz JV, Oliveira M, et al. Oral transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg Infect Dis. 2019;25:132–5.
Abad-Franch F, Diotaiuti L, Gurgel-Goncalves R, Gurtler RE. Certifying the interruption of Chagas disease transmission by native vectors: cui bono? Mem Inst Oswaldo Cruz. 2013;108:251–4.
Salvatella R, Irabedra P, Castellanos LG. Interruption of vector transmission by native vectors and “the art of the possible”. Mem Inst Oswaldo Cruz. 2014;109:122–5.
Gurgel-Gonçalves R, Galvão C, Costa J, Peterson AT. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med. 2012;2012:705326.
Vinhaes MC, de Oliveira SV, Reis PO, de Lacerda Sousa AC, Silva RA, Obara MT, et al. Assessing the vulnerability of Brazilian municipalities to the vectorial transmission of Trypanosoma cruzi using multi-criteria decision analysis. Acta Trop. 2014;137:105–10.
Brumpt E. Silva Pd: Existence du “Schizotrypanum cruzi” Chagas, 1909, à Bahia (Mata de São João) Biologie du Conorhinus megistus. Bull Soc Pathol Exotique. 1912;5:22–6.
Sherlock Í, Serafim EM. Fauna Triatominae no Estado da Bahia, Brasil: as espécies e distribuição geográfica. Rev Soc Bras Med Trop. 1972;6:265–76.
Dias JP, Bastos C, Araujo E, Mascarenhas AV, Martins Netto E, Grassi F, et al. Acute Chagas disease outbreak associated with oral transmission. Rev Soc Bras Med Trop. 2008;41:296–300.
Bastos CJ, Aras R, Mota G, Reis F, Dias JP, de Jesus RS, et al. Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl Trop Dis. 2010;4:e711.
Araujo RF, Jose Mendonca V, Rosa JA, Matos JF, Lima SC, de Araujo Figueiredo MA. Description of a newly discovered Triatoma infestans (Hemiptera: Reduviidae) foci in Ibipeba, State of Bahia Brazil. Rev Soc Bras Med Trop. 2014;47:513–6.
Mendonca VJ, Alevi KC, Pinotti H, Gurgel-Goncalves R, Pita S, Guerra AL, et al. Revalidation of Triatoma bahiensis Sherlock & Serafim, 1967 (Hemiptera: Reduviidae) and phylogeny of the T brasiliensis species complex. Zootaxa. 2016;4107:239–54.
Santana Kde S, Bavia ME, Lima AD, Guimaraes IC, Soares ES, Silva MM, et al. Spatial distribution of triatomines (Reduviidae: Triatominae) in urban areas of the city of Salvador, Bahia Brazil. Geospat Health. 2011;5:199–203.
Dias-Lima AG, Sherlock IA. Sylvatic vectors invading houses and the risk of emergence of cases of Chagas disease in Salvador, State of Bahia, northeast Brazil. Mem Inst Oswaldo Cruz. 2000;95:611–3.
Mendonca VJ, de Oliveira J, Rimoldi A, Filho JC, de Araujo RF, da Rosa JA. Triatominae survey (Hemiptera: Reduviidae: Triatominae) in the south-central region of the state of Bahia, Brazil, between 2008 and 2013. Am J Trop Med Hyg. 2015;92:1076–80.
Ribeiro G Jr, Gurgel-Goncalves R, Reis RB, Santos CG, Amorim A, Andrade SG, Reis MG. Frequent house invasion of Trypanosoma cruzi-infected triatomines in a suburban area of Brazil. PLoS Negl Trop Dis. 2015;9:e0003678.
de la Fuente AL, Dias-Lima A, Lopes CM, Emperaire L, Walter A, Ferreira A, et al. Behavioral plasticity of Triatominae related to habitat selection in northeast Brazil. J Med Entomol. 2008;45:14–9.
Ministério da Saúde/SUCAM Centro de Documentação do Ministério da Saúde. Manual de normas técnicas da campanha de controle da doença de Chagas. Brasília: Ministério da Saúde/SUCAM Centro de Documentação do Ministério da Saúde; 1980.
Ministério da Saúde Fundação Nacional de Saúde Brasília. Controle da Doença de Chagas: Diretrizes Técnicas. Brasília: Ministério da Saúde Fundação Nacional de Saúde; 1996.
Galvão C. Vetores da doença de Chagas no Brasil. Zoologia: guias e manuais de identificação series. Curitiba: sociedade Brasileira de Zoologia; 2014.
Alevi KC, Rosa JA, Azeredo-Oliveira MT. Distribution of constitutive heterochromatin in Triatoma melanocephala (Hemiptera, Triatominae). Genet Mol Res. 2014;13:7899–903.
Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–52.
Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–4.
Marcilla A, Bargues MD, Ramsey JM, Magallon-Gastelum E, Salazar-Schettino PM, Abad-Franch F, et al. The ITS-2 of the nuclear rDNA as a molecular marker for populations, species, and phylogenetic relationships in Triatominae (Hemiptera: Reduviidae), vectors of Chagas disease. Mol Phylogenet Evol. 2001;18:136–42.
Rozas M, De Doncker S, Adaui V, Coronado X, Barnabe C, Tibyarenc M, et al. Multilocus polymerase chain reaction restriction fragment-length polymorphism genotyping of Trypanosoma cruzi (Chagas disease): taxonomic and clinical applications. J Infect Dis. 2007;195:1381–8.
Brandão H, Fonseca E, Santos R, Ribeiro-Jr G, Santos CG, Cova B, Will R, Reis M. Descrição de focos residuais de Triatoma infestans (Klug, 1834) no município de Novo Horizonte, Bahia. Rev Baiana Saúde Públ. 2015;39(Suppl. 1):91–104.
Silveira EA, Ribeiro IS, Amorim MS, Rocha DV, Coutinho HS, Freitas LM, et al. Correlation between infection rate of triatomines and Chagas disease in southwest of Bahia, Brazil: a warning sign? An Acad Bras Cienc. 2016;88(Suppl. 3):1941–51.
Silveira AC, Dias JC. The control of vectorial transmission. Rev Soc Bras Med Trop. 2011;44(Suppl. 2):52–63.
Pessoa GC, Rosa AC, Bedin C, Wilhelms T, Mello Fd, Coutinho HS, et al. Susceptibility characterization of residual Brazilian populations of Triatoma infestans Klug, 1834(Hemiptera: Reduviidae) to deltamethrin pyrethroid. Rev Soc Bras Med Trop. 2015;48:157–61.
Walter A, Rego IP, Ferreira AJ, Rogier C. Risk factors for reinvasion of human dwellings by sylvatic triatomines in northern Bahia State. Brazil. Cad Saude Publica. 2005;21:974–8.
Costa J, Almeida CE, Dotson EM, Lins A, Vinhaes M, Silveira AC, Beard CB. The epidemiologic importance of Triatoma brasiliensis as a Chagas disease vector in Brazil: a revision of domiciliary captures during 1993–1999. Mem Inst Oswaldo Cruz. 2003;98:443–9.
Dale C, Almeida CE, Endonca VJ, Oliveira J, da Osa JA, Galvao C, Costa J. An updated and illustrated dichotomous key for the Chagas disease vectors of Triatoma brasiliensis species complex and their epidemiologic importance. ZooKeys. 2018;805:33–43.
Costa J, Felix M. Triatoma juazeirensis sp. nov from the state of Bahia, northeastern Brazil (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 2007;102:87–90.
Almeida CE, Folly-Ramos E, Peterson AT, Lima-Neiva V, Gumiel M, Duarte R, et al. Could the bug Triatoma sherlocki be vectoring Chagas disease in small mining communities in Bahia, Brazil? Med Vet Entomol. 2009;23:410–7.
Lardeux F, Aliaga C, Depickere S. Bias due to methods of parasite detection when estimating prevalence of infection of Triatoma infestans by Trypanosoma cruzi. J Vector Ecol. 2016;41:285–91.
Haidamak J, Shimada MK, Rocio Klisiowicz D, Reifur L. Trypanosoma cruzi vector infection rate in understimated in some localities in the state of Bahia. Rev Patol Trop. 2016;45:55.
Minuzzi-Souza TTC, Nitz N, Cuba CAC, Hagstrom L, Hecht MM, Santana C, et al. Surveillance of vector-borne pathogens under imperfect detection: lessons from Chagas disease risk (mis)measurement. Sci Rep. 2018;8:151.
Silva MB, Barreto AV, Silva HA, Galvao C, Rocha D, Jurberg J, Gurgel-Goncalves R. Synanthropic triatomines (Hemiptera, Reduviidae) in the state of Pernambuco, Brazil: geographical distribution and natural Trypanosoma infection rates between 2006 and 2007. Rev Soc Bras Med Trop. 2012;45:60–5.
Cominetti MC, Csordas BG, Cunha RC, Andreotti R. Geographical distribution of Trypanosoma cruzi in triatomine vectors in the State of Mato Grosso do Sul, Brazil. Rev Soc Bras Med Trop. 2014;47:747–55.
Almeida PS, Ceretti Junior W, Obara MT, Santos HR, Barata JM, Faccenda O. Survey of Triatominae (Hemiptera: Reduviidae) fauna in domestic environments and natural infection by Trypanosomatidae in the State of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2008;41:374–80.
Goncalves TC, Freitas AL, Freitas SP. domestic? Surveillance of Chagas disease vectors in municipalities of the state of Ceara, Brazil. Mem Inst Oswaldo Cruz. 2009;104:1159–64.
Coutinho CF, Souza-Santos R, Teixeira NF, Georg I, Gomes TF, Boia MN, et al. An entomoepidemiological investigation of Chagas disease in the state of Ceara, northeast region of Brazil. Cad Saude Publica. 2014;30:785–93.
Almeida CE, Faucher L, Lavina M, Costa J, Harry M. Molecular individual-based approach on Triatoma brasiliensis: inferences on triatomine foci, Trypanosoma cruzi natural infection prevalence, parasite diversity and feeding sources. PLoS Negl Trop Dis. 2016;10:e0004447.
Lilioso M, Folly-Ramos E, Rocha FL, Rabinovich J, Capdevielle-Dulac C, Harry M, et al. High Triatoma brasiliensis densities and Trypanosoma cruzi prevalence in domestic and peridomestic habitats in the state of Rio Grande do Norte, Brazil: the source for Chagas disease outbreaks? Am J Trop Med Hyg. 2017;96:1456–9.
Lucero DE, Ribera W, Pizarro JC, Plaza C, Gordon LW, Pena R Jr, et al. Sources of blood meals of sylvatic Triatoma guasayana near Zurima, Bolivia, assayed with qPCR and 12S cloning. PLoS Negl Trop Dis. 2014;8:e3365.
Rabinovich JE, Kitron UD, Obed Y, Yoshioka M, Gottdenker N, Chaves LF. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 2011;106:479–94.
Georgieva AY, Gordon ERL, Weirauch C. Sylvatic host associations of Triatominae and implications for Chagas disease reservoirs: a review and new host records based on archival specimens. PeerJ. 2017;5:e3826.
Ricardo-Silva A, Goncalves TC, Luitgards-Moura JF, Lopes CM, Silva SP, Bastos AQ, et al. Triatoma maculata colonises urban domicilies in Boa Vista, Roraima, Brazil. Mem Inst Oswaldo Cruz. 2016;111:703–6.
Bezerra CM, Barbosa SE, Souza RCM, Barezani CP, Gurtler RE, Ramos AN Jr, Diotaiuti L. Triatoma brasiliensis Neiva, 1911: food sources and diversity of Trypanosoma cruzi in wild and artificial environments of the semiarid region of Ceara, northeastern Brazil. Parasit Vectors. 2018;11:642.
Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Negl Trop Dis. 2015;9:e0004235.
Kjos SA, Marcet PL, Yabsley MJ, Kitron U, Snowden KF, Logan KS, et al. Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J Med Entomol. 2013;50:1126–39.
Cohen JE, Gurtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–8.
Costa J, Araújo CA, Freitas CA, Borges-Pereira J. Are members of the Triatoma brasiliensis (Hemiptera, Reduviidae) species complex able to alter the biology and virulence of a Trypanosoma cruzi Strain? Neotrop Entomol. 2015;44:186–93.
ESRI. ArcGIS Desktop: Release 10. Redlands: Environmental Systems Research Institute; 2011.
Acknowledgments
We are grateful to the Oswaldo Cruz Foundation, and the epidemiological surveillance office (SESAB-DIVEP) for the technical support and to the entomology team for coordinating the field collection of triatomines.
Funding
This study was funded by the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, no. 014 2013, PET0023/2013), PROEP/CPqGM (process 400904/2013-6), and Fiocruz/BA-IGM scientific initiation scholarship grant. This study was also supported by the Brazilian National Research Council (CNPq) by a grant to RGG. The funding sources of this study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
GRJ, MGR and RGG outlined the research project and performed gathering of data, writing, technical editing, statistical analysis calculation, Triatominae DNA purification, processing of triatomine samples, Triatominae identification, molecular evaluation, statistical analysis, georeferencing, and language editing. CGSS, GMC, CMMC, RFS, OMFS and EOLF acquired data from SESAB, gathered data, conducted proofreading and Triatominae identification and proofread the manuscript. JR, FV, FL and CD processed triatomine samples, conducted Triatominae DNA purification and molecular evaluation of samples. RFA and RBR proofread and edited the manuscript and performed georeferencing. DLPM, WNA and RBR proofread the manuscript, performed language editing, technical editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The adopted procedures were in accordance with the ethical standards of the Research Ethics Committee of the Gonçalo Moniz Institute (FIOCRUZ, Bahia, Brazil, no. 2.552.284) and with the Helsinki Declaration of 1964, revised in 1975, 1983, 1989, 1996 and 2000. The consent form was waived because the analysis was based on a state surveillance service of Bahia, Brazil. However, no personal identification data were used to ensure the complete anonymity of the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Notified and confirmed acute cases of Chagas disease in Brazil. Table S1. Confirmed cases of acute Chagas disease in Brazil between 2016 and 2019. Table S2. Reagents used for the amplification of molecular targets. Table S3. Thermocycling conditions used for amplification of molecular targets. Table S4. Sequences of the primers used.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ribeiro, G., dos Santos, C.G.S., Lanza, F. et al. Wide distribution of Trypanosoma cruzi-infected triatomines in the State of Bahia, Brazil. Parasites Vectors 12, 604 (2019). https://doi.org/10.1186/s13071-019-3849-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3849-1