Abstract
Background
Moose (Alces alces) are a culturally and economically valued species in Minnesota. However, the moose population has experienced a sudden, marked decline in their range, including extirpation in the northwest and a 66% decline in the last decade in the northeast portions of the state. Although the exact cause of this decline is unclear, parasitic metastrongylid and filarioid nematode infections are known causes of morbidity and mortality in moose across North America.
Methods
To determine if these parasitic nematodes could be contributing to the Minnesota moose population decline, we molecularly examined banked tissues obtained from moose that died of known and unknown causes for the presence of nematode DNA. Extracted brain DNA of 34 individual moose was amplified utilizing primers targeting the 18S rRNA gene and internal transcribed spacer regions of nematodes.
Results
DNA sequencing revealed that PCR products obtained from 15 (44.1%) of the moose were 99% identical to Parelaphostrongylus tenuis, a metastrongylid known to cause neurological disease and death. Additionally, brain tissue from 20 (58.8%) individuals yielded sequences that most closely aligned with Elaeophora schneideri, a parasite associated with neurological impairment but previously unreported in Minnesota. Setaria yehi, a common filarioid parasite of deer, was also detected in the brain tissue of 5 (14.7%) moose. Molecular screening of 618 captured tabanid flies from four trapping sites revealed E. schneideri was present (6%) in the Minnesota environment and transmission could occur locally. Prevalence rates among the flies ranged between 0–100% per trapping site, with Chrysops spp. and Hybomitra spp. implicated as the vectors.
Conclusions
Ultimately, these data confirm that P. tenuis is widespread in the Minnesota moose population and raises the question of the significance of E. schneideri as a contributing factor to morbidity and mortality in moose.
Similar content being viewed by others
Background
The moose (Alces alces) population in Minnesota has exhibited a recent, rapid decline, raising concerns for the future of the species in the state. Between the mid 1980’s and early 2000’s, the northwestern moose population collapsed from > 4000 moose to less than 100 animals [1]. Similarly, the northeast population has decreased by approximately 66%, from 8840 animals in 2006 to 3030 in 2018 [2]. Predation, climate change, habitat alternation and disease, particularly from parasites, have all been implicated as factors contributing to the decline [3,4,5,6,7].
Nematode parasites, particularly lungworms (Dictyocaulus spp., Protostrongylus spp.) and filarioids (Onchocercidae spp.), are known to cause morbidity and mortality in moose and other cervids. A recent study of Minnesota moose carcasses that died from unknown causes or were euthanized due to perceived illness found that 45% of the animals had lesions consistent with nematode neural migration within central nervous system (CNS) tissues [7]. Based on histological appearance, the authors concluded the pathogenic nematode species was Parelaphostrongylus tenuis (Metastrongyloidea: Protostrongylidae), a common nematode parasite of white-tailed deer (Odocoileus virginianus) distributed throughout the eastern USA [8]. Infections by P. tenuis in atypical hosts, including moose, can result in severe neurological disease and mortality due to an aberrant migration of the nematode through CNS tissues [9,10,11,12,13,14].
Parelaphostrongylus tenuis infections are common among cervids of Minnesota [15,16,17]; however, with the sudden decline in moose numbers, it is possible a newly introduced pathogen is circulating in the moose population. Recent observations have raised suspicions that Elaeophora schneideri (Spirurida: Onchocercidae), another pathogenic nematode of moose [18,19,20], may be present in the Minnesota cervid population. Reports of white-tailed deer in Minnesota exhibiting facial swellings consistent with oral food impactions and cropped ears (E. Butler, personal communication) are similar to what have been previously described in E. schneideri-infected deer [21]. In addition, there were multiple reports of moose in the northeast region of the state with poor antler development or impaired vision due to unknown causes (E. Butler, personal communication) but consistent with clinical signs and lesions previously observed in moose infected with E. schneideri [18]. Historically, this parasite was thought to be limited to the western half of North America and small pockets of the southeastern USA; however, it is possible the range of E. schneideri has extended into Minnesota, which could explain the lesions observed in deer and moose.
The life-cycle of E. schneideri involves a cervid host and a hematophagous insect vector. Mule deer (Odocoileus hemionus) and black-tailed deer (Odocoileus hemionus columbianus) are definitive hosts [22,23,24,25], and transmission occurs via the bite of a tabanid horse fly or deer fly (Diptera: Tabanidae) [26, 27]. Tabanid flies become infected when they ingest microfilariae (L1) in a blood meal. After metamorphosis in an infected fly, third-stage larvae (L3) migrate from the fly mouthparts into the host’s circulatory system, where they eventually migrate to the carotid or leptomeningeal arteries and mature into adults (L5) [27,28,29]. Microfilariae progeny generally reside within the smaller capillaries of the head and neck, where they can be acquired by a feeding tabanid fly to complete the parasitic life-cycle [23].
Although infections in definitive mule deer and black-tailed deer hosts are generally subclinical, atypical hosts, including moose, white-tailed deer, elk (Cervus canadensis), sheep (Ovis spp.) and goats (Capra hircus), can develop elaeophorosis [25]. Elaeophorosis is characterized by obstructed blood flow, endothelial damage, thrombosis, and infarction due to the presence of nematodes in the carotid and cephalic arterial system [18, 30, 31]. This disruption in arterial circulation can lead to blindness; ischemic necrosis of the brain, ears, muzzle and other cephalic tissues; poor antler development; oral food impactions; and death [18,19,20,21, 30, 32].
We investigated the potential for non-endemic parasitic nematodes, particularly E. schneideri, to be associated with CNS disease in Minnesota moose. Our objective was to determine if filarioid parasites were detectable in CNS tissue samples of moose from Wünschmann et al. [7] and tabanid flies in Minnesota. Our data reveal that E. schneideri is indeed present in the Minnesota moose herd and tabanid horsefly vectors, suggesting the parasite could contribute to morbidity and mortality in moose and transmission can occur locally. This study serves as the first documentation of E. schneideri in Minnesota and the Midwest, as well as provides new insight into the potential causes of morbidity and mortality in the Minnesota moose population with implications for future population management.
Methods
Moose sample collection and tissue histology
Carcasses from 62 Minnesota moose were necropsied and CNS tissues examined histologically for any pathological changes [7]. Briefly, tissues were collected from carcasses of moose that died of unknown causes, vehicular collisions, or were euthanized by tribal or Department of Natural Resources personnel due to various clinical signs. Sections of central nervous system (CNS) tissues were preserved in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with H&E for microscopic examination. Specimens were categorized as histologically positive (HP) if migration tracts, larvae, morulae, or cross-sections of adult nematodes were visible, whereas specimens with no pathological changes evident in the CNS were categorized as histologically negative (HN). Based on these results, 34 animals (22 HP; 12 HN) were selected for further molecular analysis.
Molecular testing of moose tissues
To screen the preserved moose CNS tissues for the presence of pathogenic nematodes, two separate 10 μm shavings were obtained from the formalin-fixed, paraffin-embedded tissue blocks for molecular analysis. Duplicate shavings were examined to increase sensitivity. Each shaving was subjected to DNA extraction according to manufacturer’s instructions (DNEasy Blood & Tissue Kit, Qiagen, Valencia, CA, USA). DNA extraction control was included to detect contamination during the DNA extraction process. Purified DNA was first screened for the presence of P. tenuis using a nested polymerase chain reaction (PCR) with the primary primer pair PTP1 (5'-CCG TCG AAT ACA TGT CAT CC-3') and PTP2 (5'-TCG TCA AGA CGA TGA TTC CC-3'); and the secondary primer pair PtIntITSF (5'-AGA ATT ACG ACA ATG GCA AC-3') and PtIntITSR (5'-ATG ATA CCC ATT GAT AAT C-3'), as previously described [13, 14, 33]. This assay is designed to selectively amplify a 110 bp portion of the second internal transcribed spacer region (ITS2) of Parelaphostrongylus spp. [33]. To screen for the presence of other nematodes in the moose CNS tissues, the Nematoda-wide primers Nem18SF (5'-CGC GAA TRG CTC ATT ACA ACA GC-3') and Nem18SR (5'-GGG CGG TAT CTG ATC GCC-3') that targets a 508 bp segment of the 18S rRNA gene were utilized as previously described [34]. All positive PCR products were purified using the Qiagen PCR Purification Kit (Qiagen, Valencia, CA, USA) and sequenced at the University of Tennessee Genomics Core (Knoxville, TN, USA).
Tabanid fly collection and molecular screening
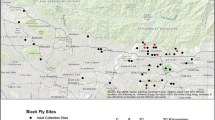
To survey for the presence of cervid nematode parasites in tabanid flies, 618 flies were collected from four locations in Minnesota (Fig. 1) using a canopy trap [35]. Sites were chosen based on their proximity to known moose habitat, remoteness and ease of access. Three of the four sites fall within the current Minnesota moose range and the fourth site, located in the Carlos Avery Wildlife Management Area in Anoka County, serves as an outside representative. Traps were operated intermittently in June-September of 2013. Captured specimens were stored at ~20 °C until they could be sorted, identified to genus, and then preserved in 70% ethanol for later DNA extraction. DNA was extracted using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA) following manufacturer's instructions. Flies were divided into groups of 10, and 5 μl of DNA from each fly was pooled into the corresponding group’s microcentrifuge tube. To screen for nematode DNA within pooled fly DNA, a PCR reaction with the Nem18S primers described above were utilized. Individual flies in PCR-positive DNA pools were subjected to an additional PCR reaction using the same Nem18S primers. All positive reactions were purified using the Qiagen PCR Purification Kit (Qiagen, Valencia, CA, USA) and sequenced at the University of Tennessee’s Genomics Core (Knoxville, TN, USA).
Phylogenetic analysis of parasite sequences
All 18S and ITS2 consensus sequence chromatograms were trimmed and edited by hand using Sequencher 5.3 (Gene Codes Corporation, Ann Arbor, MI, USA). Edited sequences were compared against the NCBI GenBank database. Due to the scarcity of published genetic data for parasitic nematodes known to infect cervids, we also compared our genetic data with sequences obtained from adult reference nematodes (Additional file 1: Table S1) that we identified morphologically and subjected to DNA extraction and PCR amplification as described above. Alignment and construction of neighbor-joining trees of 18S nematode sequences were done using MEGA 6.0 [36]. All consensus sequences were deposited in the GenBank database under the accession numbers KT020850, KT031393, KT878970, KT878971, KT878974, KT878980-KT878988, KT885226, KT885227, KT907501-KT907509 and KT93494.
Parasite prevalence estimates and statistical analysis
To estimate the prevalence of infected fly vectors, we compared the percentage of infected flies with each nematode species that was identified through DNA sequencing and phylogenetics. Our analysis also included a comparison of parasite prevalence among trapping sites, as well as among different fly genera using Dunn’s multiple comparisons test (P ≤ 0.05). Statistical analyses were performed with GraphPad Prism v.6 (GraphPad Software, La Jolla, CA, USA).
Results
Multiple nematode species detected in CNS tissues of free-ranging Minnesota moose
Using Nematoda-wide 18S primers, nematode DNA was successfully amplified and sequenced from 24 (68.6%) individual moose. When compared against GenBank, sequences most closely aligned with 18S sequences from nematode species of the family Onchocercidae, including Setaria digitata (GenBank: DQ094175.1), with a 98–99% maximum identity. Despite the high percent identity, S. digitata is not commonly associated with cervids and is not endemic to North America [25, 37,38,39]. Due to the highly conserved nature of the 18S rRNA target, we concluded the sequences from the Minnesota moose tissues indeed belonged to filarioid nematodes; however, they were most likely not S. digitata.
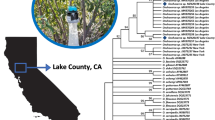
A phylogenetic comparison of 18S sequences from the Minnesota CNS moose tissues and reference specimens revealed the presence of 3 distinct species of filarioids in the moose CNS tissue samples (Fig. 2): Setaria yehi, E. schneideri and Rumenfilaria andersoni. The arterial worm, E. schneideri, was detected in 20 individual moose, 10 (50%) of which had no histological evidence of CNS nematode infections, and 10 moose had either migration tracts or nematodes visible in the CNS sections (Table 1). A second nematode species, S. yehi, was detected in one histologically-negative moose and four moose with migration tracts in the CNS. Two animals were positive for E. schneideri and S. yehi. Rumenfilaria andersoni was detected in a single moose. All nematode sequences obtained were deposited into GenBank (Additional file 2: Table S2).
Phylogenetic analysis of partial nematode18S sequences (508 bp) obtained from formalin-fixed paraffin-embedded CNS tissues of various ruminants. Tree was constructed using the maximum likelihood method and the evolutionary distances computed using the Kimura 2-parameter method. Bootstrap values ≥ 50% are shown above the branches. The tree is drawn to scale. Reference nematodes are labeled with their respective NCBI GenBank accession number. Markers indicate the detection of double infections (triangles, P. tenuis + E. schneideri; circles, S. yehi + E. schneideri; squares, P. tenuis + S. yehi)
Elaeophora schneideri present in Minnesota tabanid flies
A total of 618 tabanid flies in three genera were trapped from four locations (Table 2) and molecularly screened for the presence of filarioid nematodes. In order of descending abundance, the flies identified were Chrysops spp., Hybomitra spp. and Tabanus spp. Representatives from all three genera were collected at each of the trapping sites except for the St. Louis County location, where only Chrysops spp. were obtained.
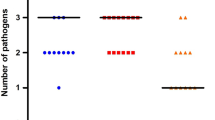
PCR screening and sequencing with Nematoda-specific 18S rRNA primers revealed 5.8% (95% CI: 4.0–7.6%) of the flies tested were positive for E. schneideri (Fig. 3). The majority of E. schneideri-sequence-positive flies were Chrysops spp. (86.2%; 95% CI: 74.9–97.5%), with Hybomitra spp. representing only 13.9% (95% CI: 2.6–25.2%) (Fig. 4). Elaeophora schneideri was not detected in the Tabanus spp. tested. Although prevalence of E. schneideri varied slightly across fly genera, these differences were not statistically significant (Friedman test with Dunn’s correction for multiple comparisons, adjusted P-value < 0.05 used to indicate a significant difference). Prevalence of E. schneideri across trapping sites varied greatly, ranging from 0% (0 of 110) at Grand Portage to 2.3% (95% CI: 0.9–3.7%) at Anoka County, to 9.3% (95% CI: 1.5–17.1%) at Lake County, and 100% (21 of 21) at the St. Louis County location (Fig. 4). However, these differences were not statistically significant (Friedman test with Dunn’s correction for multiple comparisons, adjusted P-value > 0.05).
18S rRNA gene sequencing and phylogenetic analysis reveals the presence of multiple filarioid nematodes in Minnesota tabanid horseflies. Fifty-four nematode 18S rRNA gene sequences (796 bp) were used in the analysis. Fly isolate F-369 was not included in the analysis due to poor quality sequence data. The evolutionary history was inferred using the maximum likelihood method and evolutionary distances computed using the Kimura 2-parameter method. The tree is drawn to scale. Bootstrap values are shown above the branches. Colored markers correspond to fly species and trapping location. Reference nematodes are labeled with their respective NCBI GenBank accession number
Detectable E. schneideri in Minnesota tabanid horseflies varies among fly species and trapping sites. Number of flies tested (a) and prevalence (number of positive flies / total number of flies) (b) that were PCR-positive (blue) or negative (black) for E. schneideri was determined based on 18S sequencing results for each trapping location and each of the fly genera tested
Other filarioid species detected in Minnesota tabanid flies
In addition to E. schneideri, two other species of filarioid worms were detected in the Minnesota horse flies (Fig. 3). Rumenfilaria andersoni was detected in 9 Chrysops spp. flies from the Grand Portage site. Furthermore, additional unique filarioid sequences were detected in three Chrysops spp. flies. When compared against GenBank, the unknown filarioid sequences most strongly aligned with 18S sequences from Dipetalonema spp. (GenBank: DQ531723.1) and Loa loa (GenBank: DQ094173.1) with 99% identities. Based on the sequence data, we were unable to definitively identify the genus or species of these unknown nematode(s).
Multiple E. schneideri 18S haplotypes identified in hosts and vectors of Minnesota
A comparison of all E. schneideri 18S sequences amplified from tabanid and moose hosts revealed the presence of four distinct 18S haplotypes (Additional file 2: Table S2, Additional file 3: Table S3). Two 18S haplotypes were observed among the tabanid E. schneideri sequences, with 94% (34 of 36) representing a single haplotype we denoted as “ES-1”; the other haplotype had a single representative and was labeled as “ES-2.” The sequence quality was poor for one isolate, F-369, and was therefore not included in this analysis. Analysis of the 18S rRNA gene sequences from the moose CNS tissues revealed 90 ± 13.2% (95% CI: 76.8–100%) of the sequences were identical to the ES-1 haplotype found in the flies. Two additional unique haplotypes, each with a single representative (“ES-3,” “ES-4”; Additional file 2: Table S2) were also observed.
Discussion
To our knowledge, this study is the first to report the presence of the arterial worm E. schneideri in the Midwest of the USA, specifically Minnesota, indicating E. schneideri could be an emerging pathogen in moose. Historically, E. schneideri was thought to be primarily found in the western half of North America, presumably in conjunction with the geographical range of the well-adapted mule deer and black-tailed deer definitive hosts. In these hosts, prevalence of E. schneideri is high, reaching levels of 78–100% [26, 32]. However, the parasite has also been reported in white-tailed deer in Florida, Georgia, South Carolina and Texas with a much lower prevalence of 2–10% [24, 40]. It is speculated that the emergence of E. schneideri in the southeastern USA is a consequence of the translocation of infected deer from endemic areas in the West [25, 31, 40]. At this time, it is uncertain if the emergence of E. schneideri in Minnesota is due to an importation event(s) or a natural expansion of the parasite’s geographical range. Alternatively, E. schneideri could be endemic in Minnesota moose but remained undetected due to low prevalence. Future comparative genetics studies of various geographical isolates may provide insight into the origin of Minnesota E. schneideri.
Our surveillance of tabanid flies for the presence of filarioid nematodes further confirms the presence of E. schneideri in Minnesota and suggests the nematode is being transmitted and maintained in the environment. An overall prevalence of 5.8% in the Minnesota flies was relatively high compared to the 0.3% reported in South Carolina [41] and 0.8% in Montana [42]; however, prevalence rates as high as 20% have been reported in New Mexico [26, 43]. Interestingly, we were able to sequence E. schneideri from Chrysops spp. and Hybomitra spp. flies (Fig. 2). Previous surveys implicated Hybomitra spp. as an intermediate host for E. schneideri [42,43,44], but this is the first time the parasite has been detected in Chrysops spp. flies. Although we failed to detect E. schneideri in Tabanus spp. flies, we recognize our sample size was low, and thus, it remains undetermined if Tabanus spp. contribute to the eco-epidemiology of E. schneideri in the Minnesota system.
The presence of E. schneideri could have significant health implications for Minnesota moose. Reported clinical signs of elaeophorosis in moose include sloughed ear tips [19], blindness [18, 20], neurological impairment and death [45]. Typically, this is due to the nematodes restricting blood flow in the carotids and other cephalic arteries, leading to the development of ischemic lesions [18, 20, 30]. Interestingly, neither ischemic cerebrocortical necrosis nor the presence of intra-arterial nematodes in the brain, including meninges, were observed in any of the cases. Instead, animals with abnormal histological findings exhibited lesions consistent with the metastrongylid parasite, P. tenuis. All moose with observed nematodes were sequence-positive for P. tenuis, which is expected since this organism is a common CNS pathogen in Minnesota moose [7]. However, E. schneideri was detected in moose with both normal histology and lesions consistent with nematode migration tracts. Aberrant migration has not been reported with E. schneideri, thus the observed migration tracks are most likely caused by coinfection with P. tenuis. Although these samples were PCR-negative for P. tenuis, amplification of DNA from FFPE samples lacking visible nematodes can have limited success [46]. We suspect the E. schneideri detected in the Minnesota moose CNS tissues originated in the arteries, most likely of the leptomeninges, of the cranium, and may have been dislodged during necropsy. Under these conditions, the parasites may not have caused overt gross or histological lesions. This suggests infection with E. schneideri may not necessarily result in clinical CNS disease.
Previous studies surveying hunter-killed moose for E. schneideri implied elaeophorosis is relatively mild in these animals, with many exhibiting subclinical infections [45, 47]; however, both surveys took place in endemic regions (Colorado and Wyoming) and only examined seemingly healthy individuals. We would predict co-infections with E. schneideri and P. tenuis or other parasite species would negatively impact overall moose health. We speculate that these infections could lead to a compromised immune state, allowing greater susceptibility to other pathogens, predation, or result in decreased reproductive rates. In Minnesota, where the moose population is already impacted negatively by parasites, including P. tenuis, liver flukes (Fascioloides magna) and winter ticks (Dermacentor albipictus) [7], the emergence of E. schneideri could further repress an already struggling population.
In addition to E. schneideri, we also detected the presence of other filarioid species in the moose CNS tissues and tabanid flies. Sequences from unidentified species of filarioid nematodes were amplified from three Chrysops spp. flies from the Grand Portage trapping site (Fig. 2). We were unable to determine the identity or the significance, if any, of these findings. Future studies examining the flies for infectious third stage larvae may allow these species to be differentiated based on morphological characters. Screening of the CNS tissue samples revealed S. yehi and R. andersoni were present in a select number of Minnesota moose (Table 1). Setaria yehi is a common parasite of white-tailed deer and found in the peritoneal cavity. No associations with disease in moose have been reported, although other Setaria species, namely Setaria digitata and Setaria cervi, have documented neurotropism in cattle and deer, respectively [25, 48]. Rumenfilaria andersoni, another filarioid nematode, infects moose, caribou (Rangifer tarandus) and white-tailed deer [49,50,51,52]. Adult R. andersoni reside within the lymphatic vessels of the rumen and microfilariae can be observed in the general circulatory system [49, 50]. It is unknown if infections with R. andersoni can lead to clinical or subclinical disease; only macroscopic inflammatory changes within the ruminal vessels of infected reindeer have been described [51]. Microfilariae identified as S. yehi and R. andersoni have been observed in blood samples from live-captured moose of Minnesota [52], so detection of these microfilariae in these samples is not surprising.
Conclusions
To our knowledge, this study is the first to document the presence of the pathogenic nematode E. schneideri in moose and tabanid fly populations of Minnesota, USA, indicating the occurrence of local transmission and expanding the current known distribution of E. schneideri. A better understanding of the distribution of E. schneideri is essential to help prevent the spread of this parasite to other non-endemic locations through human-mediated translocation of infected cervids, as well as its potentially negative economic impact on domestic farmers (loss of livestock, cost of treatment, etc.) or state and local governments (loss of hunting and ecotourism revenues). Furthermore, we were able to enhance our understanding of E. schneideri eco-epidemiology by implicating another genus of tabanid flies as a newly discovered vector of E. schneideri. These data will help set the foundation for future research investigating E. schneideri, particularly with regards to elaeophorosis and the potential impact it may have on moose and other cervid populations.
Abbreviations
- CNS:
-
Central nervous system
- HN:
-
Histologically negative
- HP:
-
Histologically positive
- ITS2:
-
Second internal transcribed spacer region
- ND:
-
Not done
References
Lenarz MS. Aerial moose survey. Minnesota Department of Natural Resources. 2007; https://files.dnr.state.mn.us/recreation/hunting/moose/moose_survey_2007.pdf. Accessed 8 Feb 2018
DelGiudice GD. 2018 Aerial moose survey. Minnesota Department of Natural Resources. http://files.dnr.state.mn.us/wildlife/moose/moosesurvey.pdf. 2018. Accessed 13 Feb 2018.
Murray DL, Cox EW, Ballard WB, Whitlaw HA, Lenarz MS, Custer TW, et al. Pathogens, nutritional deficiency, and climate influences on a declining moose population. Wildl Monogr. 2006;166:1–29.
Lenarz MS, Nelson ME, Schrage MW, Edwards AJ. Temperature mediated moose survival in Northeastern Minnesota. J Wildl Manage. 2009;73:503–10.
Mench LD, Fienberg J. Re-evaluating the northeastern Minnesota moose decline and the role of wolves. J Wildl Manage. 2014;78:1143–50.
Carstensen M, Hildebrand EC, Plattner D, Dexter MH, Jennelle C, Wright RG. Determining cause-specific mortality of adult moose in northeast Minnesota. In: Cornicelli L, Carstensen M, Grund MD, Larson MA, Lawrence JS, editors. Summaries of Wildlife Research Findings. St. Paul: Minnesota Department of Natural Resources; 2015. p. 133–43.
Wünschmann A, Armien AG, Butler E, Schrage M, Stromberg B, Bender JB, et al. Necropsy findings in 62 opportunistically collected free-ranging moose (Alces alces) from Minnesota, USA (2003–2013). J Wildl Dis. 2015;51:157–65.
Lankester MW. Extrapulmonary lungworms of cervids. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic Diseases of Wild Mammals. Ames: Iowa State University Press; 2001. p. 228–78.
Anderson RC, Strelive UR. The effect of Pneumostrongylus tenuis (Nematoda: Metastrongyloidea) on kids. Can J Comp Med. 1969;33:280–6.
Whitlaw HA, Lankester MW. A retrospective evaluation of the effects of parelaphostrongylosis on moose populations. Can J Zool. 1994;72:1–7.
Pugh DG, Blagburn BL, Causey MK, Wolfe DF. Clinical parelaphostrongylosis in llamas. Compend Contin Educ Vet. 1995;17:600–6.
Bender LC, Schmitt SM, Carlson E, Haufler JB, Beyer DE. Mortality of rocky mountain elk in Michigan due to meningeal worm. J Wildl Dis. 2005;41:134–40.
Gerhold RW, Keel MK, Arnold K, Hotton D, Beckstead RB. Parelaphostrongylus tenuis-associated meningoencephalitis in a sika deer (Cervus nippon). J Wildl Dis. 2010;46:287–90.
Tanabe M, Gerhold RW, Beckstead RB, de Lahunta A, Wade SE. Molecular confirmation of Parelaphostrongylus tenuis infection in a horse with verminous encephalitis. Vet Pathol. 2010;47:759.
Anderson RC. The ecological relationships of meningeal worm and native cervids in North America. J Wildl Dis. 1972;8:304–10.
Slomke AM, Lankester MW, Peterson WJ. Infrapopulation dynamics of Parelaphostrongylus tenuis in white-tailed deer. J Wildl Dis. 1995;31:125–35.
Vanderwaal KL, Windels SK, Olson BT, Vannatta JT, Moen R. Landscape influence on spatial patterns of meningeal worm and liver fluke infection in white-tailed deer. Parasitology. 2015;142:706–18.
Worley DE, Anderson CK. Elaeophorosis in moose from Montana. J Wildl Dis. 1972;8:242–4.
Madden DJ, Spraker TR, Adrian WJ. Elaeophora schneideri in moose (Alces alces) from Colorado. J Wildl Dis. 1991;27:340–1.
Pessier AP, Hamilton VT, Foreyt WJ, Parish S, McElwain TL. Probable elaeophorosis in a moose (Alces alces) from eastern Washington state. J Vet Diagn Invest. 1998;10:82–4.
Couvillion CE, Nettles VF, Rawlings CA, Joyner RL. Elaeophorosis in white-tailed deer: pathology of the natural disease and its relation to oral food impactions. J Wildl Dis. 1986;22:214–23.
Hibler CP, Adcock JL, Davis RW, Adbelbaki YZ. Elaeophorosis in deer and elk in the Gila National Forest, New Mexico. Bull Wildl Dis Assoc. 1969;5:27–30.
Weinmann CJ, Anderson JR, Longhurst WM, Connolly G. Filarial worms of Columbian black-tailed deer in California 1. Observations in the vertebrate host. J Wildl Dis. 1973;9:213–20.
Waid DD, Warren RJ, Pence DB. Elaeophora schneideri Wehr and Dickmans, 1935 in white-tailed deer from the Edwards Plateau of Texas. J Wildl Dis. 1984;20:342–5.
Anderson RC. Filarioid nematodes. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic Diseases of Wild Mammals. Ames: Iowa State University Press; 2001. p. 342–56.
Hibler CP, Gates GH, White R, Donaldson BR. Observations on horseflies infected with larvae of Elaeophora schneideri. J Wildl Dis. 1971;7:43–5.
Hibler CP, Metzger CJ. Morphology of the larval stages of Elaeophora schneideri in the intermediate and definitive hosts with some observations on their pathogenesis in abnormal definitive hosts. J Wildl Dis. 1974;10:361–9.
Hibler CP, Adcock JL, Gates GH, White R. Experimental infection of domestic sheep and mule deer with Elaeophora schneideri Wehr and Dikmans, 1935. J Wildl Dis. 1970;6:110–1.
Hibler CP, Gates GH, Donaldson BR. Experimental infection of immature mule deer with Elaeophora schneideri. J Wildl Dis. 1974;10:44–6.
Adcock JL, Hibler CP. Vascular and neuro-opthalmic pathology of elaeophorosis in elk. Pathol Vet. 1969;6:185–213.
Pence DB. Elaeophorosis in wild ruminants. Bull Soc Vect Ecol. 1991;16:149–60.
Pence DB, Gray GG. Elaeophorosis in Barbary sheep and mule deer from the Texas Panhandle. J Wildl Dis. 1981;17:49–56.
Mitchell KJ, Peters-Kennedy J, Stokol T, Gerhold RW, Beckstead RB, Divers TJ. Diagnosis of Parelaphostrongylus spp. infection as a cause of meningomyelitis in calves. J Vet Diagn Invest. 2011;23:1097–103.
Floyd RM, Rogers AD, Lambshead PJD, Smith CR. Nematode-specific PCR primers for the 18S small subunit rRNA gene. Mol Ecol Notes. 2005;5:611–2.
Hribar LJ, Leprince DJ, Foil LD. Design for a canopy trap for collecting horse flies. J Am Mosq Control Assoc. 1991;7:657–9.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
Becklund W, Walker M. Taxonomy, hosts, and geographical distribution of Setaria (Nematoda: Filarioidea) in the United States and Canada. J Parasitol. 1969;55:359–68.
Over HJ, Jansen J, van Olm PW. Distribution and impact of helminth diseases of livestock in developing countries. In: Food and agriculture organization animal production and health paper. United Nations; 1992. http://www.fao.org/3/a-t0554e.pdf. Accessed 8 Feb 2018.
Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. New York: Springer-Verlag; 1995.
Prestwood AK, Ridgeway TR. Elaeophorosis in white-tailed deer of the southeastern USA: case report and distribution. J Wildl Dis. 1972;8:233–6.
Couvillion CE, Sheppard DC, Nettles VF, Bannaga OM. Intermediate hosts of Elaeophora schneideri Wehr and Dikmans, 1935 on South Island, South Carolina. J Wildl Dis. 1984;20:59–61.
Espinosa RH. Tabanid vectors of the arterial nematode, Elaeophora schneideri, in southwestern Montana. MSc Thesis: Montana State University, Bozeman, USA; 1987.
Clark GG, Hibler CP. Horse flies and Elaeophora schneideri in the Gila National Forest, New Mexico. J Wildl Dis. 1973;9:21–5.
Davies RB. The ecology of Elaeophora schneideri in Vermejo Park, New Mexico. PhD Dissertation: Colorado State University, Fort Collins, USA; 1979.
Henningsen JC, Williams AL, Tate CM, Kilpatrick SA, Walter WD. Distribution and prevalence of Elaeophora schneideri in moose in Wyoming. Alces. 2012;48:35–44.
Dobey CL, Grunenwald C, Newman SJ, Muller L, Gerhold RW. Retrospective study of central nervous system lesions and association with Parelaphostrongylus species by histology and specific nested polymerase chain reaction in domestic camelids and wild ungulates. J Vet Diagn Invest. 2014;26:748–54.
LeVan IK, Fox KA, Miller MW. High elaeophorosis prevalence among harvested Colorado moose. J Wildl Dis. 2013;49:666–9.
Tung KC, Lai CH, Ooi HK, Yang CH, Wang JS. Cerebrospinal setariosis with Setaria marshalli and Setaria digitata infection in cattle. J Vet Med Sci. 2003;65:977–83.
Lankester M, Snider JB. Rumenfilaria andersoni n. gen., n. sp. (Nematoda: Filarioidea) in moose from northwestern Ontario, Canada. Can J Zool. 1982;60:2455–8.
Laaksonen S, Saari S, Nikander S, Oksanen A, Bain O. Lymphatic dwelling filarioid nematodes in reindeer Rangifer tarandus tarandus (Cervidae) in Finland, identified as Rumenfilaria andersoni Lankester & Snider, 1982 (Nematoda: Onchocercidae: Splendidofilariinae). Parasite. 2010;17:23–31.
Laaksonen S, Oksanen A, Hoberg E. A lymphatic dwelling filarioid nematode Rumenfilaria andersoni (Filarioidea; Splendidofilariinae), is an emerging parasite in Finnish cervids. Parasit Vectors. 2015;8:282.
Grunenwald CM, Carstensen M, Hildebrand E, Elam J, Laaksonen S, Oksanen A, et al. Epidemiology of the lymphatic-dwelling filarioid nematode Rumenfilaria andersoni in free-ranging moose (Alces alces) and other cervids of North America. Parasit Vectors. 2016;9:450.
American Society of Mammalogists. Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. J Mammal. 1998;79:1413–6.
Sikes RS, Gannon WL. Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2011;92:235–53.
American Veterinary Medical Association Panel on Euthanasia. AVMA guidelines for the euthanasia of animals. 2013. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed on 8 Feb 2018.
Acknowledgements
The authors thank Rebecca Trout-Fryxell for her help with technical advice on tabanid molecular analysis. We would like to thank Carl Betlach and Samantha Schroth for their help in processing tabanid specimens. We would also like to thank Mabre Brand for her help in processing tabanids for molecular analysis. We also thank Amanda Hand (funded by the University of Tennessee College of Veterinary Medicine) for technical editing of the manuscript. Reference nematodes were generously donated by Kimberlee Beckmen at the Alaskan Department of Wildlife, Fish and Game, John Henningsen at the Wyoming State Veterinary Laboratory, Sauli Laaksonen of the Finnish Food Safety Authority and Kevin Keel of the University of Georgia.
Funding
Funding for the research was provided by the Minnesota Department of Natural Resources Section of Wildlife and the University of Tennessee Department of Biomedical and Diagnostic Sciences. Fellowship funding for graduate student (CG) was provided by the University of Tennessee, Department of Microbiology.
Availability of data and materials
Genetic sequence data have been submitted to the GenBank database under the accession numbers KT020850, KT031393, KT878970, KT878971, KT878974, KT878980-KT878988, KT885226, KT885227, KT907501-KT907509 and KT93494. All data generated or analyzed during the current study are included in this published article and its additional files.
Author information
Authors and Affiliations
Contributions
CG, EB and RG conceived and designed the study and drafted the manuscript. CG and RG performed the molecular analysis of reference nematodes. CG performed molecular analysis of moose and tabanid samples and completed all genetic analysis. EB, MC and EH orchestrated and oversaw collection of Minnesota cervid samples and assisted in drafting of the manuscript. AW and AA performed moose necropsies and assisted in drafting of the manuscript. RM orchestrated and oversaw collection and identification of Minnesota tabanid samples and assisted with drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All protocols for the handling and collection of materials from animals followed the guidelines set by the American Society of Mammalogists [53, 54] and the American Veterinary Medical Association [55].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Reference nematodes utilized in 18S molecular analysis. Adult nematodes were identified based on morphological characters. Geographical origin and host species refer to the place and host from which the adult nematode was isolated. (DOCX 12 kb)
Additional file 2:
Table S2. Fiarioid 18S rRNA sequences obtained from Minnesota moose CNS tissues. Elaeophora schneideri Isolates with identical 18S sequences are assigned the same haplotype number. A representative sequence for each haplotype was deposited in the GenBank database. (DOCX 12 kb)
Additional file 3:
Table S3. Fiarioid 18S rRNA sequences obtained from Minnesota tabanid horseflies (n = 36; 2013). Isolates with identical 18S sequences are assigned the same haplotype number. Elaeophora schneideri sequences with ambiguous characters are labeled with ND and were not included in the haplotype analysis. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Grunenwald, C.M., Butler, E., Wünschmann, A. et al. Emergence of the arterial worm Elaeophora schneideri in moose (Alces alces) and tabanid fly vectors in northeastern Minnesota, USA. Parasites Vectors 11, 507 (2018). https://doi.org/10.1186/s13071-018-3077-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3077-0