Abstract
Background
The Australian paralysis tick, Ixodes holocyclus, causes paralysis predominantly in dogs and cats in the Eastern coastal regions of Australia. Rapid onset of effect of a parasiticide is critical to minimize the deleterious effects of these tick infestations, especially tick paralysis caused by the salivary neurotoxin. The speed of kill of a novel orally administered isoxazoline parasiticide, sarolaner chewable tablets (Simparica®), against I. holocyclus on dogs was evaluated and compared with afoxolaner (NexGard®) for 5 weeks after a single oral dose.
Methods
Twenty-four (24) dogs were randomly allocated to treatment with either placebo, sarolaner (label dose of 2 to 4 mg/kg as per dosing table), or afoxolaner (label dose of 2.7 to 6.9 mg/kg) based on pre-treatment body weights. Following artificial infestation on Day -1, dogs were examined and live ticks counted at 8, 12, 24 and 48 h after treatment on Day 0, and at 12, 24 and 48 h after subsequent re-infestations on Days 7, 14, 21, 28 and 35. Efficacy was determined at each time point relative to counts for placebo dogs based on geometric means.
Results
At 8 and 12 h time points on Day 0, sarolaner-treated dogs had significantly lower geometric mean tick counts compared to the dogs treated with afoxolaner (P ≤ 0.0303). Efficacy of sarolaner against an existing infestation was 86.2 and 96.9% compared with that of afoxolaner which had efficacy of 21.3 and 85.0% at 8 and 12 h time points, respectively. Against subsequent weekly re-infestations at 12 h time points, treatment with sarolaner resulted in significantly lower geometric mean tick counts than afoxolaner-treated dogs on all days (P ≤ 0.0077) with the efficacy ranging from 60.2 to 92.2%, compared to 5.8 to 61.0% in the afoxolaner-treated dogs. Against subsequent weekly re-infestations at the 24 h time points on Days 22 and 36, efficacy of sarolaner was significantly higher at 99.2 and 97.9%, respectively, compared with afoxolaner which had efficacy of 92.4 and 91.9% (P ≤ 0.0356). At the 48 h time points following each of the five weekly re-infestations, the mean efficacy results of sarolaner and afoxolaner treated dogs were similar on most occasions. There were no adverse reactions to treatments.
Conclusions
In this controlled laboratory evaluation, a single dose of sarolaner had a significantly faster speed of kill against an existing infestation of I. holocyclus, than afoxolaner at 8 and 12 h post-treatment. The rapid and consistent kill of ticks provided by sarolaner within 24 h after a single oral dose and following weekly re-infestations over 35 days suggests this treatment will provide highly effective, rapid and reliable control of ticks over the entire treatment interval, thereby minimizing the risk of tick paralysis in dogs.
Similar content being viewed by others
Background
Ixodes holocyclus, also known as the paralysis tick in Australia, causes tick paralysis in domestic animals, a potentially-lethal disease seen predominantly in dogs and cats [1–3], horses [4–6] and humans [7–9]. Ixodes holocyclus is widely distributed along the eastern coastal regions of Australia from North Queensland to Lakes Entrance of Victoria [1, 10–12]. The majority of cases of tick paralysis in dogs are reported during spring to early summer due to favorable climate [1, 11]. Ixodes holocyclus is suspected as a vector for transmission of Borrelia burgdorferi (sensu stricto) [13] and Rickettsia australis [14, 15] in humans, but it is rarely reported as a vector for transmission of other pathogens in domestic animals.
Tick paralysis is characterised by progressive ascending lower motor neuron (LMN) flaccid paralysis caused by the neurotoxin produced in the salivary glands of the female I. holocyclus [3, 16]. Clinical manifestations of tick paralysis have been previously reviewed and well documented and include flaccid paralysis, cardio-pulmonary complications and sometimes death [3, 17, 18]. Typically, the onset of clinical signs usually does not occur until 4 or 5 days after tick attachment [3, 17, 19]. This coincides with a sudden increase in the salivary toxin secretions which occur during the later stages of tick infestations [3, 16]. The duration of feeding, rate of growth and release of toxins however can vary depending on the age of the ticks and favorable ambient temperature and humidity [20]. The longer the duration of attachment, the greater the potential risk of paralysis, thus the sooner an attached tick can be removed, the lower the risk of paralysis [17, 19]. Therefore, an acaricide with a fast speed of kill is critical in removing any pre-existing ticks and minimising the risk of morbidity and mortality due to tick paralysis [21].
Treatment of tick paralysis typically centers around removal of attached ticks, the neutralization of toxins, the control of clinical manifestations of tick paralysis and the treatment of any anaphylactic reactions due to the administration of tick anti-sera [22]. Successful treatment outcomes also depend on locating and removing all the attached ticks on the dogs followed by the administration of an acaricidal agent [23]. A routine tick search, even by an experienced person, may be unsuccessful in locating all ticks, even in the common predilection sites [20], since ticks can be hidden in areas such as the nostril, inside the anus and ear canals, hence an acaricide with a wider systemic distribution should be the preferred choice of treatment. Systemic formulations like the new class of isoxazolines offer wider distribution to all anatomical sites in the body [21] and are therefore able to provide more reliable efficacy, unlike the topical counterparts [23] which may not reach all the anatomical sites.
The introduction of isoxazolines such as sarolaner, afoxolaner and fluralaner has resulted in an improvement in tick control in dogs in recent times. Sarolaner, a pure form of S-entamioner, is a potent ectoparaciticide [24] with a broad spectrum of activity against different tick species [25, 26], including the Australian paralysis tick, I. holocyclus (Zoetis, unpublished data).
In this laboratory study, the speed of kill of sarolaner (Simparica®) and afoxolaner (Nexgard®) was evaluated against existing I. holocyclus infestations and weekly re-infestations for a period of 5 weeks after treatment with a single dose.
Methods
The study was a blinded, negative-controlled, randomized laboratory efficacy design conducted in New South Wales, Australia. Study procedures were in accordance with the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestation on dogs and cats [27] and complied with the principles of Good Clinical Practice [28]. The protocol was reviewed and approved by the Vetx Research Animal Ethics Committee, NSW. Blinding of the study was assured through the separation of functions. All personnel conducting observations, or performing infestations and counts were blinded to treatment allocation.
Animals
Twenty-four (24) male and female, Foxhound dogs from 1 to 9 years of age and weighing from 30.1 to 46.9 kg were used in the study. Each dog was individually identified by a unique electronic transponder and had undergone an adequate wash-out period to ensure that no residual ectoparasiticide efficacy remained from any previously administered treatments. This was confirmed by the tick carrying capacity test results that showed all enrolled dogs had ≥ 21 ticks. Dogs were individually housed in indoor runs such that no physical contact was possible between them and they were acclimatized to these conditions for at least 7 days prior to treatment. Dogs were fed an appropriate maintenance ration of a commercial dry canine feed for the duration of the study. Water was available ad libitum. All dogs were given a physical examination to ensure that they were in good health at enrollment and suitable for inclusion in the study. General health observations were performed twice daily throughout the study.
Design
The study followed a randomized complete block design, with pairs of dogs as the experimental unit. Dogs enrolled in the study were immunised to the tick toxin- holocylotoxin as described previously [29] and selected based on acceptable results (tick counts of ≥ 21) to the tick carrying capacity test performed on Day -7. Prior to treatment, dogs were ranked according to pre-treatment body weights into four blocks of six (three pairs of dogs). Within each block, one pair of dogs was randomly allocated to treatment with placebo, sarolaner, or afoxolaner. There were eight dogs per treatment group.
Treatment
Body weights collected on Day -5 were used to determine the appropriate dose to be administered. On Day 0, dogs received either a placebo tablet, Simparica® (sarolaner) at the recommended dose of 2 mg/kg (range: 2 to 4 mg/kg), or NexGard® (afoxolaner) as per label directions (2.7 to 6.9 mg/kg). All doses were administered by hand pilling to ensure accurate and complete dosing. Each dog was observed for at least 2 min after treatment to ensure the dose was swallowed.
Tick infestation and assessment
The unfed adult female I. holocyclus ticks were collected from the Northern Rivers region of New South Wales, Australia approximately 2 months prior to the start of the study. The ticks were stored in dark conditions at around 12 °C and high humidity [29]. Tick infestations were performed on Days -7 (host suitability), -1, 7, 14, 21, 28 and 35. Prior to each infestation, dogs were examined to ensure they were free of ticks. Each dog was infested with 30 viable unfed female ticks at pre-defined locations (head, shoulders, dorsal midline of the body) on the dogs as described previously [29]. For Day -1 infestation, tick counts were performed at 8, 12, 24 and 48 h after treatment on Day 0 and all other tick counts were performed at 12, 24 and 48 h after each weekly infestation. Tick assessments at 8 (only on Day 0), 12 and 24 h time points were performed without removing the ticks from the dogs. After counting at the 48 h time point, all ticks were removed. Ticks were characterised as described previously [29] except moribund ticks were recorded in a separate category and included in the live counts in this study for the efficacy calculations.
Statistical analysis
The primary outcome measure was live tick counts. Data for post-treatment live (free plus attached) tick counts were summarized with arithmetic (AM) and geometric (GM) means by treatment group and time point. Tick counts were transformed by the loge(count + 1) transformation prior to analysis in order to stabilize the variance and normalize the data. Using the PROC MIXED procedure (SAS 9.3, SAS Institute Inc., Cary, NC, USA), transformed counts were analyzed using a mixed linear model for repeated measures for the 12, 24 and 48 h time points separately. The fixed effects were treatment, time point and the interaction between time point and treatment. The random effects included block, pair, animal, block by treatment by time point interaction, and error. The data for the 8 h time point (Day 0 only) were analysed with terms including the fixed effect of treatment group and the random effects of block, pair and error. Testing was two-sided at the significance level α = 0.05, with tests based on contrasts between treatment least squares means from the fitted models.
The assessment of efficacy for live ticks was based on the percent reduction in the AM and GM live tick counts for the treated groups relative to placebo, as suggested by the most recent guidelines of the WAAVP for systemic acaricides [27], and was calculated using Abbott’s formula:
As the distribution of parasite counts within each group was likely be skewed, comparison between groups was primarily based on GM live tick counts [27].
Results
There were no treatment-related adverse events during the study. Placebo-treated dogs maintained good tick infestations throughout the study with individual tick counts ranging from 15 to 30 (Tables 1, 2, 3 and 4).
Against an existing infestation, at the 8 and 12 h time points, treatment with sarolaner resulted in significantly lower GM tick counts compared to both placebo (P ≤ 0.0007) and afoxolaner treated dogs (P ≤ 0.0303). There was no significant difference between the GM live tick counts at 8 h for the afoxolaner and placebo-treated dogs (P = 0.5302). Efficacy of sarolaner against an existing infestation was 86.2 and 96.9% compared with that of afoxolaner which had efficacy of 21.3 and 85.0% at 8 and 12 h time points respectively. Efficacy of sarolaner and afoxolaner reached 100% at 24 and 48 h post-treatment respectively (Table 1 and Fig. 1).
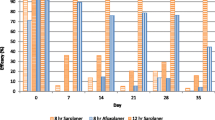
Against subsequent weekly infestations at the 12 h time points, treatment with sarolaner resulted in significantly lower GM tick counts compared to both placebo- (P ≤ 0.0048) and afoxolaner-treated dogs (P ≤ 0.0077) on all Days (7, 14, 21, 28 and 35). Efficacy of sarolaner at the 12 h time points following weekly re-infestations ranged from 60.2 to 92.2% compared to 5.8 to 61.0% in the afoxolaner treated dogs (Table 2 and Fig. 2).
Against subsequent weekly infestations at the 24 h time points on days 22 and 36, sarolaner-treated dogs had significantly lower GM tick counts than afoxolaner-treated dogs (P ≤ 0.0356). On Days 8, 15, 22, 29 and 36, treatment with sarolaner and afoxolaner resulted in significantly lower GM tick counts than placebo (P ≤ 0.0001). At 24 h following weekly re-infestations, efficacy of sarolaner ranged from 93.9 to 99.2% compared to 91.9 to 99.2% in the afoxolaner-treated dogs (Table 3 and Fig. 2).
Against weekly infestations, at the 48 h time points treatment with sarolaner and afoxolaner resulted in significantly lower GM tick counts than placebo (P ≤ 0.0001) and efficacy (GM) of sarolaner and afoxolaner treated dogs were ≥ 95.5% and ≥ 94.1% respectively (Table 4 and Fig. 2).
Discussion
A single dose of sarolaner resulted in the rapid reduction of an existing infestation of live I. holocyclus ticks with an efficacy of 86.2% at 8 h and 96.9% at 12 h post-treatment and the rapid kill of re-infestations for 5 weeks after treatment within 12 h of attachment with an efficacy of ≥ 60.2%. Although the onset of tick paralysis typically does not occur until day 4 or 5 after tick attachment, the sooner an attached tick can be killed, the lower the risk of paralysis [3, 17, 19], especially those pre-existing ticks that have already been on the dogs for 3–4 days prior to treatment. The rapid speed of kill of sarolaner as early as 8 h against an existing infestation will provide faster removal of attached ticks, thereby minimising the risk of tick paralysis. Similar faster speed of kill of sarolaner has also been demonstrated against other Ixodes tick species such as Ixodes scapularis and Ixodes ricinus [30, 31]. The faster speed of kill and persistent efficacy of sarolaner will provide excellent overall efficacy against I. holocyclus for up to 5 weeks.
Conclusions
Sarolaner had a significantly faster speed of kill at both 8 and 12 h compared with afoxolaner against existing infections of paralysis tick and had higher efficacy than afoxolaner at 12 h after re-infestation over 35 days.
The rapid and consistent kill of ticks provided by sarolaner within 24 h after a single oral dose and following weekly re-infestations over 35 days suggests this treatment will provide highly effective, rapid and reliable control of ticks over the entire treatment interval, thereby minimizing the risk of tick paralysis in dogs.
Abbreviations
- AM:
-
Arithmetic mean
- GM:
-
Geometric mean
- WAAVP:
-
World Association for the Advancement of Veterinary Parasitology
References
Eppleston KR, Kelman M, Ward MP. Distribution, seasonality and risk factors for tick paralysis in Australian dogs and cats. Vet Parasitol. 2013;196:460–8.
Schull DN, Litser AL, Atwell RB. Tick toxicity in cats caused by Ixodes species in Australia: a review of published literature. J Feline Med Surg. 2007;9:487–93.
Ross IC. An experimental study of tick paralysis in Australia. Aust Vet J. 1927;3:71–2.
Bootes BW. A fatal paralysis in foals from Ixodes holocyclus Neumann infestation. Aust Vet J. 1962;38:68–9.
Tee SY, Feary DJ. Suspected tick paralysis (Ixodes holocyclus) in a miniature horse. Aust Vet J. 2012;90:181–5.
Ruppin M, Sullivan S, Condon F, Perkins N, Lee L, Jeffcott LB, et al. Retrospective study of 103 presumed cases of tick (Ixodes holocyclus) envenomation in the horse. Aust Vet J. 2012;90:175–80.
Grattan-Smith PJ, Morris JG, Johnston HM, Yiannikas C, Malik R, Russell R, et al. Clinical and neurophysiological features of tick paralysis. Brain. 1997;120:1975–87.
Miller MK. Massive tick (Ixodes holocyclus) infestation with delayed facial- nerve palsy. Med J Aust. 2002;176:264–5.
Inokuma H, Takahata H, Fournier PE, Brouqui P, Raoult D, Okuda M, et al. Tick paralysis by Ixodes holocyclus in a Japanese traveler returning from Australia associated with Rickettsia helvetica infection. J Travel Med. 2003;10:61–3.
Brazier I, Kelman M, Ward MP. The association between landscape and climate and reported tick paralysis cases in dogs and cats in Australia. Vet Parasitol. 2014;204:339–45.
Greay TL, Oskam CL, Gofton AW, Rees RL, Ryan UM, Irwin PJ. A survey of ticks (Acari: Ixodidae) of companion animals in Australia. Parasit Vectors. 2016;9:207–17.
Jackson J, Beveridge I, Chilton NB, Andrews RH. Distributions of the paralysis ticks Ixodes cornuatus and Ixodes holocyclus in south-eastern Australia. Aust Vet J. 2007;85:420–4.
Mayne P, Song S, Shao R, Burke J, Wang Y, Roberts T. Evidence for Ixodes holocyclus (Acarina: Ixodidae) as a vector for human Lyme borreliosis infection in Australia. J Insect Sci. 2014;14:1–3.
Graves S, Unsworth N, Stenos J. Rickettsioses in Australia. Ann NY Acad Sci. 2006;1078:74–9.
Graves SR, Jackson C, Hussain-Yusuf H, Vincent G, Nguyen C, Stenos J, Webster M. Ixodes holocylus tick transmitted human pathogens in North-Eastern New South Wales, Australia. Trop Med Infect Dis. 2016;1:1–7.
Goodrich BS, Murray MD. Factors influencing the toxicity of salivary gland extracts of Ixodes holocyclus Neumann. Int J Parasitol. 1978;8:313–20.
Ilkiw JE, Turner DM, Howlett CR. Infestation in the dog by the paralysis tick Ixodes holocyclus. 1. Clinical and histological findings. Aust Vet J. 1987;64:137–9.
Atwell RB, Campbell FE, Evans EA. Prospective survey of tick paralysis in dogs. Aust Vet J. 2001;79:412–8.
Ross IC. Tick paralysis in the dog. Aust Vet J. 1934;10:182–3.
Atwell RB. Re: Ixodes holocyclus in cooler climate regions. Aust Vet J. 2015;93:N24.
Pfister K, Armstrong R. Systemically and cutaneously distributed ectoparasiticides: a review of the efficacy against ticks and fleas on dogs. Parasit Vectors. 2016;9:1–15.
Atwell RB, Campbell FE. Reactions to tick antitoxin serum and the role of atropine in treatment of dogs and cats with tick paralysis caused by Ixodes holocyclus: a pilot survey. Aust Vet J. 2001;79:394–7.
Masina S, Broady KW. Tick paralysis: development of a vaccine. Int J Parasitol. 1999;29:535–41.
McTier TL, Chubb N, Curtis MP, Hedges L, Inskeep GA, Knauer CS, et al. Discovery of sarolaner: a novel, orally administered, broad-spectrum, isoxazoline ectoparasiticide for dogs. Vet Parasitol. 2016;222:3–11.
Six RH, Everett WR, Young DR, Carter L, Mahabir SP, Honsberger NA, et al. Efficacy of a novel oral formulation of sarolaner (Simparica™) against five common tick species infesting dogs in the United States. Vet Parasitol. 2016;222:28–32.
Geurden T, Becskei C, Grace S, Strube C, Doherty P, Liebenberg J, et al. Efficacy of a novel oral formulation of sarolaner (Simparica™) against four common tick species infesting dogs in Europe. Vet Parasitol. 2016;222:33–6.
Marchiondo AA, Holdsworth PA, Fourie LJ, Rugg D, Hellmann K, Snyder DE, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) 2nd. ed.: Guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestations on dogs and cats. Vet Parasitol. 2013;194:84–97.
EMEA. Guideline on good clinical practices. VICH Topic GL9. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004343.pdf. Accessed 14 Sept. 2016.
Webster MC, Fisara P, Sargent RM. Long-term efficacy of a deltamethrin-impregnated collar for the control of the Australian paralysis tick, Ixodes holocyclus, on dogs. Aust Vet J. 2011;89:439–43.
Six RH, Young DR, Myers MR, Mahabir SP. Comparative speed of kill of sarolaner (Simparica™) and afoxolaner (NexGard®) against induced infestations of Ixodes scapularis on dogs. Parasit Vectors. 2016;9:1–6.
Six RH, Geurden T, Carter L, Everett WR, McLoughlin A, Mahabir SP, et al. Evaluation of the speed of kill of sarolaner (Simparica™) against induced infestations of three species of ticks (Amblyomma maculatum, Ixodes scapularis, Ixodes ricinus) on dogs. Vet Parasitol. 2016;222:37–42.
Acknowledgements
Zoetis would like to thank Chrissie Jackson, Drs Bob Pigott and Maurice Webster (Vetx Research, 495 Ellangowan Road, Yorklea, NSW 2470, Australia) for conducting the study.
Funding
Funding for the design, conduct of the study and data collection was provided by Zoetis Australia Research & Manufacturing Pty Ltd. Data analysis, interpretation and writing the manuscript were performed by the authors who were the Zoetis employees.
Availability of data and materials
Relevant datasets were attached in Additional file 1. Full datasets can be provided on request.
Authors’ contributions
All authors assisted with the design and conduct of the study and interpretation of the data. Manuscript was written by RP and all authors read and approved the final manuscript.
Competing interests
This study was funded by Zoetis Australia Research and Manufacturing Pty Ltd, Level 6, 5 Rider Boulevard, Rhodes, NSW 2138, Australia. RP, AH, NB, KD and SM were current or former employees of Zoetis. This study was conducted by Vetx Research (495 Ellangowan Road, Yorklea, NSW 2470, Australia), an independent contract research organisation.
Consent for publication
Unpublished Zoetis data was included as a reference and consent to publish approval of this manuscript was obtained via a Zoetis Internal Approval system.
Ethics approval
An animal ethics approval was obtained from the Vetx Research Animal Ethics Committee on 10 Dec 2015 prior to the commencement of the study. The animal ethics approval number was ZOSC1159 for the study titled “Efficacy evaluation of sarolaner and Nexgard against Ixodes holocyclus on dogs in Australia”. This study did not report on any data related to humans. All dogs enrolled in the study were owned by the Vetx Research who contracted this study.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Comparative Ixodes holocylcus individual tick counts. (XLSX 21 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Packianathan, R., Hodge, A., Bruellke, N. et al. Comparative speed of kill of sarolaner (Simparica®) and afoxolaner (NexGard®) against induced infestations of Ixodes holocyclus on dogs. Parasites Vectors 10, 98 (2017). https://doi.org/10.1186/s13071-017-2024-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2024-9