Abstract
Ludwigia adscendens subsp. diffusa (Onagraceae), an important aquatic herb widely distributed in the Nile River and canals in Egypt. The goal of the current study is to investigate the phytochemical composition of L. adscendens aerial parts n-butanol and ethyl acetate fractions and screening of its biological activities. Phytochemical investigation of L. adscendens resulted in the isolation and purification of eleven compounds belonging to flavonoids, saponins, triterpenoids, and oligosaccharides, of which one compound was identified as new using different spectroscopic techniques. Compound 2 was identified as a new compound namely, 3-O-[β-D-glucopyranoside (1 → 4) α-L-rhamnopyranoside]-23-O-feruloyl-hederagenin-28-O-[α-L-rhamnopyranoside (1 → 2) β-D-glucopyranoside], along with other 10 well know compounds. Furthermore, antidiabetic, hepatoprotective and cytotoxic activities of n-butanol and ethyl acetate fractions were investigated in vitro, revealing that ethyl acetate fraction was the most active as antidiabetic (IC50 = 62.3 µg/mL), hepatoprotective (IC50 = 80.75 µg/mL), and cytotoxic against human prostate cancer cell line (IC50 = 52.2 µg/mL). Collectively, L. adscendens aerial part is rich with a myriad of phytochemicals with potential health benefits.
Similar content being viewed by others
Introduction
Plants play a pivotal role in drug development process owing to its richness in bioactive phytochemicals with potential health benefits [1, 2]. Ludwigia L. (family Onagraceae) is an important pantropic genus widely distributed in South and North America and comprises about 82 species of flowering plants [3]. Several traditional uses including antidiabetic [4], antioxidant, antimicrobial [5], antidiarrheal [6], and anti-inflammatory activity [7] were reported for Ludwigia species [8]. Genus Ludwigia was reported for its richness in different phytochemicals such as flavonoids, saponins, phenolic compounds, and triterpenes [3, 8]. L. adscendens subsp. diffusa (Forssk.)P.H.Raven also known as Ludwigia stolonifera (Guill. & Perr.)P.H.Raven is a dominant aquatic macrophytes distributed over canals and drains branching from the Nile River in Egypt [9]. L. adscendens is important in water remediation by improving water quality by eliminating various toxic pollutants [9, 10]. With the continuous interest in the identification of new phytochemicals with novel structural and biological properties, this study was undertaken for the isolation and purification of different phytochemicals from L. adscendens aerial parts by different chromatographic techniques. Eleven compounds were isolated and identified from L. adscendens aerial parts n-butanol and ethyl acetate fractions. Moreover, the biological activity of both fractions and aerial parts total extracts were investigated showing potential antidiabetic, hepatoprotective, and cytotoxic activities.
Methods/experimental
General
NMR analysis was performed using JOEL GX-400 (400 and 100 MHz for 1H and 13C NMR), NMR Laboratory, Faculty of Pharmacy, Cairo University, Cairo, Egypt. All samples were analyzed in DMSO-d6 and CD3OD-d4 solvent. Thin layer chromatography (TLC) was performed on silica gel 60 F254 precoated aluminium sheets (20 × 20, 0.2 mm thikness) and cellulose precoated aluminium sheets (20 × 20, 0.2 mm thikness), (E. Merck, Darmstadt, Germany). Paper Chromatograpy (PC) was performed by Whatmann No.1 (Whatmann Ltd., Maidstone, Kent, England). Aluminium chloride reagent (1% in ethanol) for flavonoids and Ferric chloride reagent (1% in ethanol) were used for spots visualization. Solvent systems S1: chloroform: methanol: water ((70:30:5) & (70:30:2) v/v/v), S2: Chloroform: methanol ((80:20),(70:30)&(50:50) v/v), S3: n-butanol: acetic acid: water (BAW) (4:1:5 v/v/v, upper layer) and S4: Acetic acid: water (15:85 v/v). HR-ESI/MS analyses were carried out using a Bruker LC micro-Q-TO-F mass spectrometer, Faculty of Pharmacy, Ain Shams University, Egypt. Additionally, Microplate reader (SunRise, Tecan, USA), 96-well microtiter plates (Greiner, Germany), inverted microscope (Olympus 1 × 70, Tokyo, Japan) and Jouan® centrifuge, 1,000 – 10,000 r.p.m., France for biological studies which were performed at theMycology and Biotechnology Reginal Center, Al-Azhar University.
Plant material
Ludwigia adscendens subsp. diffusa (Forssk.) P.H.Raven syn. Ludwigia stolonifera (Guill. & Perr.) P.H.Raven (Onagraceae) aerial parts were collected from the Nile River et al.-Qanater Al-Khayriyah, El Qulyoubia governorate, Egypt, at 54VM + 52 in September 2019. The plant was botanically identified by Prof. Dr. Rim Hamdy, Botany Department, Faculty of Science, Cairo University, Egypt. A voucher specimen has been deposited at Pharmacognosy Department, Faculty of Pharmacy, Helwan university No = 31Lus1/2022. The air dried coarsely divided aerial parts (1050 g) were macerated in 5 L of 100% methanol with occasional stirring at room temperature and the process was repeated three times (3 × 5 L) till exhaustion. The methanolic extract was concentrated and dried under reduced pressure at 50 οC to give dry total extract (170 g).
Chemical reagents
Quercetin, myricetin and monosaccharaides standards were obtained from Sigma/Aldrich, USA. Dimethylsulphoxide (DMSO) was provided from Sigma, St. Louis, CA, USA. Acarbose, silymarin, doxorubicin, PC-3 cells (human prostate cancer cell line) were obtained from The Regional Center for Mycology and Biotechnology, Al-Azhar University, Egypt.
Extraction and isolation
The dried aerial parts (1050 g) of L. adscendens were macerated in 100% methanol (3 × 3L), to yield concentrated methanol extract (170 g). The dried residue (150 g) was reconstituted in 250 ml distilled water and sequentially partitioned and fractionated using different immiscible solvents (petroleum ether, chloroform, ethyl acetate and n-butanol solvents). The ethyl acetate fraction (35 g) was subjected to silica G 60 in a glass column (3 × 1.5 mm dimensions) using a step gradient chloroform and methanol mixtures. The fractions were investigated and collected according to their similarities on TLC cellulose plates using S4 solvent system and ammonia spray reagent to afford 10 main collective fractions. Fraction-III (15 g) eluted with 80% CHCl3/MeOH, was added on sephadex sub-column and eluted with BAW to afford five main sub-fractions-(1–5) according to TLC cellulose plates. Sub-fraction-3 (3 g) was further purified on sephadex sub-column to afford one pure compound 1 (15 mg). Sub-fraction-4 (6 g) was subjected to more purification on sephadex sub-column to afford two pure compounds 2 (30 mg) and 3 (20 mg). Fraction-IV (12 g) eluted with 70% CHCl3/MeOH, was further purified on sephadex column and eluted with BAW to yield five main sub-fractions according to similarity on TLC cellulose plates. Sub fractions-a (4 g) was subjected to more purification on sephadex sub-column to afford two pure compounds 4 (15 mg), and 5 (10 mg). Sub-fraction-b (3 g) was purified using sephadex LH-20 sub-column to afford one pure compound 6 (10 mg). The n-butanol fraction (35 g) was mixed with 50 ml MeOH and poured on 250 ml acetone to yield acetone precipitate (25 g) which was subjected to silica gel G 60 in a glass column using step gradient chloroform and menthol mixtures with increasing polarity from 100% CHCl3 to 100% MeOH for elution. Fractions were investigated and collected according to TLC silica plates using different solvent system and spray reagents. Fraction-III (9 g) eluted with 80% CHCl3/MeOH, was subjected to silica gel sub-column and elution with CHCl3/MeOH to afford five main sub-fractions which were collected according to TLC silica plates. Sub-fraction-A (2 g) was purified using a silica gel sub-column to afford one pure compound 7 (10 mg). Sub-fractions-B (5 g) was subjected to more purification by silica gel sub-column to afford two pure compounds 8 (15 mg) and 9 (10 mg). Fraction-IV (11 g) eluted with 70% CHCl3/MeOH, was further purified on silica sub-column and elution with CHCl3/MeOH to afford four main sub-fractions. Sub-fraction-C (4 g) was then purified by another silica sub-column to yield two pure compounds 10 (15 mg) and 11 (10 mg).
Antidiabetic activity
The antidiabetic activity of L. adscendens aerial parts fractions against acarbose was investigated in vitro using α-glucosidase-inhibitory assay as previously mentioned [11]. Briefly, in a 96-well plates a mixture of 50 μL phosphate buffer (100 mM, pH = 6. 8), 10 μL α-glucosidase (1U/mL), and 20 μL of each concentration (sample and standard) was pre-incubated for 15 min at 37 ºC. 20 μL of p-Nitrophenol (5 mM) was further added with incubation for 20 min at 37 ºC. 50 μL of Na2 CO3 (0.1 M) was added and absorbance was measured at 405 nm using Multiplate Reader. The percentage inhibition was calculated using the formula.
Where, (As) the absorbance in the presence of extract, (Ac) is the absorbance of control.
Hepatoprotective activity
The hepatoprotective effect of L. adscendens aerial part was tested in vitro [12]. In brief, 50 μL of MTT (5 mg/mL) was added to each well containing 100 μL rpm hepatocyte suspension. The plates were incubated in the dark at 37 ˚C for an additional 4 h in 5% CO2 atmosphere. 150 μL DMSO was added and absorbance was measured at 570 nm with a microplate reader. The results were expressed as percentage of viability calculated as [(ODt/ODc)] × 100%. The 50% Effective concentration (EC50) was estimated from graphic plots of the dose–response curve for each conc using Graphpad Prism software and the following equation.
Cytotoxicity activity
Cytotoxic activity of L. adscendens aerial part fractions were tested in vitro using MTT cell viability assay as previously described [13]. Briefly, in each well plate 100 µL of fresh culture RPMI 1640 medium without phenol red then 10 µL of the 12 mM MTT stock solution (5 mg of MTT in 1 mL of PBS) were added to each well. An 85 µL aliquot of the incubated media was replaced by 50 µL of DMSO and incubated at 37 ºC for 10 min. The optical density was measured at 590 nm with the microplate reader to determine the number of viable cells and the percentage of viability was calculated as the percentage of cell survival was calculated as follows:
The 50% inhibitory concentration (IC50) was estimated from graphic plots of the dose response curve for each concentration using Graphpad Prism software [14].
Results and discussion
Characterisation of isolated compounds
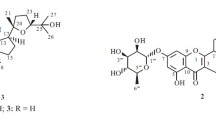
Investigation of L. adscendens aerial parts ethyl acetate and n-butanol fractions resulted in isolation of eleven compounds of which six compounds (1–6)were identified in ethyl acetate versus five compounds (7–11)from n-butanol fraction (Fig. 1). Among the isolated compounds, compound 2 was identified as a novel natural compound along with 10 known compounds. Previously isolated compounds included are octyl gallate (1)[15], 23-O-Coumaroyl-hederagenin-28-O-β-D-glucopyranoside (3) [16], quercetin-3-O-glucoside (4) [17], quercetin 3-O-α-L-rhamnoside-2''-(4'''-O-n-pentanoyl)-gallate (5) [17], myricetin-3-O-α-L-rhamnopyranoside (6) [18], hederagenin (7) [19], α-D-tetraglucoside (α-D-glucopyranosyl-(2a → 1b)-O-α-D-glucopyranosyl-(2b → 1c)-O-α-D-glucopyranosyl-(2c → 1d)-O-α-D-glucopyranoside) (8) [20], α-D-pentaglucoside (α-D-glucopyranosyl-(2a → 1b)-O-D-glucopyranosyl-(2b → 1c)-O-α-D-glucopyranosyl-(2c → 1d)-O-α-D-glucopyranosyl-(2d → 1e)-O-α-Dglucopyranoside) (9), α-D-hexaglucoside (α-D-glucopyranosyl-(2a → 1b)-O-D-glucopyranosyl-(2b → 1c)-O-α-D-glucopyranosyl-(2c → 1d)-O-α-D-glucopyranosyl-(2d → 1e)-O-α-D-glucopyranosyl-(2e → 1f)-O-α-D-glucopyranoside) (10), and α-D-heptaglucoside (α-D-glucopyranosyl-(2a → 1b)-O-D-glucopyranosyl-(2b → 1c)-O-α-D-glucopyranosyl-(2c → 1d)-O-α-D-glucopyranosyl-(2d → 1e)-O-α-D-glucopyranosyl-(2e → 1f)-O-α-D-glucopyranosyl-(2f → 1 g)-O-α-D-glucopyranoside) (11).
Compound 2 was obtained as a white amorphous powder (15 mg), with Rf = 0.35 on silica gel TLC plate, it gave violet color with 10% conc. H2SO4 spray reagent. All 1H NMR and 13C NMR spectroscopic data are summarized in (Table 1). The 1H NMR spectrum of compound 2 (Additional file 1: Fig. S1-S2) showed the presence of six singlet signals at δ ppm 0.81, 0.89, 0.79, 0.92, 0.85 and 0.87 corresponding to the methyl groups at C-24, C-25, C-26, C-27, C-29 and C-30, respectively. Moreover, the presence of a triplet signal at δ ppm 3.44 corresponding to H-3 along with the presence of a broad triplet at δ 5.25 ppm attributed to H-12 of a tri-substituted olefinic bond revealed the presence of a triterpene skeleton [21]. The aromatic region of the 1HNMR spectrum of compound 2 displayed the characteristic signals of a feruloyl moiety appearing at δ 7.22 (d), 6.82 (d), 7.4 ppm (dd) for H-2', H-5'and H-6', respectively, alongside the olefinic proton signals appearing as doublet at δ7.61 and 6.28 ppm for H-7'and H-8', respectively. The proton signal of methoxy group appeared as a singlet at δ 3.25 ppm. The 1H NMR spectrum of compound 2 showed four signals corresponding to anomeric protons at δ 5.49 (brs), 5.21 (d, J = 7.9), 5.42 (d, J = 7.9) and 5.37 (s) assigned for H-1'', H-1''', H-1'''', and H-1''''', respectively, revealing the presence of four sugar moieties (two β-D-glucoside and two α-L-rhamnoside) attached to the aglycone at different positions. Moreover, the characteristic methyl signals of rhamnoside moieties were recorded at δ ppm 1.05 (d, J = 6.1) and 1.01 (d, J = 6.2). The remaining proton signals of sugars were recorded as typical ranging from δ 3.20 to 4.13 ppm. The 13C NMR spectrum of compound 2 (Additional file 1: Fig. S3-S4) displayed the characteristic 30 carbon signals of oleanane-type triterpene moiety matched with hederagenin structure. The 13CNMR spectrum of compound 2 showed two key signals for olefinic carbons C-12 at δ 121.57 ppm, C-13 at δ 144.98 ppm and a carbonyl carbon C-28 at δ 178 ppm was detected. The downfield shift of C-23 at δ 67.57 ppm indicated its substitution with hydroxyl group. Moreover, 13C NMR spectrum showed the characteristic 9 carbon signals of ferulic acid at δ ppm 128.47, 108.12, 145.15, 148.37, 115.10, 121.69, 144.92, 115.14 and 167.96 for (C-1' to 9'), respectively alongside carbon signal of methoxy group appearing at δ ppm 55.15. The presence of the feruloyl moiety at C-23 in the aglycone was confirmed by downfield shift of H-23 at δ ppm 3.79 (d) and C-23 carbon at δ ppm 67.75 which coincided with previous literature. 13C NMR chemical shifts exhibited four anomeric signals at δ C104.52, 98.45, 93.37 and 102.13 corresponding to C-1'', C-1''', C'''' and C-1''''', respectively indicating the presence of four sugar moieties and another two carbon signals at δ18.62 and 18.62 ppm for C-6'' and C-6''''' respectively, which confirm the presence of two β-D-glucoside and two α-L-rhamnoside moieties. The bond of sugar moiety at C-28 and C-3 confirmed by spectral data of carbon at δ178.21 and 86.62 ppm and in accordance with literature [22]. The aforementioned 1H and 13CNMR data were finally confirmed using 2D correlations (Fig. 2) viz, COSY and HMBC (Additional file 1: Fig. S5 and S6). The HMBC spectrum of compound 2 showed a direct 2 J correlation between H-23 and H-8' with C-9' confirming feruloyl moiety attachment position to the aglycone part, the direct 2 J correlation of H-1'' and H-2'' with C-3 (δ 86.62) confirmed the of rhamnose moiety directly at C-3 of the aglycone part, whereas a direct 2 J correlation between H-1''' with C-4'' (δ 81.87) confirming the bond of glucose moiety to C-4'' of the rhamnose moiety. On the other hand, direct 2 J correlation between H-1''''and H-2''''with C-28 (δ 178.21) confirming bond of glucose moiety to C-28 of the aglycone moiety and a direct 2 J correlation between H-1''''' with C-2'''' (δ 77.29) confirmed bonding of rhamnose moiety at C-2'''' of the glucose moiety. A direct JH-H correlation correlation observed in the COSY spectrum was observed between the anomeric proton of rhamnose and aglycone moiety H-1'' (δ 5.49) with H-3 (δ 3.44). Other direct JH-H correlation of sugar moieties included between rhamnose moiety anomeric proton H-1'' (δ 5.49) with H-2'' (δH 4.11), and the anomeric proton of rhamnose and glucose moiety H-1''''' (δ 5.37) with H-2'''' (δ 3.56). A direct JH-H COSY correlations of feruloyl moiety between H-5' (δ 6.82) with H-6' (δ 7.4) and H-7' (δ 7.61) with H-8' (δ 6.28) of feruloyl moiety confirmed this acyl substituent in compound 2. The LC–MS showed molecular ion peak [M-H]− at m/z 1264.6241 (calcd. 1264.4470), consistent with the molecular formula to be C64H96O25− (calcd. C64H97O25). From 1 D, 2 D, and MS data and by comparison with previously reported data [21, 22] compound 2 was asigned as 3-O-[β-D- glucopyranoside (1 → 4) α-L-rhamnopyranoside]-23-O-feruloyl-hederagenin-28-O-[α-L-rhamnopyranoside(1 → 2)β-D- glucopyranoside] (Fig. 1).
Antidiabetic activity
The in vitro antidiabetic potential of L. adscendens aerial parts total extract, ethyl acetate fraction, and n-butanol fraction at different concentrations was assessed using α-glucosidase inhibition assay compared to acarbose as standard antidiabetic drug, Additional file 1: Fig. S8a. The calculated IC50 for acarbose and the different fractions of L. adscendens are listed in Table 2 and Additional file 1: Fig. S8b. The results revealed that, compared to n-butanol fraction and total extract, L. adscendens ethyl acetate fraction showed the strongest α-glucosidase enzymes inhibition effect with IC50 value of 62.30 µg/mL compared to that of acarbose (30.57 µg/mL). The activity of ethyl acetate fraction was due to it its richness in different phytochemicals such as flavonoids and triterpenoids [17]. Results were in accordance with that reported by Marzouk et al., 2007 for a potential hypoglycemic effect of L. adscendens subsp. diffusa (Jussiaea repens) aerial parts ethyl acetate extract in alloxan-induced diabetic rat model [17].
Hepatoprotective activity
Hepatoprotective activity of L. adscendens aerial parts total extract, ethyl acetate fraction, and n-butanol fraction at different concentrtions were likewise assessed on hepatocyte cell damage by MTT-assay compared to silymarin as standard hepatoprotective drug, Additional file 1: Fig. S9a. The calculated EC50 for silymarin and different fractions of L. adscendens are labelled in Table 2 and Additional file 1: Fig. S9b. The results showed that different concentrations of ethyl acetate and n-butanol fractions have moderate hepatoprotective effect against MTT hepatocyte damage with EC50 value of 80.75, 97.96 µg/mL, respectively, compared to EC50 value of 39.64 µg/mL of standard hepatoprotective drug. Such hepatoprotective potential of L. adscendens fractions is owing to its richness in antioxidant constituents such as polyphenols and flavonoids which protect hepatocyte from damage [3].
Cytotoxicity activity
The cytotoxic activity against of L. adscendens aerial parts total extract, ethyl acetate fraction, and n-butanol fraction at different concentrtions was determined in vitro against PC-3 cell line (prostate carcinoma cells). The obtained results of the different fractions were expressed as a mean value of cell growth inhibition (Additional file 1: Fig. S10a). The calculated IC50 for the different fractions are summarized in Table 2 and Additional file 1: Fig. S10b. Results showed that among tested extract and fractions, ethyl acetate fraction showed the highest cytotoxic activity against PC-3 cell line, with IC50 value of 52.2 µg/mL. The cytotoxic effect of L. adscendens was consistent with previous reports on Onagraceae species [3] such as Oenothera paradoxa revealing for a potential effect to prevent human prostate cancer cells proliferation [23].
Conclusion
Ludwigia adscendens subsp. diffusa is an important herbaceous aquatic plant in the Nile Delta region in Egypt. This study aimed towards the isolation and structural elucidation of phytochemicals from L. adscendens aerial parts ethyl acetate and n-butanol fractions. Eleven compounds were identified from ethyl acetate and n-butanol fractions of which one was identified as a novel compound assigned as 3-O-[β-D-glucopyranoside(1 → 4)α-L-rhamnopyranoside]-23-O-feruloyl-hederagenin-28-O-[α-L-rhamnopyranoside(1 → 2)β-D- glucopyranoside] (2). Biological investigation of L. adscendens aerial parts total extract, ethyl acetate fraction, and n-butanol fraction revealed that ethyl acetate fraction was most active as antidiabetic, hepatoprotective and cytotoxic. Finally, further studies are recommended to isolate phytochemicals from other classes from L. adscendens and in vivo antidiabetic and hepatoprotective studies are recommended to prove for its efficacy as revealed from this in vitro based assay to be conclusive.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Elmastas M, Erenler R, Isnac B, Aksit H, Sen O, Genc N, Demirtas I. Isolation and identification of a new neo-clerodane diterpenoid from Teucrium chamaedrys L. Nat Prod Res. 2016;30(3):299–304.
Erenler R, Telci I, Ulutas M, Demirtas I, Gul F, Elmastas M, Kayir O. Chemical constituents, quantitative analysis and antioxidant activities of E Chinacea purpurea (L.) M oench and E Chinacea pallida (N utt.) N utt. J Food Biochem. 2015;39(5):622–30.
Shawky EM, Elgindi MR, Ibrhiam HA, Baky MH. The potential and outgoing trends in traditional, phytochemical, economical, and ethnopharmacological importance of family Onagraceae: a comprehensive review. J Ethnopharmacol. 2021;281:114450.
Lin W-S, Lo J-H, Yang J-H, Wang H-W, Fan S-Z, Yen J-H, Wang P-Y. Ludwigia octovalvis extract improves glycemic control and memory performance in diabetic mice. J Ethnopharmacol. 2017;207:211–9.
Smida I, Sweidan A, Souissi Y, Rouaud I, Sauvager A, Torre F, Calvert V, Le Petit J, Tomasi S. Anti-acne, antioxidant and cytotoxic properties of Ludwigia peploides leaf extract. Int J Pharmacogn Phytochem Res. 2018;10(7):271–8.
Shaphiullah M, Bachar SC, Kundu JK, Begum F, Uddin MA, Roy SC, Khan M. Antidiarrheal activity of the methanol extract of Ludwigia hyssopifolia Linn. Pak J Pharm Sci. 2003;16(1):7–11.
Praneetha P, Reddy YN, Kumar BR. In vitro and In vivo hepatoprotective studies on methanolic extract of aerial parts of Ludwigia hyssopifolia G Don Exell. Pharmacog Mag. 2018;14(59):546.
Zhang J, Liu C, Lv Y, Wei J, Li B, Liao G, Lu R, Yang X. A pair of new isocoumarin enantiomers of Ludwigia hyssopifolia. Nat Prod Res. 2022;36(7):1749–56.
Saleh HM, Aglan RF, Mahmoud HH. Ludwigia stolonifera for remediation of toxic metals from simulated wastewater. Chem Ecol. 2019;35(2):164–78.
Baky MH, Shawky EM, Elgindi MR, Ibrahim HA. Comparative volatile profiling of Ludwigia stolonifera Aerial Parts and roots using VSE-GC-MS/MS and screening of antioxidant and metal chelation activities. ACS Omega. 2021;6(38):24788–94.
Shai L, Magano S, Lebelo S, Mogale A. Inhibitory effects of five medicinal plants on rat alpha-glucosidase: comparison with their effects on yeast alpha-glucosidase. J Med Plants Res. 2011;5(13):2863–7.
Ibrahim M, Khaja MN, Aara A, Khan AA, Habeeb MA, Devi YP, Narasu ML, Habibullah CM. Hepatoprotective activity of Sapindus mukorossi and Rheum emodi extracts: in vitro and in vivo studies. World J Gastroenterol: WJG. 2008;14(16):2566.
Eskander JY, Haggag EG, El-Gindi MR, Mohamedy MM. A novel saponin from Manilkara hexandra seeds and anti-inflammatory activity. Med Chem Res. 2014;23(2):717–24.
El Badry M, El Aasser M, El Shiekh H, Sheriff M. Evaluation of Antimicrobial, cytotoxic and larvicidal activity of Zygophyllum Coccineum North Sinai. Egypt Med Aromat Plants. 2015;4(5):214.
Latha RCR, Daisy P. Therapeutic potential of octyl gallate isolated from fruits of Terminalia bellerica in streptozotocin-induced diabetic rats. Pharm Biol. 2013;51(6):798–805.
Chang C-I, Kuo C-C, Chang J-Y, Kuo Y-H. Three new oleanane-type triterpenes from Ludwigia octovalvis with cytotoxic activity against two human cancer cell lines. J Nat Prod. 2004;67(1):91–3.
Marzouk M, Soliman F, Shehata I, Rabee M, Fawzy G. Flavonoids and biological activities of Jussiaea repens. Nat Prod Res. 2007;21(5):436–43.
Baky MH, Kamal AM, Haggag EG, Elgindi MR. Flavonoids from Manilkara hexandra and antimicrobial and antioxidant activities. Biochem Syst Ecol. 2022;100: 104375.
Abaci H, Akagac G, Nalbantsoy A, Sarikahya NB. A hederagenin-type triterpene saponin, sumbulianoside a from Cephalaria sumbuliana and its potent immunomodulatory activity against seasonal flu virus H3N2. NaT Product Res. 2021. https://doi.org/10.1080/14786419.2021.1910691.
Chung I-M, Ali M, Praveen N, Yu B-R, Kim S-H, Ahmad A. New polyglucopyranosyl and polyarabinopyranosyl of fatty acid derivatives from the fruits of Lycium chinense and its antioxidant activity. Food Chem. 2014;151:435–43.
Chang C-I, Kuo Y-H. Oleanane-type triterpenes from Ludwigia octovalvis. J Asian Nat Prod Res. 2007;9(1):67–72.
Baky MH, Gabr NM, Shawky EM, Elgindi MR, Mekky RH. A rare Triterpenoidal Saponin Isolated and Identified from Tetraena simplex (L.) Beier & Thulin (Syn. Zygophyllum simplex L.). ChemistrySelect. 2020;5(6):1907–11.
Lewandowska U, Owczarek K, Szewczyk K, Podsędek A, Koziołkiewicz M, Hrabec E. Influence of polyphenol extract from evening primrose (Oenothera paradoxa) seeds on human prostate and breast cancer cell lines. Adv Hyg Expe Med/Postepy Higieny i Medycyny Doswiadczalnej. 2014. https://doi.org/10.5604/17322693.1088036.
Acknowledgements
We would like to thank Prof. Rim Hamdy, Faculty of Science, Cairo University for her efforts in identifying the plant of study in Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study is not funded by any organization.
Author information
Authors and Affiliations
Contributions
MHB; Conceptualization, Supervision, Data curation, Investigation, Writing—review & editing. M RE; Supervision, Writing—review & editing. EMS; Data curation, Investigation, Writing original manuscript HAI; Data curation, Investigation, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The plant was botanically identified by Prof. Dr. Rim Hamdy, Botany Department, Faculty of Science, Cairo University, Egypt. A voucher specimen has been deposited at Pharmacognosy Department, Faculty of Pharmacy, Helwan university No = 31Lus1/2022.
Plant ethics
The methods in plant collection and experimentation were carried out in accordance with the guidelines prescribed by the American Society of Plant Taxonomists and adopted by the institutional research committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: Figure S1.
Magnification of 1H NMR spectrum of compound 2. Figure S2. Magnification of 1H NMR spectrum of compound 2. Figure S3. Magnification of 13C NMR spectrum of compound 2. Figure S4. Magnification of 13C NMR spectrum of compound 2. Figure S5. HMBC spectrum of compound 2. Figure S6. COSY spectrum of compound 2. Figure S7. MS spectrum of compound 2. Figure S8. (a) Hepatoprotective activity of different concentrations of silymarin and different fractions of the L. adscendens aerial parts. (b) Calculated EC50 (µg/ml) for silymarin and different fractions of L. adscendens aerial parts. Figure S9. (a) Hepatoprotective activity of different concentrations of silymarin and different fractions of the L. adscendens aerial parts. (b) Calculated EC50 (µg/ml) for silymarin and different fractions of L. adscendens aerial parts. Figure S10. (a) Cytotoxic activity of different concentrations of different fractions of L. adscendens against PC-3 cell line. (b) Calculated IC50 (µg/ml) of different fractions of L. adscendens against PC-3 cell line. Table S1. NMR Spectroscopic Data for Compound 1. Table S2. NMR Spectroscopic Data for Compounds 2, 3 and 7. Table S3. NMR Spectroscopic Data for Compounds 4, 5 and 6. Table S4. NMR Spectroscopic Data for Compounds 8, 9, 10 and 11.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baky, M.H., Elgindi, M.R., Shawky, E.M. et al. Phytochemical investigation of Ludwigia adscendens subsp. diffusa aerial parts in context of its biological activity. BMC Chemistry 16, 112 (2022). https://doi.org/10.1186/s13065-022-00909-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-022-00909-8