Abstract
Background
Phlebotomine sand flies (Diptera: Psychodidae) are insects of medical importance due to the role that some species play in the transmission of leishmaniasis. This work aimed to study some ecological aspects among sand flies fauna inhabiting two different environments: the várzea (lowland Amazonian forest) and terra firme (upland Amazonian forest), both located in Tefé Municipality, Amazonas State, Braziland to detect Leishmania infection in those phlebotomine populations.
Methods
Sand flies were collected using HP light traps. Collection took place over the course of six months: January, February, April, August, September, and October of 2013. To detect natural infection by Leishmania, DNA samples were extracted from female sand flies and submitted to Polymerase Chain Reaction (PCR) targeting the kDNA gene; Leishmania species were identified by PCR-RFLP targeting the hsp70 gene and genetic sequencing.
Results
In all, 5,716 individuals were collected, and 46 species were identified. Trichophoromyia ubiquitalis (3,330 – 58.26%) and Nyssomyia antunesi (661 – 11.26%) were the most abundant species. Species richness was greater in terra firme environments (42 species) than in the várzea environments (22 species), and forests ecotopes (43 species) were richer than peridomiciles (28 species). DNA of Leishmania was found in Th. ubiquitalis and Psychodopygus davisi, both of which inhabit the terra firme environment and sequencing analysis confirmed the presence of Leishmania (Viannia) lainsoni DNA in Th. ubiquitalis in Tefé Municipality.
Conclusions
The high abundance of Th. ubiquitalis and Ps. davisi and detection of DNA of Leishmania sp. may indicate that both species could be putative vectors for American Cutaneous Leishmaniasis (ACL) in the terra firme environment of Tefé. The sand fly fauna found in várzea is rich and diverse, exhibiting several species, nevertheless the seasonal hydric stress during part of the year that could influence the local diversity, if compared with other studies. This is the first report in Amazonas State of Th. ubiquitalis with presence of L. (V.) lainsoni DNA.
Similar content being viewed by others
Background
American Cutaneous Leishmaniasis (ACL) is an illness characterized by single or multiple skin lesions. In Brazil, there are seven species of Leishmania that cause the disease [1]. Approximately 148.315 cases were reported between 2007 and 2013 [2]. In Amazonas, the largest state in the North Region of Brazil, approximately 12.727 cases were reported during the same period; these were cases related to: Leishmania (Leishmania) amazonensis Vianna, L. (Viannia) braziliensis Vianna, L. (V.) guyanensis Floch, and L. (V.) naiffi Lainson and Shaw [3].
Human cases of ACL in the region result from direct contact between humans and female phlebotomine sand flies that are hematophagous, and transmit Leishmania by bite. These insects are mainly found in primary forests [4,5]. However, recent studies indicate that sand flies have adapted to disturbed environments, such as forest fragments, and peridomicile areas where domestic animals are present (dogs, chickens, pigs) [6-8]. Such close proximity to human habitation increases the risk of ACL infection. Approximately 260 species of phlebotomine sand flies are found in Brazil [9]; of these, 135 species are found in Amazonas [7,10-12]. Amazonas State has the highest concentration ACL vectors these include: Bichomomyia flaviscutellata (Mangabeira), Bi. olmeca nociva (Young and Arias), Ny. anduzei (Rozeboom), Ny. antunesi (Coutinho), Ny. umbratilis (Ward e Fraiha), and Trichophoromyia ubiquitalis (Mangabeira) [6,8,10].
In the Amazon basin, several human activities involve direct contact between humans and ACL vectors; these include: agriculture, timber harvest, suburb development, and, recently, the clearing of routes for oil pipelines [13-15]. In Amazonas, these activities mostly take place in two environments: the várzea and terra firme forests. Terra firme forest is characterized by permanently non-flooded areas, with 30 meter high trees and a high diversity of flora and fauna. Várzea forest is also rich in flora and fauna, however, unlike terra firme forest, it is flooded annually by white water rivers [16,17].
Phlebotomine sand flies have been well documented in Amazonas, particularly in terra firme environments. However, past research has been concerned primarily with Manaus and its neighboring localities. Information from other regions of Amazonas is scarce. In particular, phlebotomine sand flies that inhabit várzea environments have not been well documented, since few studies have been undertaken.
The Middle Solimões river basin contains both terra firme and várzea forests. Phlebotomine sand fly studies conducted in this region indicate that the most abundant species are of the genera Psychodopygus, Nyssomyia and Trichophoromyia; these genera include species considered to be ACL vectors [18,19]. In addition, cases of ACL in this region have increased considerably in recent years due to the presence of oil and gas extraction companies [20]. In Tefé Municipality, 328 cases of ACL were reported between 2007 and 2013, which is equivalent to 10.93 cases per 100,000 habitants, however, information about the species of Leishmania circulating in humans is unavailable [2]. In spite of the increased incidence of ACL in this region, few entomological studies have been undertaken to identify possible leishmaniasis vectors.
In addition, information about the natural infection of sand flies indicates that unconfirmed vectors may be present in Amazonas. Most natural infection studies in the region have been performed by dissection and visualization of trypanosomatid forms, but this method complicates the identification of Leishmania species [5,21,22], and is considered a relatively laborious technical procedure. Molecular methods like Polymerase Chain Reaction (PCR) are able to detect minimal amounts of Leishmania DNA, and allow a larger number of sand flies to be analyzed [23]; however, in Amazonas, PCR method to detect Leishmania infection has been used in a single article [24]. Our study aimed to study and compare the abundance and diversity of sand fly fauna in várzea and terra firme environments, and to detect Leishmania DNA in sand flies in an area of endemic ACL.
Methods
The Tefé Municipality (03°21’05”S, 64°42’53”W) is located in the middle of Amazonas State (AM), Brazil. It is one of ten municipalities that comprise the Middle Solimões region (Figure 1A). It has an area 23,704.488 km2, and a population of 61,453 habitants [25]. The climate is classified as Afi in the Köppen classification scheme [26]. The main vegetation consists of dense ombrophylous forest lowlands and alluvial ombrophylous forests [27].
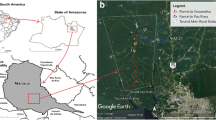
Map of study area. (A) Location of collection area in Tefé Municipality, Brazil. (B) Sand fly sampling areas in terra firme environments (1 and 2: Km 03; 3 and 4: Km 08) and várzea environments (5 and 6: Nossa Senhora do Perpétuo Socorro community; 7 and 8: Porto Vale community), Tefé Municipality, Amazonas State, Brazil. Numbers in yellow indicate forest ecotopes, and numbers in blue indicate peridomicile ecotopes. Source: Google Earth.
Phlebotomine sand flies were collected in forest and peridomicile areas, near small farms, in both terra firme and várzea environments. Collection took place in the following localities: EMADE road (Km 03 and Km 08), the community of Nossa Senhora do Perpétuo Socorro community, and the community of Porto Vale (Figure 1B). Forest and peridomicile areas were selected and analyzed in each locality. Collections were made with HP light traps over eight consecutive nights (from 6:00 pm to 7:00 am). Collections were conducted in January, February, April, August, September, and October of 2013. The collection of sand flies was authorized by Insttuto Nacional do Meio Ambiente (IBAMA) register: 5864300, protocol number: 41382.
Sand fly processing
After each capture event, sand flies were conserved in 90% alcohol and taken to a laboratory. Collected males, and the head and genitalia of females were clarified in Potassium Hydroxide solution and mounted on slides with Berlese fluid. All specimens were identified by taxonomic keys [28,29]. The taxonomical nomenclature of Galati [28] was used with the generic abbreviations proposed by Marcondes [30]. The rest of the body of females were conserved in tubes containing 90% alcohol in order to assess the posteriors for natural infection by Leishmania sp.
DNA Extraction of female sand flies and parasites
The females were grouped in pools of 10–20 specimens. Pools were divided according to month, environment, and species. The pools were processed for DNA extraction using the protocols for Blood and Tissue Qiagen DNeasy®. Samples were stored at -20° C for use in Polymerized Chain Reaction (PCR) testing.
PCR by kDNA
PCR targeting the kinetoplast region of Leishmania (PCR mkDNA) was performed using primers: Forward 5’-GGG (GT) AGGGGCGTTCT (G/C) CGAA-3’and Reverse 5-‘(G/C) (G/C) (G/C) (A/T) CTAT (A/T) TTACACCAACCCC-3’ to amplify the 120 bp fragment. The reaction mixture contained 18.7 μL of ultrapure water, 2.5 μL Buffer, 0.75 MgCl2 (2 mM), 0.38 μL prime rs (1μmol), 0.50 dNTPs (0.2 mM), 0.25 Taq Polymerase Invitrogen (1.25 U), and 2 μL of extracted DNA, for a total volume of 25 μL. Thermal cycling conditions followed those of Oliveira et al. [31].
PCR RFLP hsp70 and DNA sequencing
PCR targeting the hsp70 region of Leishmania species was performed with primers: Forward 5’-GGACGAGATCGAGCGATGGT-3’ and Reverse 5’- TCCTTCGACGCCTCCTGGTTG-3’ to amplify the 240 bp fragment. The reaction mixture contained 36.25 μL of ultrapure water, 5.0 μL Buffer, 1.5 MgCl2 (2 mM), 2 μL primers (1 μmol), 2 μL dNTPs (0.2 mM), 0.5 Taq Polymerase Invitrogen (1.25 U), and 5 μL of extracted DNA, for a total volume of 52.25 μL. The samples were placed in a thermocycler (Veriti–Applied Biossystems®) with an initial denaturation of 94°C for 4 minutes, followed by 33 cycles, of 94°C for 15 seconds (denaturation), 58°C for 30 seconds (annealing), and 72°C for 30 seconds (extension), with a final extension of 72°C for 10 minutes [32]. DNA from male sand flies was used as a negative control for each PCR. The final products amplified to 240 bp were submitted to restriction fragment length polymorphism (RFLP) by enzyme HaeIII (Invitrogen®, USA), according to manufacturer instructions.
The amplified products were visualized on a 100 bp ladder after electrophoresis using a 12% polyacrylamide gel colored with silver nitrate. The following DNA reference strains were used as positive controls: L. (L.) amazonensis (IOCL 575), L. (V.) braziliensis (IOCL 566), L. (V.) guyanensis (IOCL 565), L. (V.) lainsoni (IOCL 1045), L. (V.) naiffi (IOCL 1365), and L. (V.) shawi (IOCL 1545), obtained from Coleção de Leishmania do Instituto Oswaldo Cruz – Fiocruz/Rio de Janeiro, Brazil.
Leishmania species were identified by comparing the sequences obtained from analysis with reference sequences deposited in GenBank. Comparisons were made using BLAST program searches (Basic Local Alignment Search Tool, NCBI. Available online from: http://blast.ncbi.nlm.nih.gov.
Statistical analysis
The ranked abundance distribution (RAD) was calculated. The RAD produced a “hollow curve,” indicating that communities contain a few abundant species and many rare species [33]. The function radfit fit all models to a data frame, and the Akaike Information Criterion (AIC) was used to select the best one for the community. An analysis of variance (ANOVA) was performed to compare the number of species collected from each environment (i.e. Terra Firme and Várzea). The specificity and fidelity of sand fly species to environments was verified using the indicator value (IndVal) of species [34]; the morphotypes were excluded from this test. All analysis was done in R program, with a 5% significance level [35]. Natural infection was assessed by estimating the prevalence of infection in positive pools using Pool Screening Program (http://www.soph.uab.edu/bst/poolscreen) Version 2.0 [36]. Analysis of prevalence has been used previously to assess natural infection in Simuliidae [36], and the use of Pool Screening Program to assess sand flies has been suggested by Mártin-Sánchez et al. [37].
Results
Fauna composition of sand flies
Of the 5,716 individuals collected, 46 species were identified, including 6 morphotypes belonging to the following 11 genera: Evandromyia Mangabeira, Lutzomyia França, Micropygomyia Barretto, Nyssomyia Barretto, Pintomyia Costa Lima, Psathyromyia Barretto, Psychodopygus Mangabeira, Sciopemyia Barretto, Trichophoromyia Barretto, Trichopygomyia Barretto, and Viannamyia Mangabeira. The most abundant genera were Psathyromyia (8), Psychodopygus (7), Evandromyia (5), and Trichophoromyia (5). The most abundant species were Trichophoromyia ubiquitalis, Nyssomyia antunesi, and Nyssomyia yuilli yuilli (Table 1).
In all, 5428 specimens were collected from terra firme environments, comprising 42 species and 6 morphotypes. The most abundant species were Thichophoromyia ubiquitalis (3312 individuals – 57.94%), Nyssomyia antunesi (526 – 9.20%), Nyssomyia yuilli yuilli (259 – 4.53%), Trichophoromyia sp. (207 – 3.62%), Psychodopygus davisi (198 – 3.46%), and Trichophoromyia melloi (146 - 2.55%). In the várzea environment, 288 specimens were collected, comprising 22 species and 3 morphotypes. Of these, the most abundant species were Ny. antunesi (135 - 2.36%) and Evandromyia walkeri (87 – 1.52%) (Table 1). Both environments had high species richness, but equitability was low (Figure 2). Analysis by IndVal indicated that only four species were specific to the terra firme environment: Th. ubiquitalis, Ps. davisi, Ny. yuilli yuilli, and Th. melloi (Table 2). No species were specific to the várzea environment, but Evandromyia walkeri was found there in higher frequency.
Sand fly richness was highest in the forest ecotopes of both environments as compared with peridomiciles (ANOVA χ 2 = 3.95, p < 0.04) (Figure 3). In the terra firme environment, forest ecotopes yielded 4689 specimens and 39 species, while peridomiciles ecotopes yielded 739 individuals and 27 species. In the várzea environment, forest ecotopes yielded 263 individuals and 21 species, while peridomicile ecotopes yielded 25 individuals and 4 species (Table 1).
Detection of Leishmania DNA in phlebotomine sand flies
In total, 1,679 females (58.9% of all females collected) were grouped in 95 pools (Th. ubiquitalis – 60 pools, Ny. antunesi – 18, Ny. yuilli yuilli – 8, Ps. davisi – 4, Ps. ayrozai – 4, Ny. umbratilis - 1). Of these, 14 pools were amplified in the mkDNA region and assessed as DNA Leishmania presence confirmed: Th. ubiquitalis - 10 pools, and Ps. davisi – 4 pools. Both of these species were specific to the terra firme environment (Figure 4). The minimal infection prevalence for Th. ubiquitalis was 0. 96% (95% CI = 0.51–0.96–0.17) and all samples of Ps. davisi tested positive for infection.
PCR mKDNA. 2% agarosis gel colored with GelRed showingproducts amplified by kDNA PCR with DNA extracted from female sand flies collected in Tefé Municipality, Amazonas State, Brazil. Lines: 1 – negative control (Ultrapure water - H2O Milli-Q); 2 to 4 - Thichophoromyia ubiquitalis; 5 to 6 - Psychodopygus davisi; 7 to 13: Trichophoromyia ubiquitalis; 14 to 15: Psychodopygus davisi; 16: positive control Leishmania (Leishmania) amazonensis, 17: molecular marker 100pb (Invitrogen).
Seven samples of Th. ubiquitalis were amplified by PCR targeted to the hsp70 region. Restriction enzyme HaeIII was applied to these samples yielding the patterns of L. (V.) lainsoni and L. (V.) shawi (Figure 5). Sequencing confirmed the presence of L. (V.) lainsoni in all samples. The sequences for pool 70_hsp70 and pool 72_hsp70 were similar to the sequence of L. (V.) lainsoni (99%) deposited in GenBank under code number GU071176.1.
PCR-RFLP (234 bp) profiles for samples of Trichophoromyia ubiquitalis after disgestion of products with Hae III. Silver-stained 12.5% polyacrylamide gel. Lines: 1 - pool 65; 2 - pool 4; 3 - pool 24; 4 - pool 70; 5 - pool 72; 6 - pool 77; 7 – Ll = Leishmania (Viannia) lainsoni; 8 – Ls = Leishmania (Viannia) shawi; 9 – Ln = Leishmania (Viannia) naiffi;10 – La = Leishmania (Leishmania) amazonensis; M = 100 bp molecular weight marker 100 bp (Invitrogen).
Discussion
The species found in Tefé Municipality correspond to 34.5% of the species recorded in Amazonas [7,10-12]. Phlebotomine fauna in the Middle Solimões region is rich and diverse, being comprised of 40–50 species; of which, Th. ubiquitalis, Ny. yuilli yuilli, Ps. chagasi, and Ps. davisi are the most abundant [18,19]. Also Silva et al. [24] found Th. ubiquitalis and Ps. davisi as a dominant species in Lábrea Municipality, nearby Purus River, a Solimões River tributary. Those species composition is different from the species composition found in Manaus and its neighboring municipalities, where there are 35–50 species, the most abundant being Ny. umbratilis, Ny. anduzei, and Ny. antunesi. This shows that sand fly fauna differs throughout Amazonas State [5-8,38,39].
A high diversity of genera was found in Tefé, the highest were respectively Psathyromyia, Psyhodopygus, and Trichophoromyia, which are widely distributed throughout the Amazon region, and are also found in others states of Brazil [10,40-44]. These genera are of epidemiological importance because they are present in several regions of Brazil, and they contain some species involved or suspected in the transmission of ACL and other etiological agents [1].
There is a scarcity of data that compares sand fly fauna in terra firme and várzea environments simultaneously. However, phlebotomine fauna in Tefé is considered diverse in both environments, (31.1% in Terra firme and 16.3% in Várzea if compared with a total of species recorded to Amazonas). Some species incriminated or suspected as vectors have been recorded in the terra firme environment: Ny. anduzei, Ny. antunesi, Ny. umbratilis, Ps. davisi, Ps. h. hirsutus, and Th. ubiquitalis. This highlights the possibility that these species may participate in the transmission of ACL in both of the environments studied in Tefé. Some studies of fauna in the várzea environment have identified insects of medical importance, such as mosquitoes and biting midges [45-47]; however, there is little information about sand flies [48]. This is because the várzea environment is difficult to access during rainy seasons, and because flooding makes field work more arduous; yet flooding may have a direct impact on the habitats of insect fauna. Our results show that fauna in the várzea environment is rich, exhibiting several species such as Ny. antunesi, Th. auraensis, and Th. ubiquitalis, that are of medical importance and could pose epidemiological concerns [1,49].
In Tefé, Forest ecotopes proved to be richer and more abundant than peridomicile ecotopes. Sand flies are found mainly in forest environments probably because the resources there are more plentiful. The abundance observed in this study has been observed in other studies in the Amazon region, and these studies have found that most species reside in forest environments [8,50]. However, abundance has been observed in some species residing in localities with anthropic effects. These species include Th. ubiquitalis, Ev. walkeri, and Ny. antunesi, and this shows some adaptation to modified environments [7,8,51]. These species may have been attracted by blood meal sources such as humans, or pigs and chickens present in peridomicile areas [52-54].
Trichophoromyia ubiquitalis was the most abundant species in this study. This species is abundant in other municipalities and is widely distributed throughout the Amazon region, including the Middle Solimões region and tributaries [18,19,24], and the municipalities of Borba and Maués, near to Manaus [10]. This species also occurs in high abundance in the rest of the North Region, being found in the states of Acre, Pará, and Rondônia [44,55-57]. Recently, Silva et al. [24] found the presence of L. (L.) amazonensis DNA in Th. ubiquitalis in Lábrea Municipality, Amazonas. The Nyssomyia antunesi was the second most abundant species in this study. This species has adapted to anthropogenic environments and has been recorded as abundant in the municipalities of Lábrea, and Presidente Figueiredo, in Amazonas [7,8]. This species has been found naturally infected by L. (V.) lindenbergi in Pará State [58], and has been linked to the transmission of ACL in that region.
The detection of Th. ubiquitalis and Ps. davisi with Leishmania DNA could be an indicative of those species are putative vectors in Tefé. Previously, the presence of DNA L.(L.) amazonensis and L. (V.) braziliensis were detected respectively in the species Th. ubiquitalis and Ps. davisi [24]. Although Th. ubiquitalis exhibits low anthropophilic behavior, it is a proven vector of L. (V.) lainsoni in Pará State [57]. Psychodopygus davisi has been found infected by Leishmania spp. in Rondônia State [55,59], and naturally infected by L. (V.) braziliensis in Pará State, in Serra do Carajás, and in Peru [49,60] and with Leishmania braziliensis DNA presence in Lábrea [24]. The minimal infection prevalence of 0.96% observed in Th. ubiquitalis does not differ from that of other studies, where a 0.3–3% incidence of natural infection has been considered high [61-66].
This is the first record in Amazonas State of sand flies with presence of L. (V.) lainsoni DNA. The only previous record of this Leishmania species in the Amazonas State was a human case involving the infection of a single child in Manaus [67]. The distribution of L. (V.) lainsoni has probably been underestimated, as its presence in Brazil has only been attributed to the states of Acre, Pará [57,68], and Amazonas. The detection of DNA clew of L. (V.) lainsoni in Th. ubiquitalis, and the high abundance of this fly may indicate that this species could be a putative vector in In Tefé Municipality.
Conclusion
In the Middle Solimões region, the sand fly fauna in terra firme and várzea environments is composed of a few dominant species, and several species with few individuals. The fauna varies between ecotopes, being more abundant in forest ecotopes than in peridomicile ecotopes. The abundance of Th. ubiquitalis and its record of presence of L. (V.) lainsoni DNA may indicate that this species is a vector for ACL in Tefé Municipality, Amazonas, Brazil.
Abbreviations
- ACL:
-
American cutaneous leishmaniasis
- PCR:
-
Polymerase chain reaction
- DNA:
-
Deoxyribonucleic acid
- HP:
-
Hoover pugedo
- kDNA:
-
Kinetoplatisc DNA
- hsp70:
-
Heat shock protein 70
- bp:
-
Base pairs
- RAD:
-
Ranked abundance distribution
- AIC:
-
Akaike information criterion
- ANOVA:
-
Analyzes of variance
- INDVAL:
-
Indicator value of species
- Ll:
-
Leishmania lainsoni
- Ls:
-
Leishmania shawi
- Ln:
-
Leishmania naiffi
- La:
-
Leishmania amazonensis
References
Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–50.
Sinan. Sistema de informações de agravos de notificação. 2014. [http://dtr2004.saude.gov.br/sinanweb]. Assessed 15 Feb 2014.
Camara Coelho LI, Paes M, Guerra JAO, Barbosa MG, Coelho C, Lima B, et al. Characterization of Leishmania spp. causing cutaneous leishmaniasis in Manaus, Amazonas, Brazil. Parasitol Res. 2011;108:671–7.
Dias-Lima AG, Castellón EG, Sherlock I. Flebotomíneos (Diptera: Psychodidae) de uma floresta primária de terra firme da Estação experimental de silvicultura tropical, estado do Amazonas, Brasil. Acta Amaz. 2003;33:303–16.
Pessoa FAC, Medeiros JF, Barrett TV. Effects of timber harvest on phlebotomine sand flies (Diptera: Psychodidae) in a production forest: abundance of species on tree trunks and prevalence of trypanosomatids. Mem Inst Oswaldo Cruz. 2007;102:593–9.
Rocha LC, Freitas RA, Franco AMR. Phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) in urban rainforest fragments, Manaus - Amazonas State, Brazil. Acta Trop. 2013;126:103–9.
Figueira EAG, Silva GS, Chagas EC, Shimabukuro PHF. Phlebotomine sandflies (Diptera: Psychodidae) from Lábrea, state of Amazonas, Brazil, with a description of Evandromyia (Aldamyia) apurinan Shimabukuro, Figueira & Silva, sp. nov. Mem Inst Oswaldo Cruz. 2013;108:280–7.
Ramos WR, Medeiros JF, Julião GR, Ríos-Velásquez CM, Marialva EF, Desmouliére SJM, et al. Anthropic effects on sand fly (Diptera: Psychodidae) abundance and diversity in an Amazonian rural settlement, Brazil. Acta Trop. 2014;139:44–52.
Shimabukuro PHF, Tolezano JE, Galati EAB. Chave de Identificação Ilustrada dos Phlebotominae (Diptera, Psychodidae) do Estado de São Paulo, Brasil. Pap Avulsos Zool. 2011;51:399–441.
Alves VR, Freitas RA, Santos FL, Oliveira AFJ, Barrett TV, Shimabukuro PHF. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220–7.
Oliveira AFJ, Aguiar NO, Freitas RA, Pessoa FAC. New records of phlebotomine fauna (Diptera, Psychodidae) in the Amanã sustainable development reserve, Amazonas, Brazil. Uakari. 2013;9:55–9.
Ladeia-Andrade S, Fe NF, Sanguinette CC, Andrade Filho JD. Description of Trichophoromyia uniniensis, a new phlebotomine species (Diptera: Psychodidae: Phlebotominae) of Amazonas State Brazil. Parasit Vectors. 2014;7:400.
Guerra JAO, Talhari S, Paes MG, Garrido M, Talhari JM. Aspectos clínicos e diagnósticos da leishmaniose tegumentar americana em militares simultaneamente expostos à infecção na Amazônia. Rev Soc Bras Med Trop. 2003;36:587–90.
Guerra JAO, Ribeiro JAS, Coelho LIARC, Barbosa MGV, Paes MG. Epidemiologia da leishmaniose tegumentar na Comunidade São João, Manaus, Amazonas Brasil. Cad Saude Publica. 2006;22:2319–27.
Naiff Júnior RD, Pinheiro FG, Naiff MF, Souza I, Castro LM, Menezes MP, et al. Estudo de uma série de casos de leishmaniose tegumentar americana no município de Rio Preto da Eva, Amazonas, Brasil. Rev Patol Trop. 2009;38:103–14.
Junk WJ, Piedade MTF, Schöngart J, Cohn-Haft M, Adeney JM, Wittmann F. A classification of major naturally-occurring Amazonian lowland wetlands. Wetlands. 2011;31:623–40.
Prance GT. Notes on the vegetation of Amazonia III. The terminology of Amazonian forest types subject to inundation. Brittonia. 1979;31:26–38.
Barrett TV, Freitas RA, Albuquerque MIC, Guerrero JCH. Report on a collection of Lutzomyia sand flies (Diptera: Psychodidae) from the Middle Solimões (Amazonas, Brazil). Mem Inst Oswaldo Cruz. 1996;91:27–35.
Castellón EG, Fé NF, Buhrnheim PF, Fé FA. Flebotomíneos (Diptera, Psychodidae) na Amazônia. II. Listagem das espécies coletadas na Bacia Petrolífera no Rio Urucu, Amazonas, Brasil, utilizando diferentes armadilhas e iscas. Rev Bras Zool. 2000;17:455–62.
Costa AG, Alecrim PH, Santos JD, Conceição JKT, Brandão LF, Heckmann MIO. Aspectos epidemiológicos da infecção por leishmaniose tegumentar americana (LTA) em pacientes do município de Coari-Amazonas. [http://www.sbpcnet.org.br/livro/62ra/resumos/resumos/3276.htm]. Assessed 20 April 2013.
Pinheiro FG, Luz SLB, Franco AMR. Infecção natural por tripanosomatídeos (Kinetoplastida: Trypanosomatidae) em Lutzomyia umbratilis (Diptera: Psychodidae) em áreas de leishmaniose tegumentar americana no Amazonas, Brasil. Acta Amaz. 2008;38:165–72.
Reis SR, Gomes LHM, Ferreira NM, Nery LR, Pinheiro FG, Figueira LP, et al. Ocorrência de flebotomíneos (Diptera: Psychodidae: Phlebotominae) no ambiente peridomiciliar em área de foco de transmissão de leishmaniose tegumentar no município de Manaus, Amazonas. Acta Amaz. 2013;43:121–3.
Aransay AM, Scoulica E, Tselentis Y. Detection and identification of Leishmania DNA within naturally infected sand flies by seminested PCR on minicircle kinetoplastic DNA. Appl Environ Microbiol. 2000;66:1933–8.
Silva TRR, Assis MDG, Freire MP, Rego FD, Gontijo CMF, Shimabukuro PHF. Molecular Detection of Leishmania in Sand Flies (Diptera: Psychodidae: Phlebotominae) collected in the Caititu indigenous reserve of the municipality of Lábrea, State of Amazonas, Brazil. J Med Entomol. 2014;51:1276–82.
IBGE. Censo populacional de 2010. 2010. [http://cod.ibge.gov.br/235L5] Assessed 04 Jun 2013.
INMET. Banco de dados meteorológicos para ensino e pesquisa. 2013. [http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep]. Assessed 15 Feb 2014.
Vivona SN, Sesubiani HRV, Bruscato LP, Adileu DO. Relatório de Impactos Ambientais (RIMA), usina termelétrica de Tefé, Amazonas. Relatórios Impactos ao Meio Ambiente Relatórios Impactos ao Meio Ambiente. 2003;1:92.
Galati EAG, Rangel EF, Lainson R. Morfologia e taxonomia. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. 1st ed. Rio de Janeiro: Fiocruz; 2003. p. 23–51.
Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Assoc Publ Am Entomol Inst. 1994;1:881.
Marcondes CB. A proposal of generic and subgeneric abreviations for phlebotomine sandflies (Diptera: Psychodidae: Phlebotominae) of the world. Entomol News. 2007;118:351–6.
Oliveira JGS, Novais FO, De Oliveira CI, Da Cruz AC, Campos LF, Rocha AV, et al. Polymerase chain reaction (PCR) is highly sensitive for diagnosis of mucosal leishmaniasis. Acta Trop. 2005;94:55–9.
Da Graça GC, Volpini AC, Romero GAS, Neto MP De O, Hueb M, Porrozzi R, et al. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem Inst Oswaldo Cruz. 2012;107:664–74.
McGill BJ, Etienne RS, Gray JS, Alonso D, Anderson MJ, Benecha HK, et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol Lett. 2007;10:995–1015.
Dufrêne M, Legendre P. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr. 1997;67:345–66.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL: http://www.R-project.org/. 2014.
Katholi CR, Toe L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis. 1995;172:1414–7.
Martín-Sánchez J, Gállego M, Barón S, Castillejo S, Morillas-Marquez F. Pool screen PCR for estimating the prevalence of Leishmania infantum infection in sandflies (Diptera: Nematocera, Phlebotomidae). Trans R Soc Trop Med Hyg. 2006;100:527–32.
Dias-Lima AG, Bermúdez EC, Medeiros JF, Sherlock I. Estratificação vertical da fauna de flebótomos (Diptera, Psychodidae) numa floresta primária de terra firme da Amazônia Central, Estado do Amazonas, Brasil. Cad Saude Publica. 2002;18:823–32.
Silva DF, Freitas RA, Franco AMR. Diversidade e abundância de flebotomíneos do gênero lutzomyia (Diptera : Psychodidae) em áreas de Mata do Nordeste de Manacapuru, AM. Neotrop Entomol. 2007;36:138–44.
Azevedo AC, Costa SM, Pinto MC, Souza JL, Cruz HC, Vidal J, et al. Studies on the sandfly fauna (Diptera: Psychodidae: Phlebotominae) from transmission areas of American Cutaneous Leishmaniasis in state of Acre, Brazil. Mem Inst Oswaldo Cruz. 2008;103:760–7.
Freitas RA, Barrett TV. Descriptions of Lutzomyia (Evandromyia) georgii n. sp. and a synopsis of the series infraspinosa (Diptera: Psychodidae). Mem Inst Oswaldo Cruz. 2002;97:239–45.
Gil LHS, Araújo MS, Villalobos JM, Camargo LMA, Ozaki LS, Fontes CJF, et al. Species structure of sand fly (Diptera: Psychodidae) fauna in the Brazilian western Amazon. Mem Inst Oswaldo Cruz. 2009;104:955–9.
Machado TO, Bragança MAL, Carvalho ML, Andrade Filho JD. Species diversity of sandflies (Diptera: Psychodidae) during different seasons and in different environments in the district of Taquaruçú, state of Tocantins, Brazil. Mem Inst Oswaldo Cruz. 2012;107:955–9.
Teles CBG, Basano SA, Zagonel-Oliveira M, Campos JJ, Oliveira AFJ, Freitas AR, et al. Epidemiological aspects of American cutaneous leishmaniasis and phlebotomine sandfly population, in the municipality of Monte Negro, State of Rondonia, Brazil. Rev Soc Bras Med Trop. 2013;46:60–6.
Hutchings RSG, Hutchings RW, Sallum MAM. Culicidae (Diptera, Culicomorpha) from the western Brazilian Amazon: juami-japurá ecological station. Rev Bras Entomol. 2010;54:687–91.
Santarém MCA, Confalonieri UEC, Felippe-Bauer ML. Diversidade de Culicoides (Diptera: Ceratopogonidae) na Floresta Nacional de Caxiuanã, Melgaço, Estado do Pará, Brasil. Rev Pan-Amazônica Saúde. 2010;1:29–33.
Trindade RL, Gorayeb IS. Maruins (Diptera: Ceratopogonidae: Culicoides), após a estação chuvosa, na Reserva de Desenvolvimento Sustentável Itatupã-Baquiá, Gurupá, Pará, Brasil. Rev Pan-Amazônica Saúde. 2010;1:121–30.
Rebêlo JMM, Oliveira-Pereira YN. Flebotomíneos (Diptera, Psychodidae) de matas de terra firme e de várzea, do município de Paragominas, Estado do Pará, Brasil. Acta Amaz. 2001;31:145–54.
Valdivia HO, De Los Santos MB, Fernandez R, Baldeviano GC, Zorrilla VO, Vera H, et al. Natural Leishmania infection of Lutzomyia auraensis in Madre de Dios, Peru, detected by a fluorescence resonance energy transfer-based real-time polymerase chain reaction. Am J Trop Med Hyg. 2012;87:511–7.
Barbosa MGV, Fé NF, Marcião AHR, Silva APT, Monteiro WM, Guerra JAO. Fauna de flebotomíneos (Diptera: Psychodidae) em um foco de leishmaniose tegumentar americana na área periurbana de Manaus, Estado do Amazonas. Rev Soc Bras Med Trop. 2008;41:485–91.
Silva MNT, Castellón EG. Similaridade da fauna flebotomínica de três fragmentos florestais em área urbana do município de Manaus, Estado do Amazonas, Brasil. Rev Colomb Cienc Anim. 2010;2:85–92.
Guerra JAO, Paes MG, Coelho LIAR, Barros MLB, Fé NF, Barbosa MGV, et al. Estudo de dois anos com animais reservatórios em área de ocorrência de leishmaniose tegumentar americana humana em bairro de urbanização antiga na cidade de Manaus-AM, Brasil. Acta Amaz. 2007;37:133–7.
Legriffon CMO, Reinhold-Castro KR, Fenelon VC, Neitzke-Abreu HC, Teodoro U. Frequência de flebotomíneos em ambiente rural com boas condições de limpeza e organização, no Estado do Paraná, Brasil. Rev Soc Bras Med Trop. 2012;45:77–82.
Teodoro U, Lonardoni MVC, Silveira TCV, Dias AC, Abbas M, Alberton D, et al. Luz e galinhas como fatores de atração de Nyssomyia withmani em ambiente rural, Paraná, Brasil. Rev Saude Publica. 2007;41:383–8.
Gil LHS, Basano SA, Souza AA, Silva MGS, Barata I, Ishikawa EA, et al. Recent Observations on the sand fly (Diptera: Psychodidae) Fauna of the State of Rondônia, Western Amazônia, Brazil: the importance of psychdopygus davisi as a vector of Zoonotic Cutaneous Leishmaniasis. Mem Inst Oswaldo Cruz. 2003;98:751–5.
Silva-Nunes M, Cavasini CE, Silva NS, Galati EAB. Epidemiologia da Leishmaniose Tegumentar e descrição das populações de flebotomíneos no município de Acrelândia, Acre, Brasil. Rev Bras Epidemiol. 2008;11:241–51.
Silveira FT, Souza AA, Lainson R, Shaw JJ, Braga RR, Ishikawa EE. Cutaneous leishmaniasis in the Amazon region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará State, Brazil. Mem Inst Oswaldo Cruz. 1991;86:127–30.
Silveira FT, Ishikawa EAY, De Souza AAA, Lainson R. An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará State, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp. A new leishmanial parasite of man in the Amazon region. Parasite. 2002;9:43–50.
Grimaldi G, Momen H, Naiff RD, McMahon-Pratt D, Barrett TV. Characterization and classification of leishmanial parasites from humans, wild mammals, and sand flies in the Amazon Region of Brazil. Am J Trop Med Hyg. 1991;44:645–61.
Souza AAA, Silveira FT, Lainson R, Barata IR, Silva MGS, Lima JAN, et al. Fauna flebotomínica da Serra dos Carajás, Estado do Pará, Brasil, e sua possível implicação na transmissão da leishmaniose tegumentar Americana. Rev Pan-Amazônica Saúde. 2010;1:45–51.
Pita-Pereira D, Alves CR, Souza MB, Brazil RP, Bertho AL, Barbosa AF, et al. Identification of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridisation assay. Trans R Soc Trop Med Hyg. 2005;99:905–13.
Queiroz RG, Vasconcelos IDAB, Vasconcelos AW, Pessoa FAC, De Sousa RN, David JR. Cutaneous leishmaniasis in Ceara state in Northeastern Brazil: incrimination of Lutzomyia whitmani (Diptera: psychodidae) as a vector of Leishmania braziliensis in Baturite municipality. Am J Trop Med Hyg. 1994;50:693–8.
Michalsky EM, Guedes KS, Silva FLO, França-Silva JC, Dias CLF, Barata RA, et al. Infecção natural de Lutzomyia (Lutzomyia) longipalpis (Diptera: Psychodidae) por Leishmania infantum chagasi em flebotomíneos capturados no município de Janaúba, estado de Minas Gerais, Brasil. Rev Soc Bras Med Trop. 2011;44:58–62.
Paiva BR, Secundino NFC, Nascimento JC, Pimenta PFP, Galati EAB, Junior HFA, et al. Detection and identification of Leishmania species in field-captured phlebotomine sandflies based on mini-exon gene PCR. Acta Trop. 2006;99:252–9.
Rodríguez N, Aguilar CM, Barrios MA, Barker DC. Detection of Leishmania braziliensis in naturally infected individual sandflies by the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1999;93:47–9.
Silva EA, Andreotti R, Dias ES, Barros JC, Brazuna JCM. Detection of Leishmania DNA in phlebotomines captured in Campo Grande, Mato Grosso do Sul, Brazil. Exp Parasitol. 2008;119:343–8.
Chrusciak-Talhari A, Dietze R, Talhari CC, Silva RM, Yamashita EPG, De Oliveira PG, et al. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg. 2011;84:255–60.
da Silva AC T, Cupolillo E, Volpini AC, Almeida R, Sierra Romero GA. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of Acre, Brazil. Trop Med Int Heal. 2006;11:1388–98.
Rangel EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009;104:937–54.
Acknowledgments
AMPJ received MSc scholarships from CNPq, Grant Number 131191/2012-8. EFM received PAIC scholarships from FAPEAM, Grant Number 062.01226/2013. Financial support of FAPEAM, Grant Number 1068/2009. Also to inhabitants of the Nossa Senhora do Perpétuo Socorro and Porto Vale communities. To Fiocruz Rondônia by support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AMPJ performed the design of the study, identification, sequencing analysis and wrote the article; CBGT performed the sequencing analysis and wrote the article; APAS performed the sequencing analysis and wrote the article; MSR performed the statistical analysis and wrote the article; EFM performed the identification and wrote the article; FACP conceived the study, performed the design of the study and wrote the article; JFM conceived the study, performed the design of the study and wrote the article. All authors read and approved the final version of the manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pereira Júnior, A.M., Teles, C.B.G., de Azevedo dos Santos, A.P. et al. Ecological aspects and molecular detection of Leishmania DNA Ross (Kinetoplastida: Trypanosomatidae) in phlebotomine sandflies (Diptera: Psychodidae) in terra firme and várzea environments in the Middle Solimões Region, Amazonas State, Brazil. Parasites Vectors 8, 180 (2015). https://doi.org/10.1186/s13071-015-0789-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-015-0789-2