Abstract
Background
Within the African monitor lizard family Varanidae, two haemogregarine genera have been reported. These comprise five species of Hepatozoon Miller, 1908 and a species of Haemogregarina Danilewsky, 1885. Even though other haemogregarine genera such as Hemolivia Petit, Landau, Baccam & Lainson, 1990 and Karyolysus Labbé, 1894 have been reported parasitising other lizard families, these have not been found infecting the Varanidae. The genus Karyolysus has to date been formally described and named only from lizards of the family Lacertidae and to the authors’ knowledge, this includes only nine species. Molecular characterisation using fragments of the 18S gene has only recently been completed for but two of these species. To date, three Hepatozoon species are known from southern African varanids, one of these Hepatozoon paradoxa (Dias, 1954) shares morphological characteristics alike to species of the family Karyolysidae. Thus, this study aimed to morphologically redescribe and characterise H. paradoxa molecularly, so as to determine its taxonomic placement.
Methods
Specimens of Varanus albigularis albigularis Daudin, 1802 (Rock monitor) and Varanus niloticus (Linnaeus in Hasselquist, 1762) (Nile monitor) were collected from the Ndumo Game Reserve, South Africa. Upon capture animals were examined for haematophagous arthropods. Blood was collected, thin blood smears prepared, stained with Giemsa, screened and micrographs of parasites captured. Haemogregarine morphometric data were compared with the data for named haemogregarines of African varanids. Primer set HepF300 and HepR900 was employed to target a fragment of the 18S rRNA gene and resulting sequences compared with other known haemogregarine sequences selected from the GenBank database.
Results
Hepatozoon paradoxa was identified infecting two out of eight (25 %) V. a. albigularis and a single (100 %) V. niloticus examined. Phylogenetic analyses revealed that H. paradoxa clustered with the ‘Karyolysus’ clade, and not with those of reptilian Hepatozoon spp.
Conclusions
In addition to this being the first morphological and molecular characterisation of a haemogregarine within the African Varanidae, it is the first report of a species of Karyolysus infecting the monitor lizard family. Furthermore, this constitutes now only the third described and named Karyolysus species for which there is a nucleotide sequence available.
Similar content being viewed by others
Background
Within the apicomplexan order Adeleiorina, representatives of two haemogregarine genera, Hepatozoon Miller, 1908 and Karyolysus Labbé, 1894, are commonly reported infecting saurians. The genus Hemolivia Petit, Landau, Baccam & Lainson, 1990 on the contrary, even though reported parasitising saurian hosts, has but a single described species Hemolivia mariae Smallridge & Paperna, 1997 [1, 2]. Representatives of Hepatozoon are the most common and are cosmopolitan parasites found parasitising a wide range of vertebrate hosts from amphibians and reptiles to birds and mammals [3, 4]. Karyolysus, conversely, is known mainly as a saurian haemogregarine genus that primarily parasitises lizards of the family Lacertidae, but has also been reported from lizards of the Scincidae [1, 5–7]. Besides this discrepancy in vertebrate host preference of the species in the above haemogregarine genera, species in these genera also demonstrate different developmental patterns. Even though species of all three of the haemogregarine genera may be transmitted to the saurian host through the ingestion of the infected invertebrate vector, Hepatozoon spp. may be transmitted through a wide range of arthropod vectors (mosquitoes to ticks), whilst transmission of Hemolivia spp. and Karyolysus spp. has been recorded only through a tick and mite vector, respectively [1].
Whilst more than 30 Hepatozoon spp. have been recorded from saurians throughout Africa [8], Karyolysus spp. have mainly been reported from lacertid lizards of Europe and Asia [7]. Until Smith’s [3] revision of the Hepatozoidae, the genus Karyolysus comprised 11 species, Smith [3] reassigning two of these to Hepatozoon, now Hepatozoon berestnewi (Finkelstein, 1907) and Hepatozoon bicapsulata (Franca, 1910). To the authors’ knowledge, to date, nine species of Karyolysus are considered valid: Karyolysus lacertae Danilewsky, 1886; Karyolysus lacazei Labbé, 1894; Karyolysus biretortus Nicolle, 1904; Karyolysus zuluetai Reichenow, 1920; Karyolysus subtilis Ricci, 1954; Karyolysus octocromosomi Alvarez-Calvo, 1975; Karyolysus latus Svahn, 1975; Karyolysus minor Svahn, 1975; and the only species reported from sub-Saharan Africa Karyolysus poleensis Mutinga & Dipeolu, 1989 [3, 6, 7]. Descriptions of haemogregarine species were until recently based on morphological characteristics and life-cycle data [7]. This is particularly true of the haemogregarines described from saurians within southern Africa. Haemogregarines, specifically species of Hepatozoon, have been commonly recorded blood parasites of southern African saurians including those from lizard genera of the families Cordylidae and Varanidae [8, 9]. Within the African Varanidae, six species of Hepatozoon have been described, three of these from southern Africa (Table 1), the latter three comprising Hepatozoon varani (Laveran, 1905) from Varanus niloticus (Linnaeus, 1762) in South Africa [3, 6], Hepatozoon camarai (Dias, 1954) and Hepatozoon paradoxa (Dias, 1954) from Varanus albigularis albigularis Daudin, 1802 in Mozambique [3, 6].
The aim of the present study was thus to provide a morphological redescription of H. paradoxa and molecular data aiding in the correct taxonomic placement of this parasite.
Methods
Study area, Varanus spp. collection and blood preparation
Specimens of Varanus albigularis albigularis and Varanus niloticus were collected in daylight during the summer months of November 2013, February and November 2014, and February 2015 in the Ndumo Game Reserve (NGR) (26°52′00.0″S, 32°15′00.0″E), north-eastern KwaZulu-Natal (KZN), South Africa, bordering southern Mozambique [10]. Lizards were restrained by hand whilst blood and any haematophagous athropods were collected in situ. A small volume of blood (approximately one drop) was collected from the ventral caudal vein using an appropriately gauged (depending on the size of the lizard) sterile needle and 1 ml syringe. A small portion of the collected blood was used to prepare 2–3 duplicate thin blood smears and the remainder dropped into an equal volume of 70 % ethanol for future molecular analysis. Thin blood smears once air-dried in a dustproof container were fixed in absolute methanol and stained thereafter using a modified solution of Giemsa stain (FLUKA, Sigma-Aldrich, Steinheim, Germany) according to the methods of [11, 12].
Screening of Varanus spp. blood smears
Smears were screened under a 100× oil immersion objective on a Nikon Eclipse E800 compound microscope (Nikon, Amsterdam, The Netherlands) and images were captured with an attached Nikon digital camera and accompanying software. Haemogregarines were identified to species level by comparing morphometric data to that of previous studies on African Varanus spp. haemogregarines [9, 13–15] (see Table 1). Parasitaemia was calculated per 100 erythrocytes, with c.104 erythrocytes examined per blood smear [16–18].
DNA extraction, PCR amplification and 18S rDNA sequence analysis
Ethanol-preserved blood samples were used for molecular work. Genomic DNA of haemogregarine species was extracted from the samples using a rapid DNA extraction method as detailed in the KAPA Express Extract Kit (Kapa Biosystems, Cape Town, South Africa). Based on previous studies, amplifying fragments of the 18S rRNA gene of reptile haemogregarines of the genera Karyolysus [7], Hemolivia [12] and Hepatozoon [19], identification of the parasite of the two Varanus species, two V. a albigularis and one V. niloticus (n = 3) from the current study was completed using the primer set HepF300 (5′-GTT TCT GAC CTA TCA GCT TTC GAC G-3′) and HepR900 (5′-CAA ATC TAA GAA TTT CAC CTC TGA C-3′). The PCR reactions were run targeting a fragment (approximately 600 nt) of the 18S rRNA gene [20]. Conditions for PCR were as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles, entailing a 95 °C denaturation for 30 s, annealing at 60 °C for 30 s with an end extension at 72 °C for 1 min, and following the cycles a final extension of 72 °C for 10 min as detailed according to previous methods [12, 18]. PCR reactions were performed with volumes of 25 μl, using 12.5 μl Thermo Scientific DreamTaq PCR master mix (2×) (2× DreamTaq buffer, 0.4 mM of each dNTP, and 4 mM MgCl2), 1.25 μl of each primer, and at least 25 ng DNA. The final reaction volume was made up with PCR-grade nuclease free water (Thermo Scientific, Vilnius, Lithuania). Reactions were undertaken in a Bio-Rad C1000 Touch™ Thermal Cycler PCR machine (Bio-Rad, Hemel Hempstead, UK). Resulting amplicons were visualized under ultraviolet light on a 1 % agarose gel stained with gel red using a Bio-Rad GelDoc™ XR+ imaging system (Bio-Rad, Hemel Hempstead, UK). Two PCR products from each sample were sent to a commercial sequencing company (Inqaba Biotechnical Industries (Pty) Ltd, Pretoria, South Africa) for purification and sequencing in both directions. Resultant sequences were assembled, and chromatogram-based contigs were generated and trimmed using Geneious Ver. 7.1 [21]. Sequences were identified using the Basic Local Alignment Search Tool (BLAST) [22], and deposited in the NCBI GenBank database under accession numbers KX011039 and KX011040.
Comparative sequences for species of Hemolivia, Hepatozoon, Karyolysus, Haemogregarina, Dactylosoma Labbé 1894 and Babesiosoma Jakowska & Nigrelli, 1956 parasitising reptiles, amphibians, mammals and ticks were downloaded from GenBank and aligned to the sequences generated in this study. Adelina dimidiata Schneider, 1875, Adelina grylli Butaeva, 1996 (GenBank: DQ096835–DQ096836) and Klossia helicina Schneider, 1875 (GenBank: HQ224955) were chosen as the outgroup to root the phylogeny.
Sequences were aligned using the MUSCLE alignment tool [23] implemented in Geneious 7.1. The alignment consisted of 47 sequences, manually trimmed to a total length of 968 nt. Uncorrected pair-wise distances (p-distance), base pair differences as well as parsimony informative sites and the number thereof were identified or determined with the MEGA6 bioinformatics software program [24] for the aligned 18S rDNA sequences between all available species appearing in the phylogenetic analyses.
To infer phylogenetic relationships of the aligned dataset both Bayesian inference (BI) and Maximum likelihood (ML) methods were used. A comprehensive model test was preformed to determine the most suitable nucleotide substitution model, according to the Akaike information criterion using jModelTest 2.1.7 [25, 26]. The best model identified was the Transversion Model plus with estimates of invariable sites and a discrete Gamma distribution (TVM+I+Γ). This model was substituted with the General Time Reversible model (GTR+I+Γ) for phylogenetic analysis, as this was the most appropriate model available with the best AICc score. The BI analysis was implemented from within Geneious 7.1 using MrBayes 3.2.2 [27]. The analysis was run twice over 10 million generations for the Markov Chains Monte Carlo (MCMC) algorithm. The Markov chain was sampled every 100 cycles, and the MCMC variant contained 4 chains with a temperature of 0.2. The log-likelihood values of the sample point were plotted against the generation time and the first 25 % of the trees were discarded as ‘burn-in’ with no ‘burn-in’ samples being retained. The ML analysis was performed using RAxML Ver. 8.1.22 [28] implemented in the raxmlGUI Ver. 1.3 [29]. The alpha-parameter selected was the GTR+I+Γ model, with support assessed using 1,000 rapid bootstrap inferences. Resulting trees were combined in a 50 % majority consensus tree.
Ethics statement
This study received the relevant ethical approval (North-West University ethics approval no: NWU-00005-14-S3).
Results
Prevalence, parasitaemia and general observations of H. paradoxa in peripheral blood smears
Six adult and two juvenile Varanus albigularis albigularis (Fig. 1a, b) and one adult Varanus niloticus were captured, sampled for blood parasites and examined for possible haematophagous arthropods. The two juveniles were found to be negative for blood parasites, whilst 4/6 (67 %) adult V. a. albigularis and 1/1 (100 %) adult V. niloticus were found positive for haemogregarine infections. Two adult (33 %) (with a parasitaemia of c.5 and 20 %, respectively) V. a. albigularis and the V. niloticus (with a parasitaemia of c.0.2 %) were parasitised by a haemogregarine alike to Hepatozoon paradoxa described by Dias [9] (Figs. 1c–f and 2b–i). One of the two (17 %) V. a. albigularis (parasitaemia of 5 %) and the single V. niloticus were found to have a co-infection with another haemogregarine of a different Hepatozoon spp. (Cook, Netherlands & Smit, unpublished observations) (parasitaemia of c.0.4 and 3 %, respectively). The latter unidentified Hepatozoon species was also found infecting the remaining two parasitised V. a. albigularis (parasitaemia of c.0.1 and 0.5 %, respectively). All specimens of both species of Varanus were infested with adult and juvenile stages of the Leguan tick Amblyomma exornatum (Koch, 1844) (Fig. 1b), the two juvenile V. a. albigularis and V. niloticus demonstrating lower densities of these ectoparasites. These ectoparasites were found all over the animal, with the highest densities in the nostrils, often blocking them, also on the area surrounding the eyes, and along the edges of the mouth. It was not uncommon to find dead ticks and their remains deep within the nostril, their ability to exit blocked by later arrivals. Squashes of nymphal and adult female and male ticks provided no parasitic stages. No other haematophagous arthropods, including mites, were observed.
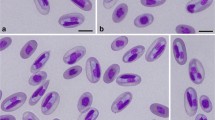
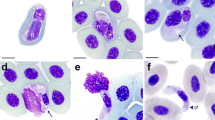
Karyolysus paradoxa (Dias, 1954) in varanid lizard Varanus albigularis albigularis Daudin, 1802. a–b Varanus albigularis albigularis. b Ticks of the species Amblyomma exornatum infesting the area above the eyes, the periphery of the mouth and deep into the nostrils (arrows). c–f Peripheral blood stages of K. paradoxa captured from the neohapantotype slide (NMB P 410). c Possible rare trophozoite stage, note that the young host erythrocyte cytoplasm and nucleus are still intact and that the parasite nucleus is visible and granular. d–f Mature gamonts within an erythrocyte in which shrinkage of the host cell is apparent and the nucleus destroyed resulting in a heavily vacuolated appearance. d Mature gamont in which folding of the gamont may be seen within the thick capsule (arrow). Scale-bar: 10 μm
Illustration of Haemogregarina paradoxa Dias, 1954 in Varanus albigularis albigularis Daudin, 1802 [9]. a-i Redrawn and adapted from Dias (1954). Illustrations representing the original description of Karyolysus paradoxa (syns. Hepatozoon paradoxa and Haemogregarina paradoxa) ex Varanus albigularis albigularis from Mozambique. a Healthy non-parasitised erythrocyte. b-i Parasitised erythrocytes, note the shrinkage of the host cell, the heavy vacuolization of the host cell nucleus, and the thick capsule surrounding the gamont which results in the gamont nucleus being invisible. Scale-bar: 10 μm
Stages of the H. paradoxa-like haemogregarine observed in peripheral blood smears from this study were compared morphologically with those observed in previous blood parasite studies of African varanids [9, 13–15] (Table 1). In size and morphology, the H. paradoxa-like stages observed during the current study conformed to those described by Dias [9] and Ball [30] (see Table 1, Figs. 1a–f, 2b–i and 3a–d, respectively). Two stages of the parasite were observed: a stage unreported by Dias [9], but possibly by Ball [30], a rare possible trophozoite stage (Figs. 1c and 3a), and what was identified as a mature intra-erythrocytic gamont stage (Figs. 1d–f, 2b–i and 3d, e).
Illustration of an unknown haemogregarine by Ball (1967) [30] in Varanus niloticus (Linnaeus in Hasselquist, 1762). a–d Redrawn and adapted from Ball (1967) (his Figs. 19–22). Illustrations representing an unknown haemogregarine found as a concurrent infection to Hepatozoon varani (Laveran, 1905) (syn. Haemogregarina varani) in a Varanus niloticus from Kenya. a Possible trophozoite stage or young stage, shrinkage and vacuolation of the host cell and host cell nucleus not yet apparent. b Possible dividing stage. c–d Mature gamont stages, note the shrinkage and heavy vacuolation of the host cell erythrocyte. c Mature gamont folded within a capsule with possible nucleus. d Folding of the gamont and the gamont nucleus not observable through the capsule. Scale-bar: 10 μm (even though not provided by Ball, we have provided a scale matching to the in text description)
Karyolysus paradoxa (Dias, 1954) Cook, Netherlands & Smit, 2016
Syns Haemogregarina paradoxa Dias, 1954; Hepatozoon paradoxa Smith, 1996.
Type-host : Varanus albigularis albigularis Daudin, 1802, Squamata: Varanidae [9].
Other hosts : Varanus niloticus (Linnaeus in Hasselquist, 1762), Squamata: Varanidae [30]; present study.
Vector : Unknown.
Type-locality : Ndumo Game Reserve (26°54′18.5″S, 32°19′24.7″E), KwaZulu-Natal, South Africa (present study).
Other localities : Maputo, Mozambique [9]. Ball [30] also described a haemogregarine morphologically alike to K. paradoxa in Marimonti, near Meru, Kenya.
Type-material : Neohapantotype, 1× blood smear from the type-host Varanus albigularis albigularis and new designated locality (26°54′18.5″S, 32°19′24.7″E), deposited in the protozoan collection of the National Museum, Bloemfontein, South Africa under accession number NMB P 410. Other voucher material deposited that includes stages of K. paradoxa, 1× blood smear from Varanus niloticus, deposited in the protozoan collection of the National Museum, Bloemfontein, South Africa under accession number NMB P 411.
Representative DNA sequences : Two sequences representing a 611 and 613 nt fragment of the 18S rRNA gene of K. paradoxa isolated from the type-host Varanus albigularis albigularis, deposited in the NCBI GenBank database under the accession numbers KX011039 and KX011040, respectively.
Redescription (Figs. 1, 2 and 3)
Trophozoite. Rare, ovoid, with vacuolated cytoplasm, measuring 6.5–6.9 × 4.3–4.7 (6.7 × 4.5) μm (n = 2); nucleus with loose chromatin, staining pink (Fig. 1c). Both trophozoites parasitising young erythrocytes, no host cell distortion visible.
Mature gamont. Rounded in shape, gamont seemingly folded within with a well-developed capsule (Fig. 1d–f), measuring 6.3–7.9 × 3.6–5.2 (7.0 × 4.4) μm (n = 20). Cytoplasm staining whitish-blue; nucleus not visible. Notable destruction of host cell cytoplasm and karyolysis of the host cell nucleus, causing an observable heavily vacuolated and foamy appearance (Fig. 1d–f).
Remarks
The haemogregarine described in this study from South African Varanus albigularis albigularis and Varanus niloticus (Fig. 1d–f) was found to be morphologically similar to Hepatozoon paradoxa described by Dias [9] from a specimen of V. a. albigularis in neighbouring Mozambique (Fig. 2b–i). It shared a number of unique characteristics including destruction of the infected host erythrocyte, consisting of dehaemoglobinisation resulting in shrinkage of the host cell and destruction of the host cell nucleus (characteristic of a number of species of Karyolysus [1]) resulting in a heavily vacuolated appearance (Figs. 1d–f and 2b–i). Additionally, the haemogregarine in this study agrees well with the size of H. paradoxa in the original description of Dias [9] (mean 7.0 × 4.4 vs 7.0 × 4.9 μm) (Table 1).
The same unique characteristics were reported of a haemogregarine found infecting a V. niloticus by Ball [30] from Kenya, measuring on average 8.1 × 5.2 μm (Fig. 3c, d). However, in Ball’s [30] study, additional, presumably younger, stages were observed (similar to the young trophozoite stage found in our study) (Figs. 1c and 3a). Ball [30] also noted a single possibly dividing stage of these trophozoites (Fig. 3b). In cells parasitised by all these possible trophozoite stages, the host erythrocyte showed no shrinkage as of yet, but was according to Ball’s report abnormal in shape and staining. At first, Ball [30] did assume this parasite to represent younger stages of another haemogregarine that has been reported parasitising African varanids Hepatozoon varani (Laveran, 1905) Smith, 1996 (Table 1). However, based on the effects of the parasite resembling H. paradoxa as described by Dias [9], its destruction of the host cell and the host cell’s nucleus, he concluded that this parasite was not H. varani. Overall, for the K. paradoxa described in this study and the parasites described in the other two studies [9, 30], the nucleus and cytoplasm was not visible owing to what appeared to be a thick enclosing capsule as seen with the gamonts of species of Hemolivia, see [12, 31] (Figs. 1d–f, 2b–i and 3c, d). Only on rare occasion in the present study and that of Ball’s [30] was the parasite seen to be folding over on itself (Figs. 1d and 3c). Otherwise, the only evidence of this behaviour was a crescent shaped stain at the centre of the oval parasite (as seen in all three reports) (Figs. 1, 2 and 3). It is based on the above unique characteristics, particularly of the mature gamont stages, that we suggest all three reports are of the same parasite species K. paradoxa.
No hapantotype, according to the International Code of Zoological Nomenclature (ICZN) Article 73.3, was designated and deposited during the original description of K. paradoxa by Dias [9]. Ball [30] also did not identify the parasite to taxon level and did not deposit any voucher material. Furthermore, our efforts to locate any original specimens or voucher material were unsuccessful. In this study, K. paradoxa was collected from Ndumo Game Reserve, northern KwaZulu-Natal, South Africa, bordering the south of Mozambique. The original description by Dias [9] was collected in the vicinity of Maputo in the southern parts of Mozambique approximately 300 km from the NGR. Additionally, K. paradoxa in the present study was collected from the same host species Varanus albigularis albigularis as in the original description by Dias [9]. Based on the above, the mature gamont size comparisons, and the unique characteristics of the mature gamonts of K. paradoxa (destruction and shrinkage of the infected host erythrocyte, destruction of the host cell nucleus resulting in vacuolation, and the thick non-staining capsule) as described above for all three reports of this parasite, which includes the original description by Dias [9], and in accordance with ICZN Article 75.3, we designate a neohapantotype. The present study also includes both the description of an additional stage of the parasite (a trophozoite) and provides sequence data (fragment 18S rDNA), which was not provided by Dias [9] in his original description of this parasite species. This neohapantotype is deposited in the protozoan collection of the National Museum, Bloemfontein, South Africa under accession number NMB P 410.
Sequence identification and phylogenetic analysis
Amplicons of 611 and 613 nt for the 18S rRNA gene of K. paradoxa were obtained from the V. a. albigularis with the seemingly pure and highest parasitaemia infection using primer sets HepF300 and HepR900. No K. paradoxa isolate was obtained from the V. niloticus, likely due to its low parasitaemia in comparison to the concurrent infection of the other unidentified Hepatozoon species (unpublished data). The details of the species used in the phylogenetic analyses and presented in the consensus tree are provided in Table 2. The topology of both the BI and ML analyses were overall similar, with discrete monophyletic clades of known and likely belonging to Karyolysus species, Hepatozoon spp. of mammals (‘intraleucocytic’ Hepatozoon spp.), the herpatofauna (‘intraerythrocytic’ Hepatozoon spp.), Hemolivia spp., Haemogregarina spp., and the Dactylosomatidae (Fig. 4). Our results showed that K. paradoxa clustered within a major monophyletic clade containing both known (morphologically and ecologically confirmed) and likely belonging to Karyolysus species, and ‘intraleucocytic’ Hepatozoon species of mammals that were sister to the Karyolysus clade. This major clade was distinct from the major monophyletic clade containing the herpatofaunal ‘intraerythrocytic’ Hepatozoon spp. and Hemolivia spp.
Phylogenetic analysis of Karyolysus paradoxa (Dias, 1954) based on 18S rDNA sequences. Bayesian inference (BI) and Maximum Likelihood (ML) analysis showing the phylogenetic relationships for two Karyolyus paradoxa isolates from the current study represented in bold, 17 Karyolysus and Hepatozoon species (representing the Karyolysus clade in blue), five mammal-infecting Hepatozoon species (representing the ‘intraleucocytic’ Hepatozoon clade in purple), eight herpatofaunal-infecting Hepatozoon species (representing the ‘intraerythrocytic’ Hepatozoon clade in green), five Hemolivia species (representing the Hemolivia clade in orange), five Haemogregarina species (representing the Haemogregarinidae clade in yellow), a Babesiosoma and Dactylosoma species (representing the Dactylosomatidae clade in pink) and two Adelina and one Klossia species (used as the outgroup in grey). All comparative sequences were downloaded from the GenBank database. Tree topologies for both the BI and ML trees were identical; the nodal support values (BI/ML) are represented on the BI tree
The parasite collected in the present study that morphologically conformed to the original description of Hepatozoon paradoxa formed a sister taxon to the larger ‘Karyolysus’ clade (containing the known and likely belonging to Karyolysus species) in the phylogenetic analysis (Fig. 4). According to evolutionary divergence estimates the present material was most closely related to known Karyolysus spp. (at 97.7 %, p-distance = 0.02) than to species of the genera Hepatozoon, Hemolivia and Haemogregarina (Table 3).
Discussion
Varanus albigularis albigularis and Varanus niloticus are known to display somewhat different habitat preferences, preferring more terrestrial and aquatic environments respectively [32]. Both V. a. albigularis and V. niloticus can be found throughout South Africa from the more tropical Indian Ocean coastal belt in the East, west to the margins of the more arid Northern and Western Cape provinces [32]. Both species often occur sympatrically, particularly in Ndumo Game Reserve, rendering the finding of the same parasite in both these species not surprising.
As the morphological and developmental characteristics typical of Haemogregarina spp. had never been observed in haemogregarines of the herpatofauna, K. paradoxa was transferred along with many other species from the herpatofauna, birds and mammals, from the genus Haemogregarina (Haemogregarinidae) to the genus Hepatozoon (Hepatozoidae) by Smith [3] during a systematic review of the Hepatozoidae. However, during the first detailed revision of K. paradoxa provided here in the present study, morphologically this species shares more characteristics with members of the family Karyolysidae. Karyolysus paradoxa peripheral blood gamonts appear to be encapsulated as in the Hemolivia and destroy the host cell nucleus as in a number of species of Karyolysus (as mentioned above in Remarks) [1, 7]. However, it is imperative to take into account that these morphological features, specifically for the latter genus, are not always present [7].
The phylogenetic position of K. paradoxa was shown to be at the base of a clade containing undescribed species of Hepatozoon, many of these the results of molecular Hepatozoon spp. surveys [33, 34], and known species of Karyolysus [7]. This may suggest that these undescribed Hepatozoon spp. might rather be species of Karyolysus. The Karyolysus spp. clade is part of a larger clade including a sister clade of Hepatozoon spp. from mammals. This topology, as seen in this study, has been observed in a number of other studies [7, 33–37]. Karadjian et al. [37], in their attempt to understand the relationships of the different haemogregarine genera, particularly in respect to the conundrum of the polyphyletic Hepatozoon clade, proposed, based on their phylogenetic findings, that a number of Hepatozoon species (most of which have not been morphologically described) might rather represent species of Karyolysus. Given that it was only since the recent identification, description and molecular characterisation of Karyolysus spp. by [7], it is only now that we are beginning to realize this possibility. Moreover, it appears that the diversity of squamates parasitised by likely species of Karyolysus is fast increasing, a scenario which is likely only to intensify in future. A recent molecular Hepatozoon spp. survey by [38] shows haemogregarine isolates from geckos of the genus Tarentola Gray, 1825 (Phyllodactylidae) are also falling within what may be seen as the ‘Karyolysus’ clade; a clade that at present also includes haemogregarines isolated from species of the families Colubridae and Lamprophiidae (both snakes), Lacertidae and Varanidae (both lizards), and Scincidae (skinks).
Furthermore, the present study shows that K. paradoxa is most closely related to known Karyolysus species, followed by species of the ‘intraleucocytic’ Hepatozoon clade and then species of the ‘intraerythrocytic’ Hepatozoon and Hemolivia clades. Karyolysus and Hemolivia morphologically still belong within the same family (Karyolisidae), however, with the use of the 18S rRNA gene these two genera in this study and in others generally fall in different major clades. Karyolysus clusters in a major clade with the ‘intraleucocytic’ mammal Hepatozoon, whilst Hemolivia clusters in a major clade with the ‘intraerythrocytic’ herpatofaunal Hepatozoon; this finding is apparent in the present study, and also in [36, 37]. It is clear that the relationship between these two genera may possibly only be resolved by using a multi-gene approach as in [39].
Conclusions
Based on the morphology and the molecular findings presented in this study, we recommend the following nomenclatural correction: Karyolysus paradoxa (Dias, 1954) (syn. Hepatozoon paradoxa (Dias, 1954) Smith, 1996, Haemogregarina paradoxa Dias, 1954) in the varanid lizards Varanus albigularis albigularis (type-host), and Varanus niloticus. Our results showed that Karyolysus paradoxa is as closely related to species within its current generic assignment in the ‘intraerythrocytic’ herpatofaunal Hepatozoon as it is with the more distantly related species of the Haemogregarina.
Besides this study representing the first morphological and molecular report of a haemogregarine within an African varanid, it is the first report of a species of Karyolysus infecting a host of the Varanidae. Furthermore, it represents the third described and named Karyolysus spp. for which there is a nucleotide sequence available. It is hoped that this study will encourage further molecular work on the Karyolysidae, particularly the genus Karyolysus.
This study also extends the host and distribution range of K. paradoxa from only a single specimen of V. a. albigularis in Mozambique to an additional two specimens in South Africa, as well as including V. niloticus as an additional host both in South Africa and Kenya. The distribution range of K. paradoxa falls within subtropical areas in South Africa, Mozambique and Kenya, and as such it would be interesting to see if this particular parasite is restricted to subtropical areas such as is the case with Hemolivia parvula (Dias, 1953) found parasitising Kinixys zombensis Hewitt, 1931 tortoises of South Africa and Mozambique; see [12], or if it is more widely distributed throughout different biomes as is the case with Hepatozoon fitzsimonsi (Dias, 1953) found parasitising several tortoise species in South Africa and Mozambique; see [19].
Even though tick squashes did not result in any observable parasitic stages future studies will focus on identifying possible vectors. Parasitic stages found in these possible vectors will be identified to species level based on both morphological and molecular findings.
With the conundrum of the larger Hepatozoon clade being polyphyletic and absorbing the Hemolivia and Karyolysus, it is important to increase the number of taxa from which we can work and ask deeper phylogenetic questions. However, besides the molecular characterisation of these species, it is still important to focus on their morphology and where possible attempt to elucidate their life-cycles in order to resolve the complex taxonomy of these organisms. More importantly, it is necessary to include another faster evolving gene or even mitochondrial genomes of these groups following [39] before we can make any well-informed decisions.
References
Telford SR. Hemoparasites of the Reptilia: Color Atlas and Text. New York: CRC Press; 2009.
Smallridge CJ, Paperna I. The tick-transmitted haemogregarinid of the Australian sleepy lizard Tiliqua rugosa belongs to the genus Hemolivia. Parasite. 1997;4:359–63.
Smith TG. The genus Hepatozoon (Apicomplexa: Adeleina). J Parasitol. 1996;82:565–85.
Merino S, Martínez J, Masello JF, Bedolla Y, Guillfeldt P. First molecular characterization of a Hepatozoon species (Apicomplexa: Hepatozoidae) infecting birds and description of a new species infecting storm petrels (Aves: Hydrobatidae). J Parasitol. 2014;100:338–43.
Davies AJ, Johnston MRL. The biology of some intraerythrocytic parasites of fishes, amphibians and reptiles. Adv Parasitol. 2000;45:1–107.
Van As J, Davies AJ, Smit NJ. Hepatozoon langii n. sp. and Hepatozoon vacuolatus n. sp. (Apicomplexa: Adeleorina: Hepatozoidae) from the crag lizard (Sauria: Cordylidae) Pseudocordylus langi from the North Eastern Drakensberg escarpment, Eastern Free State, South Africa. Zootaxa. 2013;3608:345–56.
Haklová-Kočíková B, Hižňanová A, Majláth I, Račka K, Harris DJ, Földvári G, Tryjanowski P, Kokošová N, Malčeková B, Majláthová V. Morphological and molecular characterization of Karyolysus - a neglected but common parasite infecting some European lizards. Parasit Vectors. 2014;7:555.
Van As J, Davies AJ, Smit NJ. Life cycle of Hepatozoon affluomaloti sp. n. (Apicomplexa: Haemogregarinidae) in crag lizards (Sauria: Cordylidae) and in culicine mosquitoes from South Africa. Folia Parasitol. 2015;62:008.
Dias JATS. Subsídios para o estudo dos hematozoários dos répteis de Moçambique 3: Duas novas espécies de Haemogregarina parasitas do Varanus albigularis albigularis (Daudin, 1802). Bol Soc Est Moç. 1954;87:71–9.
Netherlands EC, Cook CA, Kruger DJ, du Preez LH, Smit NJ. Biodiversity of frog haemoparasites from sub-tropical northern Kwazulu-Natal, South Africa. Int J Parasitol Parasites Wildl. 2015;4:135–41.
Cook CA, Smit NJ, Davies AJ. First record of an intraleucocytic haemogregarine (Adeleorina: Haemogregarinidae) from South African tortoises of the species Stigmochelys pardalis (Cryptodira: Testudinidae). Afr Zool. 2014;49:290–4.
Cook CA, Netherlands EC, Smit NJ. First Hemolivia from southern Africa: reassigning chelonian Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) to Hemolivia (Adeleorina: Karyolysidae). Afr Zool. 2015;50:165–73.
Laveran A. Sur une haemogregarine de Varanus niloticus. C R Seances Soc Biol. 1905;59:175–6.
Nicolle MMC, Comte C. Sur une hémogrégarine de Varanus griseus. C R Seances Soc Biol. 1906;61:310–2.
Wolbach SB. Notes on the life cycle of a hemogregarine found in a monitor (Veranus niloticus). J Med Res. 1914;29:473–88.
Cook CA, Smit NJ, Davies AJ. A redescription of Haemogregarina fitzsimonsi Dias, 1953 and some comments on Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) from southern African tortoises (Cryptodira: Testudinidae), with new host data and distribution records. Folia Parasitol. 2009;56:173–9.
Cook CA, Smit NJ, Davies AJ. Hemproteids (Apicomplexa: Haemoproteidae) from South African Tortoises (Cryptodiria: Testudinidae). J Parasitol. 2010;96:1168–72.
Netherlands EC, Cook CA, Smit NJ, du Preez LH. Redescription and molecular diagnosis of Hepatozoon theileri (Laveran, 1905) (Apicomplexa: Adeleorina: Hepatozoidae), infecting Amietia quecketti (Anura: Pyxicephalidae). Folia Parasitol. 2014;61:293–300.
Cook CA, Lawton SP, Davies AJ, Smit NJ. Reassignment of the land tortoise haemogregarine Haemogregarina fitzsimonsi Dias 1953, (Adeleorina: Haemogregarinidae) to the genus Hepatozoon Miller 1908 (Adeleorina: Hepatozoidae) based on parasite morphology, life cycle and phylogenetic analysis of 18S rDNA sequence fragments. Parasitology. 2014;141:1611–20.
Ujvari B, Madsen T, Olsson M. High prevalence of Hepatozoon spp. (Apicomplexa: Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J Parasitol. 2004;90:670–2.
Geneious software created by Biomatters. [http://www.geneious.com].
Basic Local Alignment Search Tool. [http://blast.ncbi.nlm.nih.gov/Blast.cgi].
Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;5:1792–7.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol. 2013;30:2725–9.
Guindon S, Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol. 2003;52:696–704.
Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772.
Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–5.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3.
Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–7.
Ball GH. Some blood sporozoa from East African reptiles. J Protozool. 1967;14:198–210.
Široký P, Kamler M, Frye FL, Fictum P, Modrý D. Endogenous development of Hemolivia mauritanica (Apicomplexa: Adeleina: Haemogregarinidae) in the marginated tortoise Testudo marginata (Reptilia: Testudinidae): evidence from experimental infection. Folia Parasitol. 2007;54:13–8.
Bates MF, Branch WR, Bauer AM, Burger M, Marais J, Alexander GJ, de Villiers MS. Atlas and red list of the reptiles of South Africa, Lesotho and Swaziland. Suricata 1. Pretoria: South African National Biodiversity Institute; 2014.
Maia JPMC, Harris DJ, Perera A. Molecular survey of Hepatozoon species in lizards from North Africa. J Parasitol. 2011;97:513–7.
Maia JPMC, Perera A, Harris DJ. Molecular survey and microscopic examination of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina) in lacertid lizards from the western Mediterranean. Folia Parasitol. 2012;59:241–8.
Tomé B, Maia JPMC, Harris DJ. Hepatozoon infection prevalence in four snake genera: influence of diet, prey parasitaemia levels or parasite type? J Parasitol. 2012;98:913–7.
Kvičerová J, Hypša V, Dvořáková N, Mikulíček P, Jandzik D, George Gardner M, Javanbakht H, Tiar G, Široký P. Hemolivia and Hepatozoon: haemogregarines with tangled evolutionary relationships. Protist. 2014;165:688–700.
Karadjian G, Chavatte J, Landau I. Systematic revision of the adeleid haemogregarines, with creation of Bartazoon n. g., reassignment of Hepatozoon argantis Garnham, 1954 to Hemolivia, and molecular data on Hemolivia stellata. Parasite. 2015;22:31.
Tomé B, Rato C, Perera A, Harris DJ. High diversity of Hepatozoon spp. in geckos of the genus Tarentola. J Parasitol. 2016. doi:10.1645/15-908.
Ogedengbe JD, Ogedengbe ME, Hafeez MA, Barta JR. Molecular phylogenetics of eimeriid coccidian (Eimeriidae, Eimeriorina, Apicomplexa, Alveolata): A preliminary multi-gene and multi-genome approach. Parasitol Res. 2015;114:4149–41460.
Ramdan NF, Saoud MFA, Mohammed SH, Fawzi SM. On a new haemogregarine of Varanus griseus from Egypt. Qatar Univ Sci J. 1996;16:119–25.
Laveran A, Pettit A. Contribution à l’étude des haemogregarines de quelques sauriens d’Afrique. Bull Soc Pathol Exot. 1909;2:506–14.
França C. Notes sur des hématozoaires de la Guinée Portugaise. Arqiv Real Institut de Bactériologie Câmara Pestana. 1911;3:229–38.
Todd JL, Wolbach SB. Parasitic protozoa from The Gambia 1912: second report of the expedition of the Liverpool School of Tropical Medicine to The Gambia, 1911. J Med Res. 1912;26:195–218.
Leger M, Leger A. Hématozoaires des reptiles du Haut-Senegal-Niger. Bull Soc Pathol Exot. 1914;7:488–92.
Rousselot R. 1943 Cited by Rousselot, R. 1953.
Rousselot R. Notes de Parasitologie Tropicale. I. Parasites de Sang des Animaux. Paris: Vigot Freres; 1953.
Tomé B, Maia JPMC, Harris DJ. Molecular assessment of apicomplexan parasites in the snake Psammophis from North Africa: do multiple parasite lineages reflect the final vertebrate host diet? J Parasitol. 2013;99:883–7.
Mathew JS, van den Bussche RA, Ewing SA, Malayer JR, Latha BR, Panciera RJ. Phylogenetic relationships of Hepatozoon (Apicomplexa: Adeleorina) based on molecular, morphologic, and life-cycle characters. J Parasitol. 2000;86:366–72.
Barta JR, Ogendengbe JD, Martin DS, Smith TG. Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J Eukaryot Microbiol. 2012;59:171–80.
Criado-Fornelio A, Ruas JL, Casado N, Farias NA, Soares MP, et al. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. Int J Parasitol. 2006;92:93–9.
Criado-Fornelio A, Buling A, Casado N, Gimenez C, Ruas J, et al. Molecular characterization of arthropod-borne haematozoans in wild mammals from Brazil, Venezuela and Spain. Acta Parasitol. 2009;54:187–93.
Netherlands EC, Cook CA, Smit NJ. Hepatozoon species (Adeleorina: Hepatozoidae) of African bufonids, with morphological description and molecular diagnosis of Hepatozoon ixoxo sp. nov. parasitising three Amietophrynus species (Anura: Bufonidae). Parasit Vectors. 2014;7:552.
Sloboda M, Kamler M, Bulantová J, Votýpka J, Modrý D. A new species of Hepatozoon (Apicomplexa: Adeleorina) from Python regius (Serpentes: Pythonidae) and its experimental transmission by a mosquito vector. J Parasitol. 2007;93:1189–98.
Maia JP, Crottini A, Harris DJ. Microscopic and molecular characterization of Hepatozoon domerguei (Apicomplexa) and Foleyella furcata (Nematoda) in wild endemic reptiles from Madagascar. Parasite. 2014;21:47.
Harris DJ, Damas-Moreira I, Maia JPMC, Perera A. First report of Hepatozoon (Apicomplexa: Adeleorina) in caecilians, with description of a new species. J Parasitol. 2014;100:117–20.
Dvořáková N, Kvičerová J, Papoušek I, Javanbakht H, Tiar G, Kami H, Široký P. Haemogregarines from western Palaearctic freshwater turtles (genera Emys, Mauremys) are conspecific with Haemogregarina stepanowi Danilewsky, 1885. Parasitology. 2013;141:522–30.
Dvořáková N, Kvičerová J, Hostovský M, Široký P. Haemogregarines of freshwater turtles from Southeast Asia with a description of Haemogregarina sacaliae sp. n. and a redescription of Haemogregarina pellegrini Laveran and Pettit, 1910. Parasitology. 2015;142:816–26.
Kopečná J, Jirků M, Oborník M, Tokarev YS, Lukeš J, Modrý D. Phylogenetic analysis of coccidian parasites from invertebrates: search for missing links. Protist. 2006;157:173–83.
Acknowledgements
We would like to thank Ndumo Game Reserve and Ezemvelo KZN Wildlife, for access to sites and research permits (OP 839/2014; OP 1262/2015; OP 2492/2015) for sample collection. In addition, we would like to thank Microbiology, Unit for Environmental Sciences and Management, (NWU-PC), for the use of their facilities. The financial assistance of the National Research Foundation (NRF) of South Africa to CAC (NRF Scarce Skills Postdoctoral Scholarship - Grant SFP13090332476), and the Flemish Interuniversity Council (VLIR) to ECN (VLIR-OUS project – ZEIN21013PR396) is acknowledged. Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF or VLIR.
Authors’ contributions
All authors conceived and designed the project, participated in general data analysis and in drafting the manuscript. CAC and ECN carried out the fieldwork, prepared and examined blood smears, prepared light micrographs and compiled all measurement data. ECN participated in the molecular studies and in the sequence alignment and provided support to the preparation of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cook, C.A., Netherlands, E.C. & Smit, N.J. Redescription, molecular characterisation and taxonomic re-evaluation of a unique African monitor lizard haemogregarine Karyolysus paradoxa (Dias, 1954) n. comb. (Karyolysidae). Parasites Vectors 9, 347 (2016). https://doi.org/10.1186/s13071-016-1600-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1600-8