Abstract
Background
Sugarcane (Saccharum spp.) covers vast areas of land (around 25 million ha worldwide), and its processing is already linked into infrastructure for producing bioethanol in many countries. This makes it an ideal candidate for improving composition of its residues (mostly cell walls), making them more suitable for cellulosic ethanol production. In this paper, we report an approach to improving saccharification of sugarcane straw by RNAi silencing of the recently discovered BAHD01 gene responsible for feruloylation of grass cell walls.
Results
We identified six BAHD genes in the sugarcane genome (SacBAHDs) and generated five lines with substantially decreased SacBAHD01 expression. To find optimal conditions for determining saccharification of sugarcane straw, we tried multiple combinations of solvent and temperature pretreatment conditions, devising a predictive model for finding their effects on glucose release. Under optimal conditions, demonstrated by Organosolv pretreatment using 30% ethanol for 240 min, transgenic lines showed increases in saccharification efficiency of up to 24%. The three lines with improved saccharification efficiency had lower cell-wall ferulate content but unchanged monosaccharide and lignin compositions.
Conclusions
The silencing of SacBAHD01 gene and subsequent decrease of cell-wall ferulate contents indicate a promising novel biotechnological approach for improving the suitability of sugarcane residues for cellulosic ethanol production. In addition, the Organosolv pretreatment of the genetically modified biomass and the optimal conditions for the enzymatic hydrolysis presented here might be incorporated in the sugarcane industry for bioethanol production.
Similar content being viewed by others
Background
Lignocellulosic biomass is among the most abundant biological resources on Earth. This biomass is produced as a primary product for animal feed or as residues from food crops and other agroindustrial wastes. Digestibility of the lignocellulosic biomass, which is the ease with which sugars can be released from the cell-wall polysaccharides, is a key economic target. The improvement of biomass digestibility may lead to the increased production of biofuels and other value-added products and to a better efficiency of digestion by ruminant animals [1].
Sugarcane (Saccharum spp.) is a semiperennial grass crop mainly grown for sugar and ethanol production. The processing of sugarcane generates two major biomass residues: the fibrous fraction following juice extraction from the stalks (bagasse); and the harvest residue, consisting of green tops and older leaves, known as straw [2, 3]. Both biomasses can be used as feedstock to generate heat and electricity and to produce cellulosic ethanol. In addition, the sugarcane straw is commonly used to improve soil quality [3]. The sugarcane processing can generate between 7.4 and 20 Mg ha−1 of straw in dry matter, but may reach up to 30 Mg ha−1 year−1 when high-yield sugarcane plants are used [4,5,6]. These large volumes of straw can be left on the field, where it will naturally decompose to act as a soil fertilizer, or it can be harvested to generate bioenergy. The utilization of the straw should occupy a prominent place as a feedstock for the production of cellulosic ethanol. Some studies suggest that one ton of straw corresponds to 1.2–2.8 barrel of oil equivalent [7, 8]. Increasing the efficiency and yield of this sugarcane straw conversion to bioethanol (as well as bagasse) is a major target to increase economic viability of bioethanol making it more competitive compared to fossil fuels.
The major barrier that hinders the use of plant biomass for biofuel production is the recalcitrance of the plant cell wall. In general, plant cell walls are composed of cellulosic microfibrils embedded in a matrix of hemicelluloses, pectins, lignin, and proteins, but the specific composition of cell walls depends on the plant species. In grass cell walls, the major hemicellulosic polysaccharide is xylan, which is commonly substituted with arabinofuranose (Araf) and ester-linked hydroxycinnamates. In addition, the β-(1-4)-xylose backbone can be substituted with α-(1,2)-glucuronic acid to form glucuronoarabinoxylans (GAX), which contains both arabinosyl and glucuronosyl residues on the β-1,4 xylan backbone [9]. Another distinctive feature of grass cell walls is the prevalence of two hydroxycinnamates: p-coumarate (pCA) and ferulate (FA) [10]. FA is involved in grass cell-wall crosslinking reactions through the acylation of arabinofuranosyl units that are (1-3)-linked to the xylan backbone in arabinoxylan (AX) or GAX. Ester-linked FA is coupled oxidatively in a similar manner to that of lignin monomers [11, 12], forming crosslinks with other (G)AX chains or with lignin [13,14,15,16]. This complex network of crosslinks in grass cell walls can inhibit the digestion by preventing enzymatic access to the biomass, making the cell-wall deconstruction and the release of fermentable sugars for ethanol production difficult. Therefore, decreasing FA content and consequently FA-mediated crosslinks of grass biomass has long been considered a promising strategy for increasing digestibility [1, 17].
Although the precise mechanisms by which FA is incorporated into AX are not fully elucidated, the genes responsible for this phenomenon in grasses are starting to emerge. In this sense, genes belonging to the BAHD acyl-coenzyme A (CoA) transferase superfamily are being designated as possible candidates for feruloylation of AX in grass cell walls [18]. Some studies suggested the role of BAHD genes in AX feruloylation [19,20,21], but the strongest evidence of the involvement of a BAHD gene in FA incorporation was demonstrated recently by [1]. Using Setaria viridis, an emerging plant model for grasses [22], those authors showed that suppression of SvBAHD01, a member of the BAHD acyl-CoA transferase gene family in S. viridis, was able to reduce up to 60% the levels of AX feruloylation, drastically increasing the saccharification of the biomass [1]. Therefore, silencing of SvBAHD01 orthologs may be a suitable strategy for the improvement of biomass digestibility in other species.
In industrial processes, it is essential to provide accessibility of the cellulose in the biomass to hydrolytic enzymes, to release fermentable sugars for ethanol production. This is mainly achieved by (i) using biomass feedstocks that possess less cell-wall recalcitrance; (ii) using suitable pretreatments of the raw material for hemicellulose sugars extraction; and (iii) enzymatic treatment for the cellulose conversion to glucose that will be converted to ethanol by fermentation [23]. Among the pretreatment technologies, which include Organosolv treatment [24], acid and alkali pretreatments [25, 26], and steam explosion [27], the Organosolv process has been considered as one of the most promising for cellulosic ethanol production [23, 24, 28, 29]. This type of pretreatment involves the use of an organic liquid and water to partially hydrolyze lignin bonds and lignin-carbohydrate bonds, resulting in a solid residue consisting of mainly cellulose and some hemicellulose [30, 31]. There are many advantages that emerge from the use of Organosolv pretreatment in industrial processes, mainly because of its low cost and ease of recovery, miscibility in water, and low toxicity. Therefore, the use of suitable biomass feedstocks followed by a feasible and cost-effective pretreatment and implementing enzymatic hydrolysis processes are the major bottlenecks for cellulosic ethanol production on the industrial scale.
In this study, we identify the BAHD acyl-CoA transferase gene family and describe the effects of silencing BAHD01 gene expression in a commercial sugarcane cultivar. We show that sugarcane BAHD01 RNAi lines have improved straw saccharification. In addition, we developed a predictive model for the Organosolv pretreatment and enzymatic hydrolysis analysis of sugarcane straw biomass, designed to find the optimal conditions for the screening of a large number of transgenic events.
Results
Identification of BAHD acyl-CoA gene family members and generation of silencing lines in sugarcane
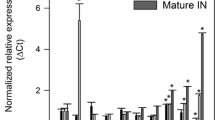
To identify BAHD01 gene in the sugarcane genome [32], we analyzed the phylogeny of BAHD genes in the ‘Mitchell Clade’ [20] for sugarcane, Setaria, Brachypodium, maize, rice, and Arabidopsis (Fig. 1a). We were able to identify four BAHD genes in the sugarcane genome, named SacBAHD01, SacBAHD03, SacBAHD05, and SacBAHD09 based on the nomenclature suggested for Setaria viridis BAHD genes [1]. It is important to mention that the selection of gene targets and transformation was initiated before the release of a more complete sugarcane genome [33]. However, to generate the phylogenetic tree presented in Fig. 1a, we also identified BAHD homologs based on this new released genome. The BAHD homologs identified in the genome released by Riacho-Panõn and Matiello [32] and Gasmeur et al. [33] are identified in the phylogenetic tree as “Sac” and “Sh,” respectively. From these genes, SacBAHD01 and SacBAHD05 presented the highest levels of expression in sugarcane leaves collected from 3- and 8-month-old plants, which were classified as young and mature leaves, respectively (Fig. 1b). SacBAHD01 was highly expressed in young leaves of sugarcane, decreasing its expression as the leaves mature, while SacBAHD05 transcript levels did not change significantly during different developmental stages.
Phylogenetic and expression analyses of candidate clade BAHD genes. a Phylogenetic tree of candidate BAHD genes identified in Arabidopsis (AT), Brachypodium (Bradi), maize (GRMZM), rice (LOC Os), sorghum (Sb), Setaria viridis (Sevir), Setaria italic (Si), and sugarcane (Sac, Riacho-Panõn and Matiello [32]; Sh, Garsmeur et al. [33]). Support for the topology is shown as fraction of bootstrap runs. BAHD names for each branch are based on Molinari et al. [46], and alternative AT names are based on Bartley et al. [20]. b Real-time qPCR analysis of the identified BAHD genes in sugarcane. Expression is relative to the high expressed reference genes GAPDH and EF1-α. Young and mature leaves correspond to tissues from three- and eight-month-old sugarcane plants, respectively (n = 5; error bars ± SEM)
To suppress the expression of SacBAHD01, a construct was designed to contain an RNAi hairpin under control of a constitutive maize ubiquitin promoter (Additional file 1: Figure S1a). RNAi BAHD01 matches 100% to a specific region of SacBAHD01 CDS sequence (Additional file 1: Figure S1b), and by comparing the sequence of this region with other BAHD gene sequences found in both sugarcane genomes [32, 33], we could verify the specificity of our RNAi BAHD01 sequence. The RNAi target sequence was formed from a 413 bp region toward the 3′ end of the CDS of SAC_BAHD01 (825-1237 bp). The longest stretch of identity with off-target BAHD genes included 24 bp (SAC_BAHD10*), 16 bp (SAC_BAHD02*), 15 bp (SAC_BAHD05), 14 bp (SAC_BAHD03, SAC_BAHD08*), 13 bp (SAC_BAHD09), 10 bp (SAC_BAHD04*), and < 10 bp (SAC_BAHD7*). Sequences marked with * were estimated from the database used rather than full-length cDNA clones. Therefore only SAC_BAHD10 has sufficient length of identity to possibly match a few short interfering RNAs produced from the hairpin compared with hundreds that would match SAC_BAHD01. Furthermore, two SNPs present in this 24 bp stretch in SAC_BAHD10 suggest that only one of several paralogs of SAC_BAHD10 actually match the RNAi. However, the SacBAHD10 gene was not expressed significantly in young and mature leaves of wild-type sugarcane SP80-3280 and did not show any compensation of its expression levels in transgenic plants, as demonstrated by RNA sequencing analysis (de Souza et al. [1]; manuscript in preparation).
SP80-3280 sugarcane variety was transformed with this construct, generating 14 independent events presenting different levels of SacBAHD01 suppression (Additional file 2: Figure S2a). We selected five of the most suppressed lines (SacBAHD01 RNAi lines 1, 2.2, 2.4, 3.1, and 4), which demonstrated silencing levels ranging from 70 to 95% (Additional file 2: Figure S2b) to perform biomass pretreatment and digestibility analysis.
Organosolv pretreatment and saccharification analysis of SacBAHD01 RNAi lines
The physical or chemical pretreatment of the biomass is pivotal to provide broad access to cellulose by hydrolytic enzymes in industrial processes. To screen for the biomasses from SacBAHD01 RNAi lines with improved digestibility, we decided on Organosolv pretreatment with ethanol as organic solvent, followed by enzymatic hydrolysis. We first established optimal enzymatic concentration to use on pretreated samples of wild-type plants by varying levels of a commercial mixture of cellulases and hemicellulases (Cellic® CTec 3; Novozymes), and demonstrating that 15 FPU/g was the minimum enzymatic activity necessary for the maximum glucose release after 48 h (Additional file 3: Figure S3). Next, we evaluated and optimized the effects of two pretreatment variables—the ethanol concentration (X1) and residence time (X2) on biomass enzymatic saccharification—using the Response Surface Methodology (RSM) based on five-level Central Composite Design (CCD), originally described by Box and Wilson [34] and successfully used for optimizing processes [35]. We used sugarcane leaves and stem tops of 12-month-old plants (straw) from transgenic lines that displayed the highest levels of SacBAHD01 suppression (events 1, 2.2, 2.4, 3.1, and 4) and nontransformed (NT) plants as biomass for Organosolv pretreatments.
Eleven experiments, under nine pretreatment conditions, were applied, according to experimental design, with ethanol concentration ranging from 30 to 100% (v/v) and residence time ranging from 0 to 240 min (Table 1). The pretreated samples were further submitted to enzymatic hydrolysis conducted in 6 and 48 h, at 15 FPU/g dry biomass. The different ethanol concentrations (X1) and the residence times (X2) of Organosolv treatments, in addition to the obtained glucose concentrations released after 6 and 48 h of saccharification of sugarcane biomass, are shown in Table 1. The difference between the responses of the experiments 9, 10, and 11 is due to an uncontrolled step such as temperature control in the reactor, for example, and has to be considered for the estimation of the model’s quality.
Based on the experimental results described above and using RSM, we were able to propose a second order model for the prediction of glucose concentration released after pretreatment of each biomass analyzed. The regression equations and correlations coefficients considering the complete model and coded values are presented in Additional file 4: Table S1. The evaluation of the variable coefficients demonstrated that the ethanol concentration (X1) and residence time (X2) in the Organosolv pretreatment strongly contributed to glucose release in 6 and 48 h of enzymatic hydrolysis. The correlation coefficients suggest that there are close agreements between the experimental results and the theoretical values predicted by the polynomial models (Additional file 4: Table S1).
Using the models as objective functions, it was demonstrated that the maximum glucose concentration released after hydrolysis for all transgenic biomasses was obtained for pretreatment variables of 30% (v/v) ethanol concentration (X1 = − 1.41) and residence time of 240 min (X2 = 1.41). For NT plants, the maximum glucose release was found for pretreatment variables of 30% (v/v) ethanol concentration (X1 = − 1.41) and residence time of 223.8 min (X2 = 1.22).
The analysis of variance (ANOVA) at 90% confidence level indicated that the models are statistically significant. Despite the difference between the responses of the experiments 9, 10, and 11, the lack of fit, associated with these repetitions, is not significant, suggesting that the regression might be used for predictive purposes. The value of pure error was small for all responses, which indicates good reproducibility of the obtained data. In this sense, the pretreatment variables X1 and X2 could be modified to predict and optimize the glucose concentration released after hydrolysis of the sugarcane straw in industrial processes. The results predicted by the models are in agreement with experimental values in all cases, which proves that the trial 11 deviation was an isolated case, but without prejudice to the models reliability.
To assure the feasibility and accuracy of the proposed models, a pretreatment validation experiment was performed, using the optimal Organosolv conditions found for transgenic biomass, i.e., 30% (v/v) ethanol concentration and 240 min of residence time. The enzymatic hydrolysis was performed under the same conditions described above, using 15 FPU of the enzymatic cocktail per gram biomass over 6 and 48 h. Experimental and predicted values of glucose release for each biomass under these conditions are shown in Table 2. As observed, the data demonstrated that experimental values for glucose release are in agreement with the predicted values, corroborating that the models proposed from RSM could reliably predict the dependent variables.
In addition, the validation assay revealed that, under optimal pretreatment conditions, the biomasses of three distinct events showed more efficient saccharification, demonstrated by higher levels of glucose release compared to NT plants (Fig. 2a). As demonstrated, SacBAHD01 RNAi lines 1, 2.2, and 2.4 presented an increase of 24%, 16%, and 11%, respectively, in biomass digestibility after Organosolv pretreatment and 48 h of enzymatic hydrolysis, compared to NT plants. Thus, these lines showing improved biomass digestibility were chosen to be submitted to a detailed cell-wall characterization.
Cell-wall characterization and digestibility of sugarcane straw biomass. a Saccharification of sugarcane straw from SacBAHD01 RNAi lines and nontransformed (NT) plants after Organosolv pretreatment, using Cellic CTec 3 (Novozymes) at 15 FPU for 6 and 48 h. b Ester-linked contents of ferulate (FA) and p-coumarate acid (pCA) in the alcohol insoluble fraction (AIR) of sugarcane straw from three independent events of SacBAHD01 RNAi lines and NT plants. c HCA conjugates in supernatant following mild acidolysis of sugarcane straw AIR. The data are represented as relative peak areas of major peaks for p-coumarate (pCA)-Ara and ferulate (FA)-Ara, previously identified by LC–MS as described by de Souza et al. [1]. n = 4; error bars ± SEM; significance of difference of transgenic lines from NT plants indicated if difference in means > least significant difference from ANOVA at p < 0.01 (*) and p < 0.001 (**)
Cell-wall HCA contents and characterization of xylan in SacBAHD01 RNAi plants
Previously, we have shown that the silencing of BAHD01 gene in the C4 model plant Setaria viridis caused a strong reduction of cell-wall-bound FA contents [1]. To verify if suppression of BAHD01 gene in sugarcane would lead to similar effects, we analyzed transgenic lines that showed increased biomass digestibility to determine cell-wall-bound hydroxycinnamate (HCA) contents in the alcohol insoluble fraction (AIR) from the straw of these plants. It was observed that FA contents of transgenic lines decreased by 50% in SacBAHD01 RNAi line 1 and circa 30% in the lines 2.2 and 2.4, compared to NT plants (Fig. 2b). It was also found that ester-linked pCA contents decreased circa 30% in cell walls of the lines 1 and 2.2, but the line 2.4 did not show statistically significant differences in total pCA contents, compared to NT plants (Fig. 2b).
Bound FA is ester-linked to arabinofuranosyl units attached to GAX of grass cell walls [36]. We found that silencing of SvBAHD01 gene led to reductions in FA bound to arabinofuranosyl units (FA-Ara) in cell-wall tissues of Setaria [1]. Using mild acidolysis with trifluoroacetic acid (TFA) of AIR, we analyzed HCA-Ara contents in transgenic sugarcane, based on the methodology described in [1]. Using peak areas in ultraviolet absorbance spectra from this method, we observed reductions in FA-Ara contents of approximately 20% for SacBAHD01 RNAi line 1 and 50% for lines 2.2 and 2.4 in the straw (Fig. 2c). pCA-Ara levels increased by 25% only in line 1, while lines 2.2 and 2.4 did not demonstrate significant changes compared with NT plants (Fig. 2c). In addition, we found no effects of SacBAHD01 silencing on monosaccharides (arabinose, xylose, galactose, and glucose) and acetyl contents of AIR samples from sugarcane straw (Additional file 5: Table S2). The reduction of FA levels in the cell walls of sugarcane SacBAHD01 silencing lines corroborated the results obtained from the suppression of this gene in the phylogenetically related plant Setaria viridis.
Gel-state 2D-NMR characterization of cell walls in SacBAHD01 RNAi plants
The total lignin content (Klason lignin) of sugarcane straw did not change between nontransformed (NT) and transgenic plants (Additional file 5: Table S2).To confirm the results obtained using biochemical analysis and to gain information on the overall aromatic composition of the unfractionated cell walls in the SacBAHD01-silenced plants, we performed gel-state 2D-NMR studies [37]. The spectral fingerprints presented in Fig. 3 demonstrate that FA levels decreased in transgenic lines compared to NT plants, corroborating the biochemical results. The decrease in FA contents in the cell walls of transgenic lines corresponded to 25%, as pCA contents decreased by 14.5%, 4%, and 7% in the lines 1, 2.2, and 2.4, respectively. It is worth mentioning that the 2D-NMR values presented here are on a lignin basis, and the integrals of small mobile components such as FA and pCA are variable in this methodology in relation to the immobile internal lignin units [1].
2D-NMR heteronuclear single-quantum coherence (HSQC) partial spectra of sugarcane straw from nontransformed (NT) plants and the three transgenic lines (1, 2.2, and 2.4). Color-coding of the contours matches that of the assigned structures; where contour overlap occurs, the colorization is only approximate. The analytical data are from volume integrals of correlation peaks representing reasonably well-resolved (except for H) C/H pairs in similar environments; thus they are from S2/6, G2, H2/6, FA2, pCA2/6, and T2´6´, with correction for those units that have two C/H pairs per unit. All relative integrals are on a G + S = 100% basis; H-units are over-quantified due to an overlapping peak from protein phenylalanine (Phe) units [38]
Discussion
The cell walls of plants from the monocotyledon family Poaceae, which include grasses such as sugarcane, commonly have the presence of two hydroxycinnamates (HCAs), p-coumarate (pCA) and ferulate (FA) [10]. Cell-wall-bound FA mostly acylates glucuroarabinoxylans (GAX) present in the hemicellulose fraction of grasses [9], while most of the pCA acylates the lignin polymer [16]. For lignin, this is now known to occur by action of BAHD transferases acylating monolignol with FA or pCA-coenzyme A (CoA) acting as donors [38,39,40,42] and other studies suggest that analogous actions of similar but distinct BAHD transferases result in GAX acylation by pCA and FA transferases [1, 19,20,21, 43]. The crosslinks promoted by the acylation of GAX by FA contribute for the inhibition of biomass saccharification by tightly binding the polysaccharide substrate to the nondigestible lignin. Therefore, decreasing the FA levels or FA-mediated crosslinking of grass biomass has been considered a promising target for increasing digestibility [17]. In fact, we have shown that the silencing of BAHD01 gene in green foxtail (Setaria viridis), a model plant for C4 grasses [22], caused large decreases in cell-wall feruloylation, thereby increasing biomass saccharification [1]. Here, we characterized the BAHD acyl-CoA transferase gene family in sugarcane (Saccharum spp.) and, using an RNAi approach, suppressed the ortholog of Setaria BAHD01 in this crop.
We were able to find six members of the candidate clade of BAHD genes [18] in the sugarcane genome (Fig. 1a). The number of BAHD genes found in the sugarcane genome appeared to be much less compared to phylogenetically related plants such as rice (10), maize (9), sorghum (9), Brachypodium (9) and Setaria (10). However, modern cultivars of sugarcane have an extremely complex genome, derived from interspecific hybridization, as is the case for the variety used in the present study (SP80-3280). Sugarcane elite cultivars might have up to 130 chromosomes, distributed among ~ 12 homo (eo)logous groups [44], with a total genome size reaching 10 Gbp [45], and such complex genome structure hinders genome sequencing, assembly and annotation. Our phylogenetic analysis is based on the recently published draft genome sequencing of the sugarcane hybrid SP80-3280 [32] and R570 variety [33], despite the long reads used in assembly of this genome, we cannot exclude the possibility that some gene sequences could be missing. Thus, it is plausible that sugarcane might have other members of the BAHD clade, which we could not identify in our analysis.
The BAHD genes identified in sugarcane were all expressed in leaf tissues from two different developmental stages, with SacBAHD01 and SacBAHD05 presenting the highest levels of expression (Fig. 1b). SacBAHD01, implicated in FA incorporation in the cell wall of grasses [1], was highly expressed in young leaves (3-month-old plants) of sugarcane, while its transcript levels decreased ~ 5-fold in mature leaves (8-month-old plants). In contrast, the other SacBAHD genes identified (03, 05, and 09) were all expressed in young and mature leaves, but their transcript levels did not drastically change during these different developmental stages. These results are in agreement with previous studies on Brachypodium BAHD genes, where BdBAHD01, 05, and 09 were highly expressed in vegetative tissues, with only BdBAHD05 and 09 being expressed in later stages of reproductive development [46]. From these four BAHD genes identified in sugarcane, BAHD01, 05, and 09 have a putative assigned role in other grasses. BAHD01 and BAHD05 proteins are potential candidates for feruloylation of GAX in Setaria and Brachypodium, respectively, while orthologs of BAHD09 are candidates for the addition of pCA to monolignols, being classified as a BAHD PMT (p-coumaroyl monolignol transferase) [40, 41, 43]. As feruloylation of GAX tends to occur in both primary and secondary grass cell walls, which are biosynthesized during the early stages of development [46], it is not surprising to see the high expression level of SacBAHD01 in young leaves of sugarcane (Fig. 1b). These results reinforce that BAHD01 is a putative candidate for feruloylation of GAX in grasses.
To test if BAHD01 silencing would have any effect on biomass digestibility, we used sugarcane straw from transgenic BAHD01 RNAi lines, comparing them to nontransformed plants. We used the sugarcane straw as biomass for the studies on digestibility because this material does not require processing, as is necessary for the production of bagasse, which is generated after the juice extraction from the stalks.
The utilization of less recalcitrant materials for bioethanol production is not sufficient to ensure maximum glucose release from the biomass. Therefore, biomass pretreatments are fundamental in industrial processes. Nevertheless, the pretreatment followed by enzymatic hydrolysis of the biomass consist in a large proportion of the costs involved in the biorefinery process, being a bottleneck for the adaptation of sugarcane mills to produce cellulosic ethanol [47]. For the pretreatments available, the Organosolv process using ethanol as solvent appears to be the most promising for many reasons: (i) low cost and ease of recovery; (ii) low energy requirement for solvent recovery; (iii) fully miscible in water; (iv) low toxicity. Usually, the Organosolv treatment of the biomass is preceded by addition of acids, which act as catalysts for the rupture of the lignin-carbohydrate complex [31, 48]. Our results demonstrated that silencing of BAHD01 in sugarcane resulted in the improvement of biomass saccharification after Organosolv pretreatment, suggesting a decrease in biomass recalcitrance (Fig. 2a). However there was no simple relationship between variation in FA or FA-Ara reduction between lines and benefit to saccharification (Fig. 2); this was also the case for the two lines of Setaria we studied in detail [1]. For instance, transgenic line 1 demonstrated highest levels of saccharification, but total FA and FA-Ara levels in this line were higher compared to lines 2.2 and 2.4 (Fig. 2). Therefore, it is possible that other factors are involved in determining saccharification efficiency. In fact, two major characteristics of cell wall—the cellulose crystalline index (CrI) and the degree of polymerization (DP) of β-1,4-glucans—have been considered essential to negatively affect biomass digestibility under various pretreatments in different species, including sugarcane [25, 26, 49, 50]. In addition, recent studies have suggested that the arabinose (Ara) substitution degree of xylans could reduce cellulose crystallinity for positively affecting biomass enzymatic digestibility under chemical pretreatments in sugarcane and other grasses [25, 51, 52]. In our studies, transgenic line 1 presented increased levels of pCA-Ara (Fig. 2c), and it is tempting to speculate that the highest levels of digestibility found for its biomass could be related to decreased CrI due to the higher degree of substitution caused by pCA-Ara. However, further investigations on cell-wall CrI and cellulose DP of our transgenic lines are required and are currently being investigated.
In the present study, we did not use catalysts before ethanol treatment, but we obtained high levels of glucose release under optimal conditions of pretreatment and enzymatic hydrolysis (Table 1; Fig. 2a). These results suggest that biomass from sugarcane BAHD01-silenced lines is a suitable material for the generation of cellulosic ethanol in industrial scale, as the increase of fermentable glucose levels reached up to 24% in line 1, compared to nontransformed plants. These high levels of saccharification were achieved under low ethanol concentration and temperature (30% v/v, 180 °C, respectively), and in a relatively short period of time (120 min).
The cell-wall characterization of sugarcane transgenic lines demonstrated that silencing of BAHD01 decreased the levels of FA and FA-Ara, with no significant changes in total lignin or monosaccharide composition (Figs. 2, 3; Additional file 5: Table S2). These results were also found when BAHD01 was silenced in Setaria, indicating that the species is in fact a suitable experimental model for grasses.
Other approaches have achieved increases in saccharification yields from sugarcane residues by silencing or knock-out of genes responsible for lignin synthesis [53, 54]. In contrast to our strategy, this alternative approach results in lower lignin which can be a valuable feedstock in biorefining; in our approach, less FA-mediated crosslinking could enable greater ease of separation of lignin from polysaccharide. Further work with field-grown sugarcane using different technologies to utilize the bagasse and straw are required to determine the best approaches. In addition, it is known that aromatic carboxylic acids such as FA are strong inhibitors of microbial growth [55]. Larsson et al. [56] demonstrated that ferulic acid strongly inhibited S. cerevisiae growth at very low concentrations (1.0 mM). As the concentration of FA in sugarcane bagasse hydrolysates can reach 1.1 mM [57], our strategy of decreasing FA levels is advantageous for industrial processes since it is possible to diminish inhibitory effects during the fermentation process.
In summary, the silencing of a BAHD acyl transferase gene in sugarcane decreased the levels of FA in cell walls, increasing the saccharification levels of its biomass, leading to great opportunities to develop new elite cultivars for bioethanol production.
Conclusions
The silencing of SacBAHD01 gene and subsequent decrease of cell-wall ferulate contents indicate a promising biotechnological approach for improving the suitability of sugarcane residues for cellulosic ethanol production. In addition, the Organosolv pretreatment of the genetically modified biomass and the optimal conditions for the enzymatic hydrolysis presented here might be incorporated in the sugarcane industry for bioethanol production.
Methods
Phylogenetic analysis
Whole genome sequences of SP80-3280 and R570 sugarcane varieties were downloaded from http://bce.bioetanol.cnpem.br/sugarcanegenome and http://sugarcane-genome.cirad.fr/ to identify potential BAHD protein sequences, respectively. Sequences from previously identified BAHD proteins in Brachypodium, rice, maize, sorghum, and Setaria [1, 18, 46] were used as query to search for the BAHD sequences in the sugarcane genome database using tblastn, with e-value of 10−10 as threshold, to identify sequences with high similarity. A custom script was then developed and executed to remove redundant sequence and retrieve the similar sequence regions from sugarcane genome. The phylogenetic analysis was performed after the alignment of the protein sequences using MUSCLE, and the phylogenetic tree was estimated by a maximum-likelihood method using FastTree 2.1.5 program [58]. The tree was visualized with the iTOL software (https://itol.embl.de/).
Expression analysis of SacBAHD genes in sugarcane leaves
Top leaves (a pool of four fully expanded leaves) of 3- and 8-month-old SP80-3280 sugarcane variety were collected for expression analysis of the identified SacBAHD genes (SacBAHD01, 03, 05 and 09) from three different plants. Total RNA was isolated using TRIzol reagent (Invitrogen, Grand Island, NY) and treated with RNaseFree RQ1 DNase (Promega, San Luis Obispo, CA) according to the manufacturer’s instructions. cDNA was synthesized from one µg of RNA using SuperScript® III kit (Invitrogen). The expression level was normalized against the sugarcane Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Elongation factor 1-alpha (EF1) genes by qRT-PCR. The reactions were performed with SYBR Green Master Mix (Applied Biosystems) under the following conditions: 95 °C for 3 min denaturation, 40 cycles at 95 °C for 10 s, and 58 °C for 45 s. Amplification specificity was verified by melt curve analysis from 55 to 95 °C. SacBAHD expression levels were calculated using the 2−∆∆Ct method [59]. The primers’ sequences used for GAPDH (CA254672), EF1 (EF581011.1), and SacBAHDs (SacBAHD1: MK614571; SacBAHD3: MK614570; SacBAHD5: MK614573; SacBAHD9: MK614572) amplifications are listed in Additional file 6: Table S3.
Generation of transgenic sugarcane plants
After the identification of BAHD01 gene in the sugarcane genome and verification of its expression in sugarcane leaves, we selected a 413 bp sequence to design a construct containing inverted repeats of this sequence flanking the maize Adh1 intron, in order to silence SacBAHD01 gene (Additional file 1: Fig. S1a). In this construct, synthesized by DNA Cloning Service (Hamburg, Germany), the SacBAHD01 RNAi cassette is under control of the maize ubiquitin promoter, and the bar gene used as selectable marker is under control of the rice actin 1 promoter. This construct was used to transform embryogenic callus of SP80-3280 sugarcane variety, following our published protocol [60].
Molecular analysis of transgenic plants
Genomic DNA from regenerated sugarcane plantlets resistant to the herbicide bialaphos was extracted using a modified CTAB method [61]. The gene insertion was confirmed by conventional PCR using specific primers designed for the bar gene amplification (Additional file 6: Table S3). The candidate transgenic events were submitted to analysis by real-time PCR of the target gene SacBAHD01, using GAPDH and EF1-α as reference for normalization of transcripts as described above. Plants from independent transgenic events demonstrating the highest levels of SacBAHD01 suppression were vegetatively propagated, and leaves from 8-month-old plants were collected for confirmation of the stability of BAHD01 suppression. Nontransformed (NT) plants, which consist of plants from cell tissue culture, were included in all studies. After confirmation, the straw of these plants was collected for further analysis.
Organosolv pretreatment and enzymatic hydrolysis
Sugarcane leaves (four fully expanded top leaves) and stem tops (around 30 cm) of 12-month-old plants (straw) were collected for pretreatment. The straw is composed of approximately 60% leaves and 40% stem tops. The straw was chopped, and the samples were pretreated using Organosolv process and ethanol as organic solvent. The pretreatment was conducted in 19-L batch reactor PARR 4555 using 3 L of solvent and 5 g (dry weight) of each biomass placed in baskets (nontransformed (NT) plants, and five independent SacBAHD01 RNAi lines). The starting point of the residence time was considered as temperature in the reactor reached 180 °C. Different concentrations of ethanol (ranging from 30 to 100%) and residence times (ranging from 0 to 240 min) were used in our studies.
In order to evaluate and optimize the effects of ethanol concentration (X1) and residence time (X2) in enzymatic saccharification, after pretreatment, we applied the Response Surface Methodology (RSM) based on Central Composite Design (CCD). RSM is a tool based on statistical theory that provides reliable information about the process, reducing the number of experiments or repetitions and improving the quality of the information. A CCD design is a specific type of experimental design that contains a factorial design with center points, which is augmented by a group of axial points that allow estimation of curvature. When the distance from the center of the design region to a factorial point is ± 1 unit for each factor, the distance from the center to axial point, considering the rotatability of factorial plan, is |α| = (2k)1/4 > 1, where k is a number of factors. Then, axial points represent two new extreme levels totalizing five levels (− α, − 1, 0, + 1, + α) for each factor. This technique is also useful to analyze factors simultaneously and optimize more than one response at a time [34, 35]. This experimental design considered two independent variables (X1 and X2), three replicates at the center point, alpha for rotatability of 1.4, resp experiments completely randomized. Glucose concentrations released after 6 and 48 h of enzymatic hydrolysis, considering four replicates, were used as the responses in CCD. Table 1 shows coded and original values used in CCD experiments. The original values have to be coded to keep the proportionality between the effects of the variables. When working with coded values, these are independent of the variables values and vary steadily from − 1.41 to + 1.41. The value of the center point (level 0) is determined considering the average distance between levels − 1.41 and + 1.41. Levels + 1 and − 1 are proportional to the levels previously determined.
For enzymatic hydrolysis, pretreated samples were dried in an oven overnight at 40 °C. Enzymatic saccharification assays were performed in 2 mL tubes with 5% (w/v) dry biomass in 100 mM citrate buffer, pH 5.0. The cellulase/hemicellulase cocktail Cellic® CTec 3 (Novozymes, Lyngby, Denmark) was added at the desired filter paper activity units FPU/g biomass (ranging from 3 to 30 FPU). The reaction was incubated in a Thermomixer microplate incubator (Eppendorf, Germany) operated at 50 °C and agitation speed of 800 rpm. Samples were withdrawn after 6 and 48 h, followed by centrifugation at 10,000g for 15 min. Sugars in enzyme hydrolysates were analyzed by high-performance liquid chromatography system HPLC (Agilent, Palo Alto, CA-USA), equipped with a refractive index detector and Aminex HPX-87H ion exchange column (Bio-Rad, Hercules, CA-USA). The mobile phase contained 5 mM H2SO4, at a flow rate of 0.6 mL min−1, heated at 45 °C. The experiments were carried out in four technical replicates, and the statistics were applied based on the data collected from three different plants.
The statistical analysis was employed to verify significant differences between NT and independent transgenic plants after hydrolysis, using the Tukey test at 95% probability. The software Statistica™ 12.0 (Statsoft, Palo Alto, Ca-USA) was used for the design of experiments, regression, graphical analyses, and analyses of variance. The second-degree polynomials (Eq. 1) were calculated to estimate the response of the dependent variables, X1 and X2. Significance level was considered to be of 90%.
where Y is the predicted response; X1 is organic solvent concentration in encoded value; X2 is residence time in encoded value; b0 is offset term; b1, b2 are linear effects; b11, b22 are squared effects; and b12 are the interaction terms. Canonical analysis and eigenvalues were used to locate the stationary point of the responses and to determine whether it represents a maximum point. To validate the model predictions, an additional pretreatment was carried out under conditions predicted by the model, followed by enzymatic hydrolysis.
Quantitation of cell-wall-bound hydroxycinnamate (HCA) content and determination of HCA conjugates released by mild acidolysis
The content of the ester-linked cell-wall-bound hydroxycinnamates (p-coumarate and ferulate) was determined essentially as described [62], using the straw of 12-month-old nontransformed (NT) plants and sugarcane transgenic lines 1, 2.2, and 2.4 as samples. First, the biomass was chopped and sieved to obtain a fine powder, and then freeze-dried. The determination of HCA conjugates was performed as described by de Souza et al. using 10 mg freeze-dried ground tissue [1]. Briefly, the alcohol-insoluble residue (AIR, prepared using extractions as described for cell-wall-bound HCA) was treated with 1.2 mL 50 mM trifluoroacetic acid for 4 h at 99 °C with agitation at 750 rpm. After centrifugation for 10 min at 16,000g 2× 500 μL aliquots of supernatant were freeze-dried. The pellet was washed twice with water and freeze-dried. Released HCA-conjugates from one 500 μL aliquot of supernatant were dissolved in 250 µL 50% methanol, 0.1% formic acid, and 10 μL separated as for cell-wall-bound HCA except using a binary gradient with methanol (solvent A) and 0.1% formic acid (solvent B), under the following conditions: isocratic 100% B, 0–1 min; linear 100% to 0% B, 1–21 min; isocratic 0% B, 21–23 min; and linear 0% to 100% B, 23–28 min at a flow rate of 1 mL min−1. The results for HCA-Arabinose (HCA-Ara) were expressed as relative peak areas (absorbance at 280 nm) corresponding to major peaks for p-coumarate (pCA)-Ara and ferulate (FA)-Ara, previously identified by LC–MS [1]. The statistical analysis was performed using ANOVA at p < 0.05 (*) and p < 0.001 (**), with n = 3.
Cell-wall characterization by solution-state two-dimensional NMR
Sugarcane cell walls were characterized using solution-state 2D NMR according to procedure described by [37]. Aliquots of approximately 500 mg (in triplicate) of freeze-dried ground tissue (straw) of each biological replicate from NT control and events 1, 2.2, and 2.4 were weighed and extracted overnight (minimum of 8 h) with a mixture acetone/water (95:5 v/v) on a Soxhlet apparatus (~ 70 °C). The extract-free samples were oven-dried at 50 °C for 48 h. The dried extract-free plant material was submitted to a ball-milling procedure. Aliquots of 200 mg of each sample were milled in 20 mL jars with 10 × 10 mm ball bearings in a Fritsch® Planetary micro mill Pulverisette 7 premium line equipment, according to following milling protocol: 5 × 5 min with 5 min pauses in between. After the ball-milling procedure, the preparation for NMR analysis for all samples was carried out according to [37] for gelling samples without derivatization, and their conditions used for acquisition of the NMR spectra and processing. The acquisition of the NMR spectra were performed at Laboratory of Nuclear Magnetic Resonance of Federal University of Sao Carlos (São Carlos-SP/Brazil), on a 600 MHz Bruker® AVANCE III spectrometer system equipped with a 5 mm TCI cryoprobe with ATMA® (Automatic Tuning and Matching).
Total lignin content and monosaccharide composition
The total lignin content (Klason lignin) and the monosaccharide and acetyl composition of AIR samples were determined following the protocol described by the National Renewable Energy Laboratory of the US Department of Energy [63].
Availability of supporting data
The authors ensure the availability of supporting data.
Change history
08 June 2019
After the publication of the article [1], it was brought to our attention that the GenBank Accession number for the EF1 is missing in Methods section. Please find the accession number in the erratum below.
References
de Souza WR, Martins PK, Freeman J, Pellny TK, Michaelson LV, Sampaio BL, et al. Suppression of a single BAHD gene in Setaria viridis causes large, stable decreases in cell wall feruloylation and increases biomass digestibility. New Phytol. 2018;218:81–93.
Menandro LMS, Cantarella H, Franco HCJ, Kölln OT, Pimenta MTB, Sanches GM, et al. Comprehensive assessment of sugarcane straw: implications for biomass and bioenergy production. Biofuels Bioprod Bioref. 2017;11:488–504.
Sindhu R, Gnansounou E, Binod P, Pandey A. Bioconversion of sugarcane crop residue for value added products—an overview. Renew Energ. 2016;98:203–15.
Vitti AC, Franco HCJ, Trivelin PCO, Ferreira DA, Otto R, Fortes C, et al. Nitrogênio proveniente da adubação nitrogenada e de resíduos culturais na nutrição da cana-planta. Pesq Agropec Bras. 2011;46:287–93.
Carvalho JLN, Otto R, Franco HCJ, Trivelin PCO. Input of sugarcane post-harvest residues into the soil. Sci Agric. 2013;70:336–44.
Franco HCJ, Pimenta MTB, Carvalho JLN, Magalhães PSG, Rossell CEV, Braunbeck OA, et al. Assessment of sugarcane trash for agronomic and energy purposes in Brazil. Sci Agric. 2013;70:305–12.
Rípoli TCC, Molina WF Jr, Rípoli MLC. Energy potential of sugar cane biomass in Brazil. Sci Agric. 2000;57:677–81.
Santos FA, Queiróz JH, Colodette JL, Fernandes SA, Guimarães VM, Rezende ST. Potencial da palha de cana-de-açúcar para produção de etanol. Quim Nova. 2012;35:1004–10.
Hatfield RD, Rancour DM, Marita JM. Grass cell walls: a story of cross-linking. Front Plant Sci. 2017;7:2056.
Harris PJ, Trethewey JAK. The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochem Rev. 2010;9:19–33.
Ralph J, Helm RF, Quideau S, Hatfield RD. Lignin feruloyl ester cross-links in grasses. 1. Incorporation of feruloyl esters into coniferyl alcohol dehydrogenation polymers. J Chem Soc Perk T. 1992;21:2961–9.
Ralph J, Grabber JH, Hatfield RD. Lignin-ferulate cross-links in grasses: active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohyd Res. 1995;275:167–78.
Ishii T. Structure and functions of feruloylated polysaccharides. Plant Sci. 1997;127:111–27.
Ralph J, Hatfield RD, Grabber JH, Jung HG, Quideau S. Helm RF Cell wall cross-linking in grasses by ferulates and diferulates. In: Lewis NG, Sarkanen S, editors. Lignin and lignan biosynthesis. Washington: American Chemical Society; 1998. p. 209–36.
Ralph J, Bunzel M, Marita JM, Hatfield RD, Lu F, et al. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem Rev. 2004;3:79–96.
Ralph J. Hydroxycinnamates in lignification. Phytochem Rev. 2010;9:65–83.
de Oliveira DM, Finger-Teixeira A, Mota TR, Salvador VH, Moreira-Vilar FC, Molinari HBC, et al. Ferulic acid: a key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol J. 2015;13:1224–32.
Mitchell RAC, Dupree P, Shewry PR. A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol. 2007;144:43–53.
Piston F, Uauy C, Fu LH, Langston J, Labavitch J, Dubcovsky J. Down-regulation of four putative arabinoxylan feruloyl transferase genes from family PF02458 reduces ester-linked ferulate content in rice cell walls. Planta. 2010;231:677–91.
Bartley LE, Peck ML, Kim SR, Ebert B, Manisseri C, Chiniquy DM, et al. Overexpression of a BAHD acyltransferase, OsAt10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol. 2013;161:1615–33.
Buanafina MMD, Fescemyer HW, Sharma M, Shearer EA. Functional testing of a PF02458 homologue of putative rice arabinoxylan feruloyl transferase genes in Brachypodium distachyon. Planta. 2016;243:659–74.
Brutnell TP, Wang L, Swartwood K, Goldschmidt A, Jackson D, Zhu X-G, et al. Setaria viridis: a model for C4 photosynthesis. Plant Cell. 2010;22:2537–44.
Mesa L, Gonzáleza E, Carab C, Gonzáleza M, Castrob E, Mussatto SI. The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Chem Eng J. 2011;168:1157–62.
Borand MN, Karaosmanoglu F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: a review. J Renew Sustain Energy. 2018;10:033104.
Hu M, Yu H, Li Y, Li A, Cai Q, Liu P, et al. Distinct polymer extraction and cellulose DP reduction for complete cellulose hydrolysis under mild chemical pretreatments in sugarcane. Carb. Polym. 2018;202:434–43.
Li Y, Liu P, Huang J, Zhang R, Hu Z, Feng S, et al. Mild chemical pretreatments are sufficient for bioethanol production in the transgenic glucosidase-overproduced rice straw. Green Chem. 2018;20:2047–56.
Mokomele T, da Costa Sousa L, Balan V, van Rensburg E, Dale BE, Görgens JF. Ethanol production potential from AFEX™ and steam-exploded sugarcane residues for sugarcane biorefineries. Biotechnol Biofuels. 2018;4(11):127.
Bozell JJ, Black SK, Myers M, Cahill D, Miller WP, Park S. Solvent fractionation of renewable woody feedstocks: organosolv generation of biorefinery process streams for the production of biobased chemicals. Biomass Bioenerg. 2011;35:4197–208.
Park YC, Kim TH, Kim JS. Effect of organosolv pretreatment on mechanically pretreated biomass by use of concentrated ethanol as the solvent. Biotechnol Bioproc E. 2017;22:431–9.
McDonough TJ. The chemistry of organosolv delignification. Tappi J. 1992;76:186–93.
Zhao X, Cheng K, Liu D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol. 2009;82:815–27.
Riaño-Pachón DM, Mattiello L. Draft genome sequencing of the sugarcane hybrid SP80-3280. F1000 Res. 2017;6:861.
Garsmeur O, Droc G, Antonise R, Grimwood J, Potier B, Aitken K, et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat Commun. 2018;9:2638.
Box GEP, Wilson KB. On the experimental attainment of optimum conditions. J Royal Stat Soc. 1951;13:1–45.
Myers RH, Montgomery DC. Response surface methodology: Process and product optimization using designed experiments. 2nd ed. New York: Wiley; 2002.
Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–89.
Kim H, Ralph J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org Biomol Chem. 2010;8:576–91.
Kim H, Padmakshan D, Li Y, Rencoret J, Hatfield RD, Ralph J. Characterization and elimination of undesirable protein residues in plant cell wall materials for enhancing lignin analysis by solution-state NMR. Biomacromolecules. 2017;18:4184–95.
Withers S, Lu FC, Kim H, Zhu YM, Ralph J, Wilkerson CG. Identification of grass-specific enzyme that acylates monolignols with p-coumarate. J Biol Chem. 2012;287:8347–55.
Marita JM, Hatfield RD, Rancour DM, Frost KE. Identification and suppression of the p-coumaroyl CoA: hydroxycinnamyl alcohol transferase in Zea mays L. Plant J. 2014;78:850–64.
Petrik DL, Karlen SD, Cass CL, Padmakshan D, Lu FC, Liu S, et al. p-Coumaroyl-CoA: monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J. 2014;77:713–26.
Karlen SD, Peck ML, Zhang C, Smith RA, Padmakshan D, Helmich KE, et al. Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci Adv. 2016;2:1–9.
Sibout R, Le Bris P, Legee F, Cezard L, Renault H, Lapierre C. Structural redesigning Arabidopsis lignins into alkali-soluble lignins through the expression of p-coumaroyl-coA: monolignol transferase PMT. Plant Physiol. 2016;170:1358–66.
Grivet L, Arruda P. Sugarcane genomics: depicting the complex genome of an important tropical crop. Curr Opin Plant Biol. 2002;2:122–7.
Le Cunff L, Garsmeur O, Raboin LM, Pauquet J, Telismart H, Selvi A, et al. Diploid/polyploid syntenic shuttle mapping and haplotype-specific chromosome walking toward a rust resistance gene (Bru1) in highly polyploid sugarcane (2n approximately 12×x approximately 115). Genetics. 2008;180:649–60.
Molinari HB, Pellny TK, Freeman J, Shewry PR, Mitchell RA. Grass cell wall feruloylation: distribution of bound ferulate and candidate gene expression in Brachypodium distachyon. Front Plant Sci. 2013;4:50.
Silveira MHL, Vanelli BA, Chandel AK. Second generation ethanol production: potential biomass feedstock, biomass deconstruction, and chemical platforms for process valorization. In: Chandel AK, Silveira MHL, editors. Advances in sugarcane biorefinery technologies, Commercialization, Policy Issues and Paradigm Shift for Bioethanol and By-Products. Berlin: Springer; 2018. p. 135–52.
Duff SJB, Murray WD. Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour Technol. 1996;55:1–33.
Kumari S, Das D. Improvement of gaseous energy recovery from sugarcane bagasse by dark fermentation followed by biomethanation process. Bioresour Technol. 2015;194:354–63.
Wang YT, Fan CF, Hu HZ, Li Y, Sun D, Wang YM, et al. Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol Adv. 2016;34:997–1017.
Wu ZL, Zhang ML, Wang LQ, Tu YY, Zhang J, Xie GS, et al. Biomass digestibility is predominantly affected by three factors of wall polymer features distinctive in wheat accessions and rice mutants. Biotechnol Biofuels. 2013;6:183.
Chen HM, Zhao J, Hu TH, Zhao XB, Liu DH. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: substrate digestibility, fermentability and structural features. App. Energy. 2015;150(S298):224–32.
Jung JH, Kannan B, Dermawan H, Moxley GW, Altpeter F. Precision breeding for RNAi suppression of a major 4-coumarate:coenzyme A ligase gene improves cell wall saccharification from field grown sugarcane. Plant Mol Biol. 2016;92:505–17.
Kannan B, Jung JH, Moxley GW, Lee SM, Altpeter F. TALEN-mediated targeted mutagenesis of more than 100 COMT copies/alleles in highly polyploid sugarcane improves saccharification efficiency without compromising biomass yield. Plant Biotech J. 2018;16:856–66.
Jönsson LJ, Martín C. Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol. 2016;199:103–12.
Larsson S, Quintana-Sáinz A, Reimann A, Nilvebrant N-O, Jönsson LJ. Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2000;84:617–32.
Martín C, Galbe M, Nilvebrant N-O, Jönsson LJ. Comparison of the fermentability of enzymatic hydrolysates of sugarcane bagasse pretreated by steam explosion using different impregnating agents. Appl Biochem Biotechnol. 2002;98–100:699–716.
Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8.
Basso MF, da Cunha BADB, Ribeiro AP, Martins PK, de Souza WR, Oliveira NG, et al. Improved genetic transformation of sugarcane (Saccharum spp.) embryogenic callus mediated by Agrobacterium tumefaciens. Curr Prot Plant Biol. 2017;2:221–39.
Molinari HBC, Marur CJ, Daros E, De Campos MKF, De Carvalho JFRP, Pereira LFP, et al. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant. 2007;130:218–29.
Freeman J, Ward JL, Kosik O, Lovegrove A, Wilkinson MD, Shewry PR, Mitchell RAC. Feruloylation and structure of arabinoxylan in wheat endosperm cell walls from RNAi lines with suppression of genes responsible for backbone synthesis and decoration. Plant Biotechnol J. 2017;15:1429–38.
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, et al. Determination of structural carbohydrates and lignin in biomass. Golden: NREL; 2008.
Acknowledgements
Not applicable.
Funding
This work is supported in part by Coordination for the Improvement of Higher Education Personnel (CAPES‐Embrapa) and Embrapa Macroprogram SEG (02.12.01.008.00.00).
Author information
Authors and Affiliations
Contributions
WRS, TFP, RACM, DSRG, PAOM, and HBCM designed the experiments and conceptualized the data; KED, PKM, FV, APR, and BADBC performed sugarcane transformation and molecular characterization of transgenic events; BLS, PAOM, and KED performed 2D-NMR experiments and analysis; TRS and EFF performed bioinformatics analysis; WRS and TFP performed all biochemical analyses; WRS and TFP wrote the original draft of the manuscript, and WRS wrote the final version of the manuscript; AKK, PAOM, EFF, RACM, DSRG, and HBCM revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
The authors have consented for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article is revised. GenBank Accession number for the EF1 was missing in Methods section. The sequence number EF581011.1 has been included.
Additional files
Additional file 1: Figure S1.
BAHD01 RNAi cassette and alignment of region targeted by the cassette.
Additional file 2: Figure S2.
Expression analysis and silencing levels of transgenic SacBAHD01 RNAi lines.
Additional file 3: Figure S3.
Enzymatic hydrolysis (saccharification) of wild-type sugarcane straw after Organosolv pretreatment.
Additional file 4: Table S1.
Spreadsheet containing quadratic model equations, in coded values, for 6 and 48 h enzymatic hydrolysis for events and NT plants.
Additional file 5: Table S2.
Determination of Klason lignin, monosaccharide and acetyl composition of AIR from straw of control and SacBAHD01 RNAi transgenic plants.
Additional file 6: Table S3.
Primers used in this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
de Souza, W.R., Pacheco, T.F., Duarte, K.E. et al. Silencing of a BAHD acyltransferase in sugarcane increases biomass digestibility. Biotechnol Biofuels 12, 111 (2019). https://doi.org/10.1186/s13068-019-1450-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-019-1450-7