Abstract
Background
Among the third-generation biodiesel feed stock, oleaginous marine yeasts are the least studied microorganisms for such purpose.
Results
Wild strains yeasts were isolated from various Tunisian marine sources including fish waste (Candida tenuis CtTun15, Debaryomyces hansenii DhTun2015, Trichosporon asahii TaTun15 and Yarrowia lipolytica YlTun15) and seawater (Rhodotorula mucilaginosa RmTun15). Following incubation with ethyl methanesulfonate (EMS: 75 mM) for various periods of time (T15, T30, T45, T60 min), the cell viability of these strains responded differentially according to yeast species. For instance, mutated CtTun15 did not survive after 30 min of EMS treatment; higher resistances were observed in DhTun2015 (45 min), in YlTun15, RmTun15 and in TaTun15 (60 min) but with significant decreased cell viabilities (survival rate: 6.02, 3.16, 11.22, 11.58, 7.70%, respectively). For all surviving mutated strains, the optima of biomass and lipid yields were detected after 96 h in YPD culture; but derived from strains submitted to different period of EMS incubation. In most mutated strains, the maximum biomass (BP) and lipid (LP) productivities coincided and were observed after 30 min of EMS incubation. Only CtTun15 showed different optima of BP and LP (after 30 min and 15 min, respectively). The fatty acids (FA) compositions considered essential in the prediction of biodiesel criteria; were highly affected by EMS mutagenesis. Essentially, 30- and 45-min EMS incubation induced the highest levels of PUFA and MUFA in YlTun15, RmTun15 and TaTun15 with non-significant differences in the different times. However, CtTun15 and DhTun2015 mutant strains responded differently, with the highest levels of MUFA observed following 15 and 45 min; and that of PUFA after 30 and 45 min, respectively.
Conclusion
The methyl-esterification of FA from the three mutated yeast strains (30 min—YlTun15, RmTun15 and TaTun15) yielded biodiesel with physical proprieties consistent with the International Standard System. However, investigations are needed for up-scaling biodiesel production.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Intensive investigations are currently oriented toward the exploration of renewable energy sources as substitutes to fossil fuels [1, 2]. Among such sources, oleaginous microorganisms that can accumulate more than 20% lipids dry weight represent promising substitutes [3]. Moreover, the quality of their lipid allows their potential use in commercial production of oil for food, chemical and energy applications [4,5,6]. For instance, biodiesel as type of bio-fuels produced mainly by a transesterification process, are used in some European countries in a way similar that of to the mineral diesel and, with some additives, applicable with various engines [7].
Yarrowia, Candida, Rhodotorula, Rhodosporidium, Cryptococcus, Trichosporon, and Lipomyces were reported as genera of oleaginous yeast [8, 9] and each are also known as single-cell oil (SCO). Meng [6] reported that some species of Rhodosporidium, Rhodotorula, and Lipomyces are able to accrue lipids to more than 70% of their dry weight. The accumulation of fat in yeast is affected by several parameters that may be grouped as physical (e.g., pH, temperature, light) and chemical (e.g., carbon and nitrogen sources) [10, 11]. However, the TAGs synthesized by oleaginous yeasts consist primarily of C16 and C18, such as C16:0, C16:1, C18:0 C18:1 and 18:2 [12, 13], with varying amounts of shorter (C14) and longer (C26) fatty acid chains, which have key roles in protein modification [14]. The fatty acid (FA) composition of the microbial lipids was found to be similar to vegetable oil, which is commonly used in biodiesel production. Thus, microbial lipids can be used as a potential raw material for biodiesel production [15]. To enhance lipid and FA content in yeast, several techniques were developed including the classical random metagenesis. For such a purpose, ethyl methanesulfonate (EMS) is amongst the most common alkylating agents used in yeast as mutation-inducing agents. Thus, EMS has been used to induce the over-production of metabolites in microalgae, including eicosapentaenoic acid (EPA) [16], astaxanthin, carotenoids [17,18,19,20] and hydrogen [21]. In contrast, EMS-random mutagenesis has also been used to develop mutant strains of microalgae devoid of EPA [22].
The aim of this study was to determine the effect of chemical mutagenesis with EMS on the cell viability, and the lipid and biomass productivity of five oleaginous yeasts, Rhodotorula mucilaginosa RmTun15, Y. lipolytica YlTun15, Trichosporon asahii TaTun15, Debaryomyces hansenii DhTun2015 and Candida tenuis CtTun15. Based on a thorough literature survey and using the fatty acid profile of each of the wild and mutated strains, along with the physical proprieties of Biodiesel, allowed us to predict which of the strain that can produce the best quality of Biodiesel.
Results and discussion
Determination of the optimal concentration of EMS
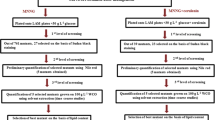
A preliminary study was carried out to determine the optimum of growth for each wild yeast strain as well as the number of colonies formed. During a cultivation period of 144 h in YPD, all species showed an optimum growth after 120 h, but with different values (Table 1). To determinate the effect of mutagenesis, the wild species were subjected to various levels of EMS (25, 75 and 100 mM) in different volumes (15, 50 and 100 µl) during a fixed period (20 min). Using ANOVA test, the experimental data were used to identify the specificity of EMS for the different marine organisms and their effects on their growth and viability (Additional file 1: Table S1). As single factors, the concentration and the volume of the mutant agent have significant effects on both colonies numbers counted and growth (p < 10−4) after 48 and 120 h, respectively, with the number of colonies inversely correlated with the concentration of EMS. The combined effects of both factors were equally proved in all mutated strains as the F-value was significantly higher than p-value [23].
The relationship between independent and dependent variables has been illustrated by the three-dimensional (3D) representation (Table 2). To each plot and for each strain, individual contour curve corresponded to an infinity responses of the tested variables combination including their impact on cell viability (number of colony) and growth (OD at 600 nm). Hence, for all strains, the number of colonies decreased with increased EMS concentration and volume. However, the cell growth increased with both rising variables up to 75 mM EMS level than showed significant decrease. As shown in Tables 2 and 3, the lethal doses and volumes for CtTun15 species corresponded, respectively, to 75 mM/100 μl in experiment 1; 100 mM/50 µl in experiment 2; and 100 mM/100 μl in experiment 5. Such lethal doses/volumes were depicted in experiment 5 for the remaining strains but additionally for DhTun15 in experiment 1. In experiment 9, where EMS volume was 50 μl and concentration was 75 mM, all surviving mutated yeast strains presented the best growth. The combination defined in experiment 9 was considered as optimal as it reflected the best growth in all strains, although the high mortality rate was recorded in such conditions indicating the mutagenic efficiency of the procedure as suggested in other study [24].
Effect of EMS on cell growth and biomass productivity
During the last decades, mutagenesis on marine yeast was performed using various agents such as irradiation [25], heavy-ion [26] or UV/EMS/NTG on a random mutation [20]. Studies on marine yeast related to the effect of EMS are scarce and the present work is the first study using such agent following various times of exposure. Using the optimal condition of EMS concentration (75 mM) and volume (50 µl), the effect of EMS on cell growth of the different yeast strains following various time of exposure is shown in Table 4 where codes were added for each strain at each time of EMS exposure. Statistical analysis (p < 0.05) showed that each strain reacted differently, but with similar trend of change. Thus, each strain has a specific response that can be summarized with cell death rate that ranged from 13.17 to 88.78%; 12.54 to 88.42% and 21.86 to 92.30%, respectively, for YlTun15, RmTun15 and TaTun15 between 15 and 60 min. For D. hansenii DhTun2015, the mortality rate varied between 22.07% at 15 min and 96.84% at 45 min, reaching 100% at 60 min. Regarding C. tenuis CtTun15, the total mortality rate was reached at 45 min and increased from 54.46% at 15 min to 93.89% at 30 min. It is worth noting that the effect of EMS is also related to its concentration in the medium [27]. Mobini-Dehkordi et al. [28] found that EMS mutagenesis affects cell viability of the yeast Saccharomyces cerevisiae reaching 43% after 45 min of exposure to EMS. Tapia et al. [25] reported that after 40-min irradiation, the colony number of the oleaginous yeast Lipomyces starkeyi was reduced to approximately to 5% of the total colonies present in control plates. Ma et al. [26] found that heavy-ion mutagenesis had the same effect on the mortality rate of Nannochloropsis that was enhanced from 15 to 89% with increasing irradiation doses. Doan et al. [29] and Anandarajah et al. [30] in EMS mutagenesis for Nannochloropsis had also reported similar phenomenon. Chlorella cells exposed to 0.28-M EMS showed a survival rate of 50% [31] and Zhang et al. [24] observed that 0.6–0.8 M of EMS was the optimal mutagenic range for Desmodesmus sp. S81 and G41 with survival rate of 30.66 ± 0.57% and 39.28 ± 1.13%, respectively, for 30 min of treatment. Nojima et al. [32] found for the strain Chlorococcum sp. FFG039 that the treatment with 0.25–0.5-M EMS or MNNG results in a survival rate between 0 and 10%. The effect of EMS on survival rate was also detected in the red algae P. yezoensis, the percentage of living cells decreased upon exposure to 1 and 2 mM of EMS to 52 and 10% at day 5, respectively, and was null for higher concentrations of EMS (3 and 4 mM) [33].
Figure 1 summarizes the effect of the mutant EMS on the growth and biomass productivity of all studied strains. For all wild species, the optimal yeast productions were obtained after 120 h of YPD culture with a biomass productivity of 0.15; 0.16 and 0.27 mg/ml/h for CtTun15, DhTun2015 and TaTun15. For YlTun15 and RmTun15, the biomass productivity was 0.37 mg/ml/h for both wild strains.
The effect of EMS treatment on the growth (OD) and biomass productivity (BP) of wild and mutated strain of a Rhodotorula mucilaginosa (RmTun15-Accession Number MF327252); b Yarrowia lipolytica (YlTun15-Accession Number MF327143); c Trichosporon asahii (TaTun15-Accession Number KY509046); d Debaryomyces hansenii (DhTun2015-Accession Number KY5083343) and e Candida tenuis (CtTun15-Accession Number KY558632). (n = 6 in each case, Vertical bar = ± SE)
Thus, the optimum growth for all mutated strains was after 96 h of culture, with significantly higher rates than the wild strains. The kinetics monitoring of the biomass productivity suggests that for all mutated strains, the maximum growth coincided with the optimum of the biomass recovered at 96 h. Figure 1a, b showed that BP augmented significantly to reach 0.68 and 0.61 mg/ml/h for MR-2 and MY-2, respectively. For MT-2, MD-2 and MC-2, the biomass productivities were estimated to be 0.29; 0.19 and 0.21 mg/ml/h and which were significantly higher than the wild strain (Fig. 1c–e). The noticeably increased growth rate of mutants may be explained by the fact that some mutants have an enhanced metabolic pathway to improve cell growth. Analogous improvements of growth using random mutagenesis were mentioned to be in various other mutant microorganisms, including Rhodotorula sp. [20]; Ulva fasciata [34], Scenedesmus dimorphus [35]; Chlamydomonas reinhardtii [36], Chlorococcum sp. FFG039 [32] and the red alga PyE2 [33]. According to Tapia et al. [25], the reduction of cell growth is correlated with the inhibition of lipid biosynthesis metabolism that is stimulated by a different mutant agent such as UV, EMS, and MNNG.
Effect of EMS on lipid content and productivity
The effect of the treatment with EMS was also detected on the yield of lipid and lipid productivity. The lipid yield (L), lipid content (wt%) and final lipid productivity (LP) are shown in Table 5. Thus, the increase in biomass induced an increase in the lipid yield with a maximum of 3.21 ± 0.09 g/100 g of wet biomass of the strain MC-1. The final lipid productivity increased significantly from 0.022 in wild CtTun15 to 0.027 mg/ml/h in MC-1 and then decreased to 0.024 mg/ml/h in MC-2. Wild strain of D. hansenii DhTun2015, MD-1 and MD-2 had a mean yield of 2.19 g/100 g of wet biomass (mean LP 0.019 mg/ml/h) and then decreased significantly to 1.36 ± 0.19 g/100 g wet biomass with a lipid productivity of 0.112 mg/ml/h in MD-3. The wild strain R. mucilaginosa RmTun15 and Y. lipolytica YlTun15, known as oleaginous yeasts [37] produced 8.18 ± 0.02 and 10.32 ± 0.21 g/100 g lipid (wet biomass). Among the four mutant strains of RmTun15, MR-2 showed the highest lipid productivity with 0.130 mg/ml/h. This mutant strain showed 11.82 ± 0.33 g/100 g of wet biomass, which was significantly higher than the lipid yield of the wild strain, MR-1, MR-3 and MR-4. The mutant strains showed a higher lipid content than the wild strain (36%) with a maximum of 46%, 38% and 39% for MR-2, MR-3 and MR-4, respectively. An increased lipid yield (13.22 ± 0.11 g/100 g of wet biomass) and a high lipid productivity (0.130 mg/ml/h) were found in MY-2 strain. Concerning TaTun15, a significant rise of 0.82% was noticed in MT-1 when compared to the wild strain. For MT-2, MT-3 and MT-4, however, the mean lipid yield was 5.77 g/100 g (wet biomass) and a mean lipid productivity of 0.058 mg/ml/h. According to Dai et al. [4], R. glutinis accumulated lipids up to 49.25% on a cellular biomass basis with biomass yield of 29.77 g/l. Tapia et al. [25] found that for L. starkeyi mutated, oleaginous yeast, the lipid content productivity varied from 0.023 ± 0.001 g/l/h to 0.032 ± 0.002 g/l/h, lipid content ranged from 29.88 ± 1.71 to 39.60 ± 1.30%. Doan and Obbard [29] found that for Nannochloropsis sp. the mutagenesis with EMS increased the total lipid content of mutant strain from 16.2 ± 2.6% in day 10 to 50.8 ± 6.8% in day 18 representing 1.5 to 2 times higher than that of wild strain (7.6 ± 1.8% in day 10 to 34.0 ± 3.8% in day 18). Kitahara et al. [38] reported that all mutant strains of Rhodosporidium toruloides showed higher lipid productivity than the wild type (WT). In this study, the biomass and lipid yield of wild RmTun15 were higher than that for R. mucilaginosa [39]. The mutated strain XR-2, obtained by a combined mutagenesis of ARTP and NTG of R. toruloides, had an increased lipid and carotenoids productions [40]. The chemical mutant MNNG increased the lipid and the biomass production of Y. lipolytica NCIM 3589 as mentioned by Katre et al. [41]. Also, lipid production and the lipid productivity of oleaginous yeast R. toruloides were augmented by UV light mutagenesis [42]. In addition, random mutagenesis with UV and selection using cerulenin were effective to improve the lipid productivity of the mutated strain of Cryptococcus curvatus NBRC-0732 and Rhodosporodium toruloides NBRC 10033 [43]. It was also reported that for Trichosporon sp. cultivated in glucose as carbon source, the lipid yield was 21.45 g/l which was much higher than the lipid yield of both wild and mutated TaTun15 as well as the other studied strains either T. cutaneum CTM 30125 (2.8 g/l) [44, 45]. Sarayloo et al. [46] increased the lipid productivity of a Chlorella vulgaris (UV715-EMS25) mutant strain to 91 mg/l/days; the lipid content and biomass were, respectively, 67% and 35% higher than those of the wild type (WT). It is worth noting that several studies were made including engineering metabolic and phenotypes of strain to increase the biomass and the lipid production [47,48,49,50,51,52,53].
In this study, the classical mutagenesis technique using ethylmethane sulfonate was applicable to increase lipid content and production of different strain of marine yeast (RmTun15; CtTun15; DhTun2015, TaTun15 and YlTun15) and select mutated strain with a high single-cell oil (SCO) content suitable for Biodiesel production.
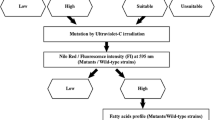
Effect of EMS on FAMES profile
According to fatty acids methyl-esters (FAMEs) composition, microorganisms can be used in various applications (e.g., nutraceutical) or for Biodiesel production [54, 55]. To evaluate the effect of the chemical mutagenesis using EMS on the quality and the composition of oil, we determine the profile of fatty acid from lipid produced by the wild and mutant strains (Fig. 2). The fatty acid composition of CtTun15-wild strain showed a high content of saturated fatty acid (32.78%), of which C15 accounted for 27% of the total identified FA. Moreover, this strain is rich in PUFA with essentially C16:2 (15.5%) and C18:2 (15.26%). Changes in the FA profile and concentration occurred following mutagenesis. Thus, for MC-1 and MC-2, the C15:0 fraction decreased significantly with an average of 10.87%; while C18:0 increased from 3.74 to 5.54% and 9.45% for MC-1 and MC-2, respectively. The C18:2 represented 22.66% at MC-1 and about 30% at MC-2. The level of MUFA doubled in MC-1 (9.36%) and then decreased to 6% for MC-2, while remaining higher than the control strain (4%). The increase in the PUFA level is dependent on the occurrence and detection of new PUFAs such as C18: 2, C20: 1; C20: 4, C20: 5, C22: 5, and C22: 6. As for RmTun15, the control strain profile is distributed mainly in SFA (38%), mostly C16 (27%) and C18 (6%), and PUFAs (45%), where C16: 2 and C18:2 represent 29 and 8%, respectively, of the total fatty acids. After mutagenesis, the lipid profile changed and there was a significant increase in MUFA levels with an average of 43.53% represented by C18: 1 (30.55%) in MR-2 and MR-3. In the MR-4 strain, the FA composition changed as the wild and MR-1 strains remained statistically different and the percentage of SFA and PUFA increased to 43 and 45%, respectively, whilst MUFA decreased to 2%. The lipid profile analysis of the wild strain DhTun2015 showed that the SFA fraction represented 66% of which C15:0 fatty acid was predominant (47%) and MUFA and PUFA represented 15 and 18%, respectively. After mutagenesis, the saturated fatty acid composition of MD-1 is similar to the wild type (with 47% C15:0). This content decreased significantly in MD-2 and MD-3. The PUFA fraction increased significantly from 18% in wild strain to 20; 23 and 32% in MD-1, MD-2 and MD-3, respectively.
For TaTun15, there was a significant decrease in SFA content in MT-2 (22%) and MT-3 (18%) compared to the wild species with an average of 20%. This decrease is mainly dependent on the fall of C15:0 followed by C16:0 and C18:0. For MT-2 and MT-3, the average of the MUFA and PUFA fractions were 34 and 18% respectively with C22: 5, representing 10% of the total identified PUFA fraction. Concluding with YlTun15, a relatively balanced profile of FA was found in MY-2 and MY-3 with 21% AGS; 40% MUFA and 33% PUFA. Regarding MY-1 and MY-4, a profile similar to that of wild strain was found with the fraction SFA > PUFA > MUFA. The spectacular variation in the fatty acid profile of the different mutant strains can be related to the modification and the regulation of the gene involved in the biosynthesis of fatty acid [46, 56].
Comparing these results with those of other studies, it was found that even the mutated strains possess a profile rich in fatty acid with 18 and 16 carbon atoms as described for R. mucilaginosa TJY15a [57]; C. curvatus [58], R. toruloides Y4 [5]; S. cerevisiae [28], R. toruloides cultivated in glucose and glycerol [59]; the mutated and wild Nannochloropsis sp. [30]; Chlorella minutissima [60]; Mortierella isabellina [61], Y. lipolytica in POME [62], R. mucilaginosa [63] and L. starkeyi [64]. Khot and Ghosh [65] found a similar result for the strain R. mucilaginosa IIPL32. Brar et al. report that the fatty acid profile of wild Trichosporon sp. was rich in C16 (25.52%), C18 (12.95%), C18:1 (50.05%) and C18:2 (7.92%) or, for the wild T. cutaneum, Guerfali et al. found that the fatty acid profile was mainly C16, C18 and C18:1 with 32%, 9.2% and 51%, respectively [44, 45].
Ethyl methanesulfonate is a suitable mutagen for related purposes [66, 67]. In addition, French et al. [68] reported that EMS is a powerful chemical mutagen and its effect on cells is allied to its concentration in a medium. This study may conclude that chemical mutagenesis with ethyl methane sulfonate affects not only biomass productivity and lipid productivity but also the lipid profile, which guides the application of the strains according to desired, ends (Biofuel, PUFA production, etc.). For a better understanding of lipid metabolism in both mutated and wild strain of RmTun15; DhTun2015; CtTun15; TaTun15 and YlTun15, RNA sequencing and comparison of differential gene expression will be investigated in the future.
Biodiesel quality
According to international standards, biodiesel must follow some criteria such as ASTM 6751-3 (USA), EN 14214 (Europe) and Bureau of Indian Standard (IS 15607-05) for biodiesel [27]. The fatty acid profile determines the quality of biodiesel and it has been recommended that higher proportions of methyl esters of both C16 and C18 SFAs and MUFAs with reasonable amounts of PUFAs are required for a good quality of Biodiesel [1, 65, 69]. Table 6 shows the characteristics of the biodiesel from the wild RmTun15, YlTun15 and TaTun15 and their mutant strain MR-2, MR-3, MY-2, MY-3, MT-2, MT-3. The choice of such strains was based on their optimum lipid yield and fatty acid profile. Biodiesel proprieties can be classified into different categories with the most important being those related to engines, weather conditions, transport and depositing [70]. The cetane number (CN) was considered as a primary indicator of fuel quality that gives idea on the speed and the compression needed for the ignition. In this study, CN varied significantly from wild to mutant stain. Thus, CN increased from 45.26 ± 2.62 in RmTun15 to mean value of 55 min in MR-2 and MR-3 values that are near to the recommended value of standard fuel. The mean value of CN in MY-2 and MY-3 was 32.65 min which statistically is < wild strain CN (59.53 ± 0.39 min). However, for TaTun15, this parameter increased meaningfully from 50.96 ± 4.36 to 67.15 ± 1.29 in MT-2 and 60.08 ± 0.94 in MT-3. To evaluate the stability to oxidation of biodiesel, the iodine value (IV) was estimated. For RmTun15, MR-2 and MR-3, the mean value of this index was 107.43 ± 2.86 gI2/100 g. The iodine value (IV) increased expressively from 48.53 ± 1.54 in the wild strain to 163.22 ± 5.35 gI2/100 g in My-2 and MY-3. The wild T. asahii TaTun15 and the mutated strain MT-3 have a mean value of 89.66 ± 1.46 gI2/100 g that is statistically lower than MT-2 (60.04 ± 0.45 gI2/100 g). Regarding the saponification value (SV) or the acid value, the statistical analysis showed that all wild strains (TaTun15, RmTun15 and YlTun15) have a mean value of 213.81 ± 3.47 mg KOH which decreased significantly in mutated MY-2 and 3 (195.19 ± 1.02 mg KOH); MR-2 and 3 (153.23 ± 3.74 mg KOH) and MT-2and 3 (146.78 ± 2.29 mg KOH). This variation can be explained by the modification of the fatty acid composition with an increase of the medium and long-chain fatty acid. The oxidation stability was evaluated for the mutated strain with a mean value of 19.74 ± 1.10 h for MR-2 and MR-3; 20.94 ± 2.26 h for MT-2 and MT-3 and 18.26 ± 1.03 h for MY-2 and MY-3. All mutated strains have a density of 0.85 g/cm3 and a kinematic value varying from 3.69 to 4.35 ± 0.09 mm2/s that was confirmed to the value fixed in the international standard parameter of Biodiesel. The determination of the physical parameter of biodiesel (CV, IV, SN, HHV, viscosity, oxidation stability) of some genus of oleaginous yeast such as Rhodotorula [71,72,73], Rhodosporidium [14, 59, 74, 75], Cryptococcus [75], Debaryomyces [75], Yarrowia [75, 76], Trichosporon [44, 45] and Papiliaterma [77] predicted the good quality of biodiesel generated by those species. Comparing the results of biodiesel predicted to the different strains of this study, we found a similarity with the genus mentioned in some indices such as the cetane number, the iodine value, the kinematic value and cold filter plugging point which was close to the standard parameter of biodiesel (ASTM D6751, EN14214, Indian Biodiesel IS15607 and the commercial biodiesel). According to Knothe [78], the quality of biodiesel depends on the fatty acid composition of the biolipids. Conferring to Khot and Ghosh [65], high monounsaturated fat content is a good quality of biodegradable product. Dai et al. [4] reported that R. glutinis could be a potential strain for biodiesel production according to his lipid profile. Li et al. [57] confirmed that R. muciloginosa TJY15a constitute a good feedback for biodiesel production. Arora et al. [59] and Khot and Ghosh [65] reported, respectively, the same conclusion for C. minutissima cultivated in optimal condition of lipid productivity (NLPL) and for the oleaginous yeast R. mucilaginosa IIPL32.
Conclusion
The modification induced by the mutant EMS changed the biomass and the lipid productivity of the strains TaTun15; YlTun15; DhTun2015 CtTun15 and RmTun15. This has made it possible to provide competent strains that can be oriented towards various uses (production of carotenoids, EPA, etc.). The fatty acid compositions confirm the oleaginous proprieties of the identified yeasts that were similar to other oily microbes. In addition, depending on the fatty acid profile and the physical properties of the biodiesel, it was concluded that the mutated strain incubated with EMS (75 mM) for 30 min of R. mucilaginosa RmTun15 (MR2), T. asahii TaTun15 (MT-2; MT-3) and Y. lipolytica YlTun15 (MY-2), constitute a potential for the production of Biodiesel. Further study will be conducted to isolate and identify the genes responsible for the over-production of lipid and fatty acid.
Methods
Microorganisms
Five strains were used in this study: D. hansenii DhTun2015 (Acc. Num KY5083343.1) and Y. lipolytica YlTun15 (Acc. Num MF327143.1) isolated from Tunisian farmed Dicentrarchus labrax scales and gills, respectively; C. tenuis CtTun15 isolated farmed Sparus aurata coproduct Acc. Num KY558632.1); R. mucilaginosa RmTun15 isolated from shallow water (La Goulette, Tunisia) (Acc. Num MF327252.1) and T. asahii TaTun15 isolated from shrimp’s waste (Acc. Number KY509046.1).
Chemicals
Chemicals used in this study were: Yeast Extract (Biokar Diagnostics, France); Peptone (Biokar Diagnostics, France); Glucose (Sigma, Germany); Chlormaphenicol (Biomatik, USA); Ethyl Methane Sulfonate (Sigma, Germany); Sodium Thiosulfate (Sigma, Germany); Sodium Phosphate (Sigma, Germany); Sodium Chloride (Sigma, Germany); 2,6-Di-tert-butyl-4-methylphenol (BHT) (Sigma, Germany); Methanol (Carlo-Herba, France); Hydrochloric acid 37% (Carlo Herba, France); n-Hexane (VWR chemical, France); Dichloromethane (VWR chemical, France); 2,2-dimethoxypropane (Sigma, Germany); Hydrochloric acid solution (Sigma, Germany); Sodium Methoxide solution (Sigma, Germany) and Nonadecanoic acid (Sigma, Germany).
Culture media
From the plate culture of each strain stored at 4 °C, a loop of cell mass was transferred aseptically to 30-ml YPD media (2% peptone, 2% glucose, 1% yeast extract and 100 ppm chloramphenicol) and then incubated in an orbital shaker (LabWit ZWY-240) at 160 rpm and 30 °C for reactivation. Then, from this preculture, YPD cultures were prepared in 100-ml Erlenmeyer flasks (50-ml working volume) with a starting OD of 0.4 at 600 nm. 100 ppm chloramphenicol was added to the culture medium which was incubated at 26° C with a stirring speed of 160 rpm. Samples were taken at 24-h intervals, in triplicate, to determine cell growth, biomass, and lipid accumulation.
Chemical mutagenesis with ethyl methane sulfonate (EMS)
The yeast strains were treated with EMS following the method elaborated in Hahn Lab [79, 80]. Briefly, the different species were grown in a YPD medium at 30 °C for 12 h. For each culture, 108 washed cells were placed in 15-ml tubes and suspended in 5-ml phosphate buffer (0.1 M, pH:7). Under the hood, EMS was added to each tube, except the non-mutagenized control, and the cells were incubated on a roller at 30° C for varying time points between 0 min and 1 h. At each time point, 8 ml of sterile 5% sodium thiosulfate was added to inactivate the EMS and to stop the mutagenesis. After centrifugation, the cells were resuspended in distilled water, from which plating was applied on YPD agar for 2–3 days until colonies appeared. Finally, the plate was stored at 4° C to await further analysis. Mutated strains are designed as mentioned in Table 3.
Experimental design
Optimizing concentration and volume of EMS for the different marine yeast species was carried out using the surface response method and Statistica 13.3 software. The following values were adopted for determining dependent and independent variables: x-concentration of EMS varying from 25 to 100 mM; y-volume of EMS (ml) from 15 to 100 µl, z1-number of colonies forming (CFU) and z2-growth measured by the optical density at 600 nm OD (600 nm). In this study, the experiment was planned using the 3**(2-0) full factorial design, 3 blocks, 9 runs in the Statistica 13.3 software. Central composite design was used for planning, and 9 experiments were performed for two independent factors as shown in Table 3. According to the presented plan, 9 random experiments were performed for each species with three replicates for each experiment.
Biomass assessment
Biomass was measured by absorbance and dry biomass [60]. For dry biomass preparation, 1-ml aliquots of each culture was taken and transferred to pre-weighted tubes and pelleted. After 12 h at 80 °C, dry biomass weight was determined by calculating the difference between the final and the initial pre-weighted tube. The absorbance readings were realized by taking 1 ml of aliquots during culture at 24-h intervals, properly diluted with water and measured at 600 nm using a spectrophotometer (LLG-uniSPEC 2 UV/VIS)
Determination of lipid content
The lipids content in the yeast was quantified according to a modified Folch’s method [81] with some modifications. The modification was done during the pretreatment of the strain. The fresh biomass, previously incubated overnight at − 80 °C, was mixed with 5 ml of HCl (2 M), incubated for 20 min in ultrasonic ice bath, and then centrifuged at 10,000 rpm, (4 °C) for 10 min to eliminate cellular debris. The supernatant was mixed with 5 ml of NaCl (0.73%) and 20 ml of Folch solution (dichloromethane/methanol/0.01%BHT), and then centrifuged at 8000 rpm for 10 min at 4 °C. The supernatant was collected into a pre-weighed screw-capped glass tubes and evaporated under vacuum with an Evaporator–concentrator (MiVac, GeneVac, England) at room temperature. The lipid yield was determined according to the formula:
where wB is the weight of wet biomass; w1 is the final weight of screw-capped glass tube containing the lipid fraction and w2 is the initial weight of the screw-capped glass tube.
To follow lipid production in the different strains of yeast, some parameters were followed:
Fatty acid composition
The methylation of fatty acids was performed to obtain the methyl ester derivatives for analysis by gas chromatography, as described by Griffiths et al. [82]. The lipid fraction was diluted in toluene. To 500 µl of the reaction mix, in screw-capped glass tubes, 50 µl of nonadecanoic acid (C19:0) was added to the reaction as an internal standard. Subsequently, 0.1 ml of 2,2-dimethoxypropane and 1 ml of sodium methoxide were added and the sample was mixed briefly then placed in a shaking incubator (900 rpm) at 80 °C for 30 min. The mixture was cooled for 10 min at room temperature and 1 ml of HCl/methanol was added before repeating the incubation. After cooling, 1 ml of bidistilled water and 0.5 ml of n-Hexane for GC were added and the tubes were mixed by vortexing. The upper hexane layer containing FAMEs extract was transferred to vials for GC and kept at − 20 °C until analysis. The analysis of the fatty acids was carried out by GC HP model 6890 equipped with an INNOWAX polar capillary column (30 m in length and 0.25 μm in diameter), a Flame Ionization Detector (FID) and an injector splitted. The volume of injection was 1 μl. Fatty acids were identified by comparing the retention times of FAME with SUPELCO™ 19-component FAME mixture (PUFA no. 3, Sigma-Aldrich). Three replicate GC analyses were performed.
Determination of biodiesel quality
Biodiesel properties were determined using the following formulae, as described by [60, 83,84,85,86]:
with M is the molecular mass of each fatty acid component, DB is the number of double bonds, FC is the % of each fatty acid component, MUFA is the weight % of monounsaturated fatty acids and PUFA is the weight % of poly unsaturated fatty acid.
Statistical analysis
Data were subjected to Analyses of Variance at the 5% level using SPSS 24.0 software and the Tukey test was performed to separate differences among means.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
20 June 2019
The original version of the article [1] unfortunately contained a mistake in author’s second name. The name of the author has been corrected from Thomas Breuck to Thomas Brück in this correction article.
Abbreviations
- ARTP:
-
atmospheric and room temperature plasma
- B:
-
biomass
- BP:
-
biomass productivity
- EMS:
-
ethyl methanesulfonate
- EPA:
-
eicosapentaenoic acid
- FA:
-
fatty acid
- FAME:
-
fatty acid methyl ester
- IV:
-
iodine value
- L:
-
lipid yield
- LP:
-
final lipid productivity
- MNNG:
-
N-methyl-N′-nitro-N-nitrosoguanidine
- MUFA:
-
monounsaturated fatty acid
- PUFA:
-
polyunsaturated fatty acid
- SCO:
-
single cell oil
- SFA:
-
saturated fatty acid
- SV:
-
saponification value
- WT:
-
wild type
- YPG:
-
yeast–peptone–glucose
References
Patel A, Arora N, Mehtani J, Pruthi V, Pruthi PA. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew Sustain Energy Rev. 2017;77:604–16.
Su HF, Lin JF, Tan FR. Progress and perspective of biosynthetic platform for higher-order biofuels. Renew Sustain Energy Rev. 2017;88:801–8267.
Ageitos JJ, Vallejo JA, Veiga-Crespo P, Villa TG. Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol. 2011;90:1219–27.
Dai C-C, Tao J, Xie F, Dai Y-J, Zhao M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr J Biotechnol. 2007;18:2130–4.
Li Y, Zhao Z, Bai F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microbial Technol. 2007;41:312–7.
Meng X, Yang JM, Xu X, Zhang L, Nie QJ, Xian M. Biodiesel production from oleaginous microorganisms. Renew Energy. 2009;34:1–5.
Joshi G, Pandey JK, Rana S, Rawat DS. Challenges and opportunities for the application of biofuel. Renew Sustain Energy Rev. 2017;79:850–66.
Thiru M, Sankh S, Rangaswamy V. Process for biodiesel production from Cryptococcus curvatus. Bioresour Technol. 2011;102:10436–40.
Dey P, Maiti MK. Molecular characterization of a novel isolate of Candida tropicalis for enhanced lipid production. Appl Microbiol. 2013;114:1357–68.
Rakicka M, Lazar Z, Dulermo T, Fickers P, Nicaud JM. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol Biofuels. 2015;8:104.
Guinde-Cimermann N, Plemenitaš A, Buzzini P. Changes in lipids composition and fluidity of yeast plasma membrane as response to cold. In: Buzzini P, Margesin R, editors. Cold-adapted yeasts. Berlin: Springer; 2014. p. 225–42.
Tehlivets O, Scheuringer K, Kohlwein SD. Fatty acid synthesis and elongation in yeast. Biochim Biophys Acta Mol Cell Biol Lipids. 2007;1771:255–70.
Beopoulos A, Nicaud JM, Gaillardin C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol. 2011;90:1193–206.
Nasirian N, Mirzaie M, Cicek N, Levin BB. Lipid and carotenoid synthesis by Rhodosporidium diobovatum, grown on glucose versus glycerol, and its biodiesel properties. Can J Microbiol. 2018;64:277–89.
Yu D, Wang X, Fan X, Ren H, Hu S, Wang L, Shi Y, Liu N, Qiao N. Refined soybean oil wastewater treatment and its utilization for lipid production by the oleaginous yeast Trichosporon fermentans. Biotechnol Biofuels. 2018;11:299.
Chaturvedi R, Fujita Y. Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol Res. 2006;53:208–19.
Huesemann MH, Hausmann TS, Bartha R, Aksoy M, Weissman JC, Benemann JR. Biomass productivities in wild type and pigment mutant of Cyclotella sp. (diatom). Appl Biochem Biotechnol. 2009;157:507–26.
Choi GG, Bae MS, Ahn CY, Oh HM. Enhanced biomass and gamma-linolenic acid production of mutant strain Arthrospira platensis. J Microbiol Biotechnol. 2008;2008(18):539–44.
Mendoza H, de la Jara A, Freijanes K, Carmona L, Ramos AA, Duarte VD, Varela JCS. Characterization of Dunaliella salina strains by flow cytometry: a new approach to select carotenoid hyperproducing strains. Electron J Biotechnol. 2008;11:1–13.
Cong L, Chi Z, Li J, Wang X. Enhanced carotenoid production by a mutant of the marine yeast Rhodotorula sp hidai. J Ocean Univ China (oceanic and coastal sea research). 2006;6:66–71.
Flynn T, Ghirardi ML, Seibert M. Accumulation of O-2-tolerant phenotypes in H-2-producing strains of Chlamydomonas reinhardtii by sequential applications of chemical mutagenesis and selection. Int J Hydrog Energy. 2002;27:1421–30.
Schneider JC, Livne A, Sukenik A, Roessler PG. A mutant of Nannochloropsis deficient in eicosapentaenoic acid production. Phytochemistry. 1995;40:807–14.
Gan CY, Latiff AA. Extraction of antioxidant pecticpolysaccharide from mangosteen (Garcinia mangostana) rind: optimization using response surface methodology. Carbohydr Polym. 2011;83:600–7.
Zhang Y, He M, Zou S, Fei C, Yan Y, Zheng S, Rajper AA, Wang C. Breeding of high biomass and lipid producing Desmodesmus sp. by ethylmethane sulfonate-induced mutation. Bioresour Technol. 2016;207:268–75.
Tapia EV, Anschau A, Coradini ALV, Franco TT, Deckmann AC. Optimization of lipid production by the oleaginous yeast Lipomyces starkeyi by random mutagenesis coupled to cerulenin screening. AMB Express. 2012;2:64.
Ma Y, Wang Z, Zhu M, Yu C, Cao Y, Zhang D, Zhou G. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioressour Technol. 2013;136:360–7.
Atadashi IM, Aroua MK, Aziz AA. High quality biodiesel and its diesel engine application: a review. Renew Sustain Energy Rev. 2010;2010(14):1999–2008.
Mobini-Dehkordi M, Nahvi I, Zarkesh-Esfahani H, Ghaedi K, Tavassoli M, Akada R. Isolation of a novel mutant strain of Saccharomyces cerevisiae by an ethyl methane sulfonate-induced mutagenesis approach as a high producer of bioethanol. J Biosci Bioeng. 2008;105:403–8.
Doan TTY, Obbard JP. Enhanced intracellular lipid in Nannochloropsis sp. via random mutagenesis and flow cytometric cell sorting. Algal Res. 2012;1:17–21.
Anandarajah K, Mahendraperumal G, Sommerfeld MHuQ. Characterization of microalga Nannochloropsis sp. mutants for improved production of biofuels. Appl Energy. 2012;96:371–7.
Manandhar-Shrestha K, Hildebrand M. Development of flow cytometric procedures for the efficient isolation of improved lipid accumulation mutants in a Chlorella sp. microalga. J Appl Phycol. 2013;25:1643–51.
Nojima D, Ishizuka Y, Muto M, Ujiro A, Kodama F, Yoshino T, Maeda Y, Matsunaga T, Tanaka T. Enhancement of biomass and lipid productivities of water surface-floating microalgae by chemical mutagenesis. Mar Drugs. 2017;2017(15):151.
Lee HJ, Choi J. Isolation and characterization of a high-growth-rate strain in Pyropia yezoensis induced by ethyl methane sulfonate. J Appl Phycol. 2018. https://doi.org/10.1007/s10811-018-1426-1.
Chen YC. The hormesis of the green macroalga Ulva fasciata with low-dose 60 Cobalt gamma radiation. J Phycol. 2011;47:939–43.
Choi JI, Yoon M, Joe M, Park H, Lee SG, Han SJ, Lee PC. Development of microalga Scenedesmus dimorphus mutant with higher lipid content by radiation breeding. Bioprocess Biosyst Eng. 2014;37:2437–44.
Lee BS, Choi GG, Choi Y-E, Sung MJ, Park MS, Yang JW. Enhancement of lipid productivity by ethyl methane sulfonate mediated random mutagenesis and proteomic analysis in Chlamydomonas reinhardtii. Korean J Chem Eng. 2014;31:1036–42.
Pan LX, Yang DF, Shao L, Li W, Chen GG, Liang ZQ. Isolation of the oleaginous yeasts from the soil and studies of their lipid-producing capacities. Food Technol Biotechnol. 2009;2009(47):215–20.
Kitahara Y, Yin T, Zhao X, Wachi M, Du W, Liu D. Isolation of oleaginous yeast Rhodosporidium toruloides mutants tolerant of sugarcane bagasse hydrolysate. Biosci Biotechnol Biochem. 2014;78:336–42.
Enshaeieh M, Abdoli A, Madani M. Single cell oil (SCO) Production by Rhodotorula mucilaginosa and its environmental benefits. J Agric Sci Technol. 2015;17:387–400.
Zhang C, Shen H, Zhang X, Yu X, Wang H, Xiao S, Wang J, Zhao ZK. Combined mutagenesis of Rhodosporidium toruloides for improved production of carotenoids and lipids. Biotechnol Lett. 2016;38:1733–8.
Katre G, Ajmera N, Zinjarde S, RaviKumar A. Mutants of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil as a biofactory for biodiesel production. Microb Cell Fact. 2017;16:176.
Yamada R, Kashihara T, Ogino H. Improvement of lipid production by the oleaginous yeast Rhodosporidium toruloides through UV mutagenesis. World J Microbiol Biotechnol. 2017;33:99.
Yamada R, Yamauchi A, Kashihara T, Ogino H. Evaluation of lipid production from xylose and glucose/xylose mixed sugar in various oleaginous yeasts and improvement of lipid production by UV mutagenesis. Biochem Eng J. 2017;128:76–82.
Brar KK, Sarma AK, Aslam M, Polikarpov I, Chadha BS. Potential of oleaginous yeast Trichosporon sp. for conversion of sugarcane bagasse hydrolysate into biodiesel. Bioresour Technol. 2017. https://doi.org/10.1016/j.biortech2017.03.155.
Guerfali M, Ayadi I, Belhassen A, Gargouri A, Belghith H. Single cell oil production by Trichosporon cutaneum and lignocellulosic residues bioconversion for biodiesel synthesis. Process Saf Environ Protect. 2018. https://doi.org/10.1016/j.psep.2017.11.002.
Sarayloo E, Simsek S, Unlu SY, Cevahir G, Erkey C, Kavakli IH. Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Biores Technol. 2018;2018(250):764–9.
Tai M, Stephanopoulos G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab Eng. 2013;15:1–9.
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, et al. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun. 2014;5:3131.
Hull CM, Loveridge EJ, Rolley NJ, Donnison IS, Kelly SL, Kelly DE. Co-production of ethanol and squalene using a Saccharomyces cerevisiae ERG1 (squalene epoxidase) mutant and agro-industrial feedstock. Biotechnol Biofuels. 2014;7:133.
Liu J, Chu Y, Cao X, Zhao Y, Xie H, Xue S. Rapid transesterification of microamount of lipids from microalgae via a micro- mixer reactor. Biotechnol Biofuels. 2015;8:229.
Qiao K, Imam Abidi SH, Liu H, Zhang H, Chakraborty S, Watson N, et al. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica. Metab Eng. 2015;29:56–65.
Abghari A, Mazak C, Chen S. Combinatorial engineering of Yarrowia lipolytica as a promising cell biorefinery platform for the de novo production of multi-purpose long chain dicarboxylic acids. Fermentation. 2017;3:40.
Niehus X, Crutz-Le Coq AM, Sandoval G, Nicaud JM, Ledesma-Amaro R. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials. Biotechnol Biofuels. 2018;11:11.
Sharma KK, Schuhmann H, Schenk PM. High lipid induction in microalgae for biodiesel production. Energies. 2012;5:1532–53.
Lohman EJ, Gardner RD, Pedersen T, Peyton BM, Cooksey KE, Gerlach R. Optimized inorganic carbon regime for enhanced growth and lipid accumulation in Chlorella vulgaris. Biotechnol Biofuels. 2015;8:82.
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv. 2014;32:1476–93.
Li M, Liu G-L, Chi Z, Chi Z-M. Single cell oil production from hydrolysate of cassava starch by marine-derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenerg. 2010;34:101–7.
Hassan M, Blanc PJ, Granger LM, Pareilleux A, Goma G. Influence of nitrogen and iron limitations on lipid production by Cryptococcus curvatus grown in batch and fed-batch culture. Process Biochem. 1996;31:355–61.
Xu J, Zhao X, Wang W, Du W, Liu D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem Eng J. 2012;2012(65):30–6.
Arora N, Patel A, Pruthi PA, Pruthi V. Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour Technol. 2016. https://doi.org/10.1016/j.biortech.2016.02.112.
Harde S, Wang Z, Horne M, Zhu J, Pan X. Microbial lipid production from SPORL-pretreated Douglas fir by Mortierella isabellina. Fuel. 2016;175:64–74.
Louhasakul Y, Cheirsilp B, Prasertsan P. Valorization of palm oil mill effluent into lipid and cell-bound lipase by marine yeast Yarrowia lipolytica and their application in biodiesel production. Waste Biomass Valoriz. 2016;7:417–26.
Yen H-W, Liao Y-T, Liu Y-X. Cultivation of oleaginous Rhodotorula mucilaginosa in airlift bioreactor by using seawater. J Biosci Bioeng. 2016;121:209–12.
Islam MA, Yousuf A, Karim A, Pirozzi D, Khan MR, Wahid Z. Bioremediation of palm oil mill effluent and lipid production by Lipomyces starkeyi: a combined approach. J Cleaner Prod. 2017;172:1–9.
Khot M, Ghosh D. Lipids of Rhodotorula mucilaginosa IIPL32 with biodiesel potential: oil yield, fatty acid profile, fuel properties. J Basic Microbiol. 2017;57:345–52.
Wahlbom CF, Zyl WH, Jonsson LJ, Han-Hagerdal B, Otero RR. Generation of the improved recombinant xylose-utilizing Saccharomyces cerevisiae TMB 3400 by random mutagenesis and physiological comparison with Pichia stipitis CBS 6054. FEMS Yeast Res. 2003;3:319–26.
Khattab AA, Bazaraa WA. Screening, mutagenesis and protoplast fusion of Aspergillus niger for the enhancement of extracellular glucose oxidase production. J Ind Microbiol Biotechnol. 2005;32:289–94.
French CT, Ross CD, Keysar SB, Joshi DD, Lim CU, Fox MH. Comparison of the mutagenic potential of 17 physical and chemical agents analyzed by the flow cytometry mutation assay. Mutat Res. 2006;602:14–25.
Verma P, Sharma MP, Dwivedi G. Impact of alcohol on biodiesel production and properties. Renew Sustain Energy Rev. 2016;56:319–33.
Barabás I, Todorut I-A. Biodiesel quality: standards and properties. Rijeka: InTech; 2011. p. 392.
Easterling ER, French WT, Hernandez R, Licha M. The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis. Bioresour Technol. 2009;100:356–61.
Galafassi S, Cucchetti D, Pizza F, Franzosi G, Bianchi D, Compagno C. Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour Technol. 2012;111:398–403.
Jiru TM, Steyn L, Pohl C, Abate D. Production of single cell oil from cane molasses by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 as a biodiesel feedstock. Chem Cent J. 2018;12:91.
Tanimura A, Takashima M, Sugita T, Endoh R, Kikukawa M, Yamaguchi S, Sakuradani E, Ogawa J, Shima J. Selection of oleaginous yeasts with high lipid productivity for practical biodiesel production. Bioresour Technol. 2014;153:230–5.
Munch G, Sestric R, Sparling R, Levin DB, Cicek N. Lipid production in the under-characterized oleaginous yeasts, Rhodosporidium babjevae and Rhodosporidium diobovatum, from biodiesel-derived waste glycerol. Bioresour Technol. 2015;185:49–55.
Katre G, Raskar S, Zinjarde S, Kumar R, Kulkarni BD, Kumar AR. Optimization of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy. 2018;142:944–52.
Wang G, Liu L, Liang W. Single cell oil production from hydrolysates of inulin by a newly isolated yeast Papiliotrema laurentii AM113 for biodiesel making. Appl Biochem Biotechnol. 2018;184:168–81.
Knothe G. A technical evaluation of biodiesel from vegetable oils vs. algae: will algae-derived biodiesel perform? Green Chem. 2011;13:3048–65.
Warfield L. EMS mutagenesis of yeast. Hahn. 1998. http://www.koko.gov.my/CocoaBioTech/Mutagenesis4.html. Accessed 20 Jan 2016.
Winston F. EMS and UV mutagenesis in yeast. Curr Protoc Mol Biol. 2008. https://doi.org/10.1002/0471142727.mb1303bs82.
Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509.
Griffiths MJ, Van Hille RP, Harrison STL. Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids. 2010;45:1053–60.
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol. 2009;100:261–8.
Franscisco EC, Neves DB, Lopes EJ, Franco TT. Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biodiesel quality. J Chem Technol Biotechnol. 2010;85:395–403.
Islam MA, Magnusson M, Brown RJ, Ayoko GA, Nabi MDN, Heimann K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies. 2013;6:5676–702.
Wu H, Miao X. Biodiesel quality and biochemical changes of microalgae Chlorella pyrenoidosa and Scenedesmus obliquus in response to nitrate levels. Bioresour Technol. 2014;170:421–7.
Arous F, Frikha F, Triantaphyllidou IE, Aggelis G, Nasri M, Mechichi T. Potential utilization of agro-industrial wastewaters for lipid production by the oleaginous yeast Debaryomyces etchellsii. J Cleaner Prod. 2016;133:899–909.
Acknowledgements
The authors would like also to thank Prof. Roger Uglow from the University of Hull (UK) for his contribution to revising the manuscript.
Funding
This work was conducted within the project “Biotechnologie Marine Vecteur d’Innovation et de Qualité BIOVecQ PS1.3_08” co-financed by the cross-border IEVP Italy-Tunisia program and the Ministry of Higher Education and Scientific Research-Tunisia. These funding bodies had no role in the design of this study or any role during the analysis, the interpretation of the data and in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
BB, and SS conceived and designed the experiments; BB conducted the experimental analysis and wrote the manuscript; SS supervised, validate the data analyses and co-wrote the manuscript, AS discussed the protocol and provided the chemicals used in mutagenesis/methylation analysis, and TB previously trained BB and discussed the protocol. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article is revised. The name of third author in author group has been corrected to Thomas Brück and his affiliation and ORCID have been updated.
Additional file
Additional file 1: Table S1.
Analysis of variance (ANOVA)of the experiment (p = 0.05). (SS: sum of squares, dF: degrees of freedom, MS: mean square, F: statistics, p: Probability). Summarizes the results of fitting the models of the experimental design to the data by the F test and p value.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bessadok, B., Santulli, A., Brück, T. et al. Species disparity response to mutagenesis of marine yeasts for the potential production of biodiesel. Biotechnol Biofuels 12, 129 (2019). https://doi.org/10.1186/s13068-019-1459-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-019-1459-y