Abstract
The discoveries recommend that the photoinduced conditions of fluorescein-determined go about as impetus for photochemically combining polysubstituted quinolines in ethanol at room temperature under air environment by means of revolutionary Friedländer hetero-annulation of 2-aminoaryl ketone and α-methylene carbonyl compound. This study lays out an original capability for photochemically orchestrating fluorescein. This non-metallic organic dye is economically accessible and modest, producing great outcomes, accelerating the cycle, and achieving a high compound economy. The turnover number (TON) and turnover recurrence (TOF) of polysubstituted quinolines have been determined. This cycle will likewise run on a gram scale, demonstrating the chance of modern applications.
Similar content being viewed by others
Introduction
Visible light, as a rich, effectively open, and sustainable clean energy source has ignited a ton of interest in supporting reactant natural blend reactions [1,2,3,4,5,6,7,8,9]. Noticeable light helped responses, in contrast with conventional manufacturing methods, meet the necessities of moderate reaction conditions, simplicity of activity, and ecological agreeableness. Most natural atoms, then again, can’t retain noticeable light, and the response must be supported by utilizing the right photocatalyst.
Organic dyes, which have shown identical photocatalytic interest in a couple of cycles, were utilized as an engaging choice to change metal buildings [10,11,12,13], attributable to their minimal expense and absence of poisonousness. Fluorescein has as of late been involved by Chu and colleagues for extremist buildup cyclization of benzimidazoles utilizing apparent light catalysis [14].
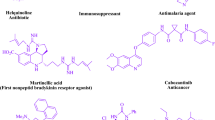
Quinolines have many pharmacological and natural impacts [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Various systems are accessible [44,45,46,47,48,49,50,51,52,53,54,55,56,57]. These treatments brought about a huge number of occurrences. Restrictions on the utilization of metal impetuses, cruel response conditions, costly reagents, monotonous workup, low yield, extended response time, and natural peril are instances of engineered rules.
Due to the previously mentioned challenges and our anxiety about harmless to the ecosystem techniques, most researchers have been charmed by the quest for straightforward, effective, and harmless to the ecosystem ways to deal with support natural responses in green conditions. Given the prior worries and our goal to create polysubstituted quinolines, reading on naturally safe impetuses for the right blend of nitrogen heterocyclic buildings under green conditions is crucial. The utilization of a non-metallic natural color, fluorescein, in the previously mentioned photochemical blending process is given another job in this review. Photoinduced states delivered by fluorescein have been displayed to work as an impetus for photochemically revolutionary producing polysubstituted quinolines. Apparent light guides the Friedländer hetero-annulation [58] of 2-aminoaryl ketone and α-methylene carbonyl compound in ethanol at room temperature and in an air climate. This is a fruitful one-pot response that was completed in an exceptionally proficient, unobtrusive, and direct way.
Experimental
General technique
A combination of 2-aminoaryl ketone (1, 1.0 mmol) and α-methylene carbonyl compound (2, 1.5 mmol) in EtOH (3 mL) was added fluorescein (0.5 mol%) and mixed at encompassing temperature under white LED (12 W) light. Attention was utilized to follow the response’s turn of events, with the eluent being n-hexane/ethyl acetate (3:2). The subsequent material was screened and washed with water after the response, and the rough strong was solidified again from ethanol to create the unadulterated substance without extra purging. If we could manufacture the aforementioned compounds using gram scale methods we would want to test if we could scale up to the level required for pharmaceutical process R&D. One experiment used 50 mmol of 2-aminobenzophenone and 75 mmol of acetylacetone. Using a typical filtration technique, the product was collected after only 8 min of the reaction. This material has a 1HNMR spectrum that suggests that it is spectroscopically pure. The products were ordered after spectroscopic information was analyzed. The products were ordered in the wake of looking at spectroscopic information (1HNMR). 1HNMR files for compounds 3c and 3k are provided in the Additional file 1.
Results and discussion
To start, Table 1 sums up the consequences of a review into the superior reactivity of 2-aminobenzophenone (1.0 mmol) and dimedone (1.5 mmol) in EtOH (3 mL) after the light at room temperature. A follow measure of 3a was found at room temperature for 45 min without the utilization of a photocatalyst (Additional file 1: Table S2). Fluorescein, Na2 eosin Y, phenanthrenequinone, erythrosin B, alizarin, rose Bengal, 9H-xanthen-9-one, acenaphthenequinone, riboflavin, xanthene, and rhodamine B were explored under comparative circumstances. In yields going from 48–96%, the improvement of this occasion and the formation of the matching item 3a were seen agreeably. Fluorescein outflanked other organophotocatalysts in this cycle, as per our discoveries. The yield was expanded to 96% by adding 0.5 mol% fluorescein. What’s more, item yields in DMF, toluene, THF, DMSO, CHCl3, and CH2Cl2 were low (Additional file 1: Table S3). The yield and pace of the response rose as the response progressed in H2O/EtOH, H2O, MeOH, solvent-free, CH3CN, EtOAc. In EtOH, the response went extremely well, giving 96% under similar circumstances. The yield was assessed under different lighting conditions and displayed to rise to some degree because of white light. A control exploration uncovered that even without a light source, a hint of the synthetic could be recognized. The revelation underlines the significance of fluorescein and apparent light in the item’s turn of events. Also, the best conditions were found by fluctuating the white LED illumination powers. Additional file 1: Table S3 shows that when white 12 W LED illumination was utilized, the best outcomes were gotten. This approach can be utilized on different substrates, as exhibited in Table 2 and Fig. 1. (More data is provided in Additional file 1: Tables S2 and S3).
Table 3 likewise remembers data for turnover number (TON) and frequency of turnover (TOF). The higher the TON and TOF mathematical qualities are, the less catalyst is used and the higher the yield, and the catalyst becomes more effective as the value grows.
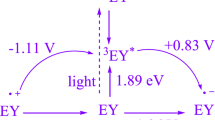
The preferred mechanism is denoted in Fig. 2. The visible light can be changed in part by the application of more energy to speed up this reaction. This fluorescein, according to earlier studies [14], uses visible light as a source of renewable energy to build acceptable catalytic methods employing a single-electron transfer (SET) pathway. Through an energy transfer (EnT) between Fl*− and -methylene carbonyl compound 2 regenerates the ground-state fluorescein and the intermediate A. When this radical anion A is nucleophilically added to 2-aminoaryl ketone 1, a reactive intermediate B is formed. Then, a SET pathway promotes visible light-triggered fluorescein*, which produces the cation radical C. The cyclized dehydrated is then added for a total of 3.
The photoredox cycle is started out whilst dye inside the ground state is irradiated with visible light to provide the high-energy excited state of dye (Dye*). The system of seen mild photoredox catalysis is supplied by the use of separate paths from dye inside the excited state (Dye*). Within the presence of a sacrificial electron acceptor, Dye* reductive’s belongings can be hired. In different phrases, Dye* leads the unconventional cation species of Dye as an electron donor. Within the presence of a sacrificial electron donor, Dye* also works as an electron acceptor [59].
Conclusion
At long last, the photoinduced conditions of fluorescein-determined go about as an impetus for photochemically combining polysubstituted quinolines by extremist Friedländer hetero-annulation of 2-aminoaryl ketone and α-methylene carbonyl compound in EtOH at a surrounding temperature in an air environment. This study lays out a clever capability for photochemically combining fluorescein, a non-metallic natural color that is economically accessible and reasonable while creating great outcomes, accelerating the interaction, and achieving a high iota economy. This is an effective one-pot response that was acted in an exceptionally proficient, moderate, and direct way.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Supplementary Information file.
References
Mohamadpour F. Catalyst-free, visible light irradiation promoted synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones in aqueous ethyl lactate. J Photochem Photobiol, A. 2021;407:113041.
Mohamadpour F. Visible light irradiation promoted catalyst-free and solvent-free synthesis of pyrano[2,3-d]pyrimidine scaffolds at room temperature. J Saudi Chem Soc. 2020;24:636–41.
Mohamadpour F. Catalyst-free and solvent-free visible light irradiation-assisted Knoevenagel-Michael cyclocondensation of aryl aldehydes, malononitrile, and resorcinol at room temperature. Monatshefte für Chemie-Chem Mon. 2021;152:507–12.
Wang Z, Wang L, Wang Z, Li P, Zhang Y. A practical synthesis of α-bromo/iodo/chloroketones from olefins under visible-light irradiation conditions. Chin Chem Lett. 2021;32:429–32.
Xie X, Wang L, Zhou Q, Ma Y, Wang ZM, Li P. Visible-light-induced novel cyclization of 2-(2-(arylethynyl) benzylidene)-malononitrile derivatives with 2, 6-di (tert-butyl)-4-methylphenol to bridged spirocyclic compounds. Chin Chem Lett. 2022;33:5069–73.
Ma Y, Gao F, Xiao W, Li N, Li S, Yu B, Chen X. Two transition-metal-modified Nb/W mixed-addendum polyoxometalates for visible-light-mediated aerobic benzylic C-H oxidations. Chin Chem Lett. 2022;33:4395–9.
Xiang P, Sun K, Wang S, Chen X, Qu L, Yu B. Direct benzylation reactions from benzyl halides enabled by transition-metal-free photocatalysis. Chin Chem Lett. 2022;33:5074–9.
Ma CH, Zhao L, He X, Jiang YQ, Yu B. Visible-light-induced direct 3-ethoxycarbonylmethylation of 2-aryl-2H-indazoles in water. Org Chem Front. 2022;9:1445–50.
Huang X, Liu S, Liu G, Tao Y, Wang C, Zhang Y, Li Z, Wang H, Zhou Z, Shen G, Xue Z. An Unprecedented 2-fold interpenetrated lvt open framework built from Zn6 ring seamed trivacant polyoxotungstates used for photocatalytic synthesis of pyridine derivatives. Appl Catal B. 2023;323:122134.
Mohamadpour F. New role for photoexcited organic dye, Na2 eosin Y via the direct hydrogen atom transfer (HAT) process in photochemical visible-light-induced synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones under air atmosphere. Dyes Pigm. 2021;194:109628.
Mohamadpour F. Synthesis of polyfunctionalized dihydro-2-oxypyrroles catalyzed by 1, 2, 3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) as a novel donor-acceptor fluorophore. Sci Rep. 2022;12:16911.
Mohamadpour F. Methylene blue as a photo-redox catalyst: the development synthesis of tetrahydrobenzo[b]pyran scaffolds via a single-electron transfer/energy transfer. Front Chem. 2022;10:934781.
Mohamadpour F. A new role for photoexcited Na2 eosin Y as direct hydrogen atom transfer (HAT) photocatalyst in photochemical synthesis of dihydropyrano[2,3-c]pyrazole scaffolds promoted by visible light irradiation under air atmosphere. J Photochem Photobiol, A. 2021;418:113428.
Li Z, Song H, Guo R, Zuo M, Hou C, Sun S, He X, Sun Z, Chu W. Visible-light-induced condensation cyclization to synthesize benzimidazoles using fluorescein as a photocatalyst. Green Chem. 2019;21:3602–5.
Heilbronn ED. Inhibition of cholinesterases by tetrahydroaminacrin. Acta Chem Scand. 1961;15:1386–90.
Maayani S, Weinstein H, Ben-Zvi N, Cohen S, Sokolovsky M. Psychotomimetics as anticholinergic agents—I: 1-cyclohexylpiperidine derivatives: anticholinesterase activity and antagonistic activity to acetylcholine. Biochem Pharmacol. 1974;23:1263–81.
Srivastava SK, Chauhan PM, Bhaduri AP, Fatima N, Chatterjee RK. Quinolones: novel probes in antifilarial chemotheraphy. J Med Chem. 2000;43:2275–9.
Muscia GC, Bollini M, Carnevale JP, Bruno AM, Asis SE. Microwave-assisted Friedländer synthesis of quinolines derivatives as potential antiparasitic agents. Tetrahedron Lett. 2006;47:8811–5.
Maguire MP, Sheets KR, McVety K, Spada AP, Zilberstein A. A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J Med Chem. 1994;37:2129–37.
Suzuki M, Iwasaki H, Fujikawa Y, Kitahara M, Sakashita M, Sakoda R. Synthesis and biological evaluations of quinoline-based HMG-CoA reductase inhibitors. Bioorg Med Chem. 2001;9:2727–43.
Desai C, Macchi D, Patel D. Quinoline derivatives as antitubercular. Indian J Chem Sect B Org Chem Incl Med Chem. 1996;35:871–3.
Castelli MV, Kouznetsov VV, López SN, Sortino M, Enriz RD, Ribas JC, Zacchino S. In vitro antifungal activity of new series of homoallylamines and related compounds with inhibitory properties of the synthesis of fungal cell wall polymers. Bioorg Med Chem. 2003;11:1531–50.
Singh M, Singh MP, Ablordeppey S. In vitro studies with liposomal cryptolepine. Drug Dev Ind Pharm. 1996;22:377–81.
Ebisu H, Nishikawa M, Tanaka M, Okazoe T, Morizawa Y, Shinyama H, Nakamura N. Pharmacologic profiles of GA0113, a novel quinoline derivative angiotensin II AT1-receptor antagonist. J Cardiovasc Pharmacol. 1999;34:526–32.
Muruganantham N, Sivakumar R, Anbalagan N, Gunasekaran V, Leonard JT. Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol Pharm Bull. 2004;27:1683–7.
Roma G, Di Braccio M, Grossi G, Mattioli F, Ghia M. 1, 8-Naphthyridines IV. 9-Substituted N, N-dialkyl-5-(alkylamino or cycloalkylamino)[1,2,4] triazolo [4,3-a][1,8] naphthyridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur J Med Chem. 2000;35:1021–35.
Savini L, Chiasserini L, Pellerano C, Filippelli W, Falcone G. Synthesis and pharmacological activity of 1,2,4-triazolo [4,3-a] quinolines. Il Farmaco. 2001;56:939–45.
Johnson JV, Rauckman BS, Baccanari DP, Roth B. 2, 4-Diamino-5-benzylpyrimidines and analogs as antibacterial agents. 12. 1, 2-Dihydroquinolylmethyl analogs with high activity and specificity for bacterial dihydrofolate reductase. J Med Chem. 1989;32:1942–9.
Chen YL, Fang KC, Sheu JY, Hsu SL, Tzeng CC. Synthesis and antibacterial evaluation of certain quinolone derivatives. J Med Chem. 2001;44:2374–7.
Sadana AK, Mirza Y, Aneja KR, Prakash OM. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo [4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo [4,3-a] quinolines as antibacterial agents. Eur J Med Chem. 2003;38:533–6.
Kidwai M, Bhushan KR, Sapra P, Saxena RK, Gupta R. Alumina-supported synthesis of antibacterial quinolines using microwaves. Bioorg Med Chem. 2000;8:69–72.
Kayirere MG, Mahamoud A, Chevalier J, Soyfer JC, Crémieux A, Barbe J. Synthesis and antibacterial activity of new 4-alkoxy, 4-aminoalkyl and 4-alkylthioquinoline derivatives. Eur J Med Chem. 1998;33:55–63.
Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–92.
Perzyna A, Klupsch F, Houssin R, Pommery N, Lemoine A, Hénichart JP. New benzo [5,6] pyrrolizino [1,2-b] quinolines as cytotoxic agents. Bioorg Med Chem Lett. 2004;14:2363–5.
Lamazzi C, Léonce S, Pfeiffer B, Renard P, Guillaumet G, Rees CW, Besson T. Expeditious synthesis and cytotoxic activity of new cyanoindolo [3,2-c] quinolines and benzimidazo [1,2-c] quinazolines. Bioorg Med Chem Lett. 2000;10:2183–5.
Kaczmarek Ł, Peczyńska-Czoch W, Osiadacz J, Mordarski M, Sokalski WA, Boratyński J, Marcinkowska E, Glazman-Kuśnierczyk H, Radzikowski C. Synthesis, and cytotoxic activity of some novel indolo [2,3-b] quinoline derivatives: DNA topoisomerase II inhibitors. Bioorg Med Chem. 1999;7:2457–64.
Martirosyan AR, Rahim-Bata R, Freeman AB, Clarke CD, Howard RL, Strobl JS. Differentiation-inducing quinolines as experimental breast cancer agents in the MCF-7 human breast cancer cell model. Biochem Pharmacol. 2004;68:1729–38.
Kolokythas G, Pouli N, Marakos P, Pratsinis H, Kletsas D. Design, synthesis and antiproliferative activity of some new azapyranoxanthenone aminoderivatives. Eur J Med Chem. 2006;41:71–9.
Heitsch H. Non-peptide antagonists and agonists of the bradykinin B2 receptor. Curr Med Chem. 2002;9:913–28.
Dubé D, Blouin M, Brideau C, Chan CC, Desmarais S, Ethier D, Falgueyret JP, Friesen RW, Girard M, Girard Y, Guay J. Quinolines as potent 5-lipoxygenase inhibitors: synthesis and biological profile of L-746,530. Bioorg Med Chem Lett. 1998;8:1255–60.
Ma ZZ, Hano Y, Nomura T, Chen YJ. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles. 1997: 541–6
Ma ZZ, Hano Y, Nomura T, Chen YJ. Alkaloids and phenylpropanoids from Peganum nigellastrum. Phytochemistry. 2000;53:1075–8.
Ma ZZ, Hano Y, Nomura T, Chen YJ. Two new quinazoline-quinoline alkaloids from Peganum nigellastrum. Heterocycles. 1999;8:1883–9.
Shirini F, Yahyazadeh A, Mohammadi K, Khaligh NG. Solvent-free synthesis of quinoline derivatives via the Friedländer reaction using 1, 3-disulfonic acid imidazolium hydrogen sulfate as an efficient and recyclable ionic liquid catalyst. C R Chim. 2014;17:370–6.
Lekhok KC, Bhuyan D, Prajapati D, Boruah RC. Zinc triflate: a highly efficient reusable catalyst in the synthesis of functionalized quinolines via Friedlander annulation. Mol Divers. 2010;14:841–6.
Reddy BP, Iniyavan P, Sarveswari S, Vijayakumar V. Nickel oxide nanocompounds catalyzed synthesis of poly-substituted quinolines via Friedlander hetero-annulation reaction. Chin Chem Lett. 2014;25:1595–600.
Zolfigol MA, Salehi P, Ghaderi A, Shiri M. A catalytic and green procedure for Friedlander quinoline synthesis in aqueous media. Catal Commun. 2007;8:1214–8.
Wu J, Xia HG, Gao K. Molecular iodine: a highly efficient catalyst in the synthesis of quinolines via Friedländer annulation. Org Biomol Chem. 2006;4:126–9.
Zhang XL, Wang QY, Sheng SR, Wang Q, Liu XL. Efficient Friedländer synthesis of quinoline derivatives from 2-aminoarylketones and carbonyl compounds mediated by recyclable PEG-supported sulfonic acid. Synth Commun. 2009;39:3293–304.
Shaabani A, Soleimani E, Badri Z. Triflouroacetic acid as an efficient catalyst for the synthesis of quinoline. Synth Commun. 2007;37:629–35.
Garella D, Barge A, Upadhyaya D, Rodriguez Z, Palmisano G, Cravotto G. Fast, solvent-free, microwave-promoted friedländer annulation with a reusable solid catalyst. Synth Commun. 2009;40:120–8.
Narasimhulu M, Reddy TS, Mahesh KC, Prabhakar P, Rao CB, Venkateswarlu Y. Silica supported perchloric acid: a mild and highly efficient heterogeneous catalyst for the synthesis of poly-substituted quinolines via Friedländer hetero-annulation. J Mol Catal A: Chem. 2007;266:114–7.
Reddy BS, Venkateswarlu A, Reddy GN, Reddy YR. Chitosan-SO3H: an efficient, biodegradable, and recyclable solid acid for the synthesis of quinoline derivatives via Friedländer annulation. Tetrahedron Lett. 2013;54:5767–70.
Dabiri M, Baghbanzadeh M, Nikcheh MS. Oxalic acid: an efficient and cost-effective organic catalyst for the Friedländer quinoline synthesis under solvent-free conditions. Monatshefte für Chemie-Chem Mon. 2007;138:1249–52.
Yadav JS, Reddy BS, Sreedhar P, Rao RS, Nagaiah K. Silver phosphotungstate: a novel and recyclable heteropoly acid for Friedländer quinoline synthesis. Synthesis. 2004;2004:2381–5.
Khaligh NG, Mihankhah T, Johan MR. Synthesis of quinoline derivatives via the Friedländer annulation using a sulfonic acid functionalized liquid acid as dual solvent-catalyst. Polycyclic Aromat Compd. 2020;40:1223–37.
Mohamadpour F. The development of Friedländer heteroannulation through a single electron transfer and energy transfer pathway using methylene blue (MB+). Sci Rep. 2022;12:7253.
Friedlaender P. Ueber o‐Amidobenzaldehyd. Berichte der deutschen chemischen Gesellschaft.
Miyabe H. Organic reactions promoted by metal-free organic dyes under visible light irradiation. In: Visible-light photocatalysis of carbon-based materials. 2017; IntechOpen.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of the Apadana Institute of Higher Education.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FM conceived and designed the experiments. FM conducted the experiments and interpreted the results. FM participated in analyzing the data and writing the paper. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Figure S1. 1HNMR Spectrum of compound of 3c. Figure S2. 1HNMR Spectrum of compound of 3K. Table S1. Comparison of 1HNMR data. Table S2. Photocatalyst optimization table. Table S3. Solvent and visible light optimization table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohamadpour, F. Visible-light-driven radical Friedländer hetero-annulation of 2-aminoaryl ketone and α-methylene carbonyl compound via organic dye fluorescein through a single-electron transfer (SET) pathway. BMC Chemistry 16, 116 (2022). https://doi.org/10.1186/s13065-022-00910-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-022-00910-1