Abstract
The development of new antidiabetes agents is necessary to obtain optimal glycemic control and overcome its complications. Different quinazolin-4(3H)-one bearing phenoxy-acetamide derivatives (7a–r) were designed and synthesized to develop α-glucosidase inhibitors. All the synthesized derivatives were evaluated against α-glucosidase in vitro and among them, compound 7b showed the highest α-glucosidase inhibition with an IC50 of 14.4 µM, which was ∼53 times stronger than that of acarbose. The inhibition kinetic studies showed that the inhibitory mechanism of compound 7b was a competitive type towards α-glucosidase. Also, molecular docking studies analyzed the interaction between the most potent derivative and α-glucosidase. Current findings indicate the new potential of quinazolin-4(3H)-ones that could be used for the development of novel agents against diabetes mellitus.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) has become one of the important issues in recent years with the rising obesity crisis categorized as 6th most frequent cause of global mortality [1]. DM can be classified into three major types: type I DM (insulin-dependent) due to immune-mediated β cells destructions; type II DM (non-insulin-dependent) due to an insulin secretory defect and insulin resistance as well as gestational diabetes that develops during pregnancy [2]. T2DM accounts for around 90–95% of the diabetic population and has become a major health problem worldwide [3]. All three types of DM are characterized by chronic high glucose levels which stimulate the generation of ROS that has a pivotal role in diabetic complications [4] Reducing postprandial hyperglycemia could prevent and minimize the risk of micro-and macro-vascular complications [5, 6].
In this context, inhibiting the activities of carbohydrate digestive enzymes is considered effective management of T2DM to retard glucose absorption [7]. α-glucosidase is known as a key enzyme responsible for the digestion of carbohydrates situated in the brush border of the small intestine of humans so that the di- and oligosaccharides undergo hydrolysis to glucose for intestinal absorption [8, 9].
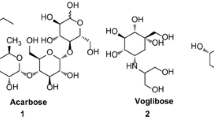
Acarbose, miglitol, and voglibose are important anti-diabetic drugs used in clinical treatment as hypoglycemic compounds [5], but the efficacy of these is drugs a matter of debate due to unwanted side effects including flatulence, meteorism, abdominal distention, and the possibility of diarrhea [10, 11]. Also, it was reported that diabetic patients can develop resistance to current regimens. As a consequence, novel inhibitors are required to improve DM treatment. Numerous studies have described the inhibition of α-glucosidase induced by triazole [12], chromene [12], pyridine [13], quinolone [3], cyanoacetohydrazide [7], and benzimidazole [14] based compounds.
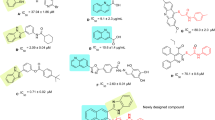
Quinazoline is a fused heterocyclic system and a core structure in a large variety of compounds that exhibited diverse biological activities, including anti-cancer, anti-microbial, anti-tubercular, anti-melanogenesis and anti-convulsant activities [15, 16]. Furthermore, recent studies demonstrated the α-glucosidase inhibitory activity of quinazoline-based derivatives [17, 18]. In this regard, substituted quinazolin-4(3H)-one derivative linked to coumarin (A) nucleus exhibited promising inhibition. SARs showed that quinazolinone derivatives lacking a coumarin ring reduce inhibitory activity; however, quinazolinone-coumarin hybrids significantly increase the inhibitory activities confirming the substitution on the quinazoline ring improved the potencies [19]. Quinazolinone-1,2,3-triazole derivatives (compound B) were exhibited as potent α-glucosidase inhibitors with no toxicity against breast cancer cell line MCF-7 [20]. Analog C with phenoxy-quinazolinone pharmacophore is another potent competitive α-glucosidase inhibitor with Ki value of 44 µM [21].
Recently, it was shown that aryl-substituted phenoxy-acetamide scaffolds were reported as potent α-glucosidase inhibitors. C=O moiety of acetamide as the key skeletons anchoring can stabilize α-helices, β-sheets, and other secondary structures of biological macromolecules through participation in various forms of interaction including hydrogen bonding, nucleophile–carbonyl, carbonyl–carbonyl (CO/CO) interaction [22]. Assessments on diphenylimidazole core attached to the various N-aryl acetamides (Fig. 1, Compound D) showed higher inhibitory activities with IC50 values of 55.6–149.2 µM than the activity of acarbose. In silico study exhibited several hydrophobic and H-bound interactions interaction with the binding site [23]. Also, phenoxybiscoumarin linked to different phenyl acetamides (Fig. 1, Compound E) was reported as an α-glucosidase inhibitor with an IC50 value of 41.73 to > 750 μM compared with acarbose (IC50 = 750.0 μM). Interestingly, the critical role of acetamide moiety was confirmed via H-bound interaction with the active site of the enzyme [24]. In our previous study novel series of benzimidazole-bearing phenoxy acetamide derivatives were developed as α-glucosidase inhibitors with IC50 values between 99.6 ± 3.1 to > 750 μM. In silico studies showed that the phenoxy linker of potent inhibitor exhibited H-bound interaction with Asp616 and/or Asp282 [14].
Keeping in view of the importance of quinazolin-4(3H)-one ring and the phenoxy-acetamide moiety to establish different forms of interactions with the α-glucosidase binding site as well as provide a suitable site for derivitization to evaluate the SARs, this work focused on the design and synthesis of quinazolin-4(3H)-one bearing aryl substituted phenoxyacetamide derivatives. The inhibition of all derivatives against α-glucosidase as well as kinetic studies was performed to describe the inhibition pattern. In silico studies were also considered to gain a better understanding of the interactions of the most potent derivative with the α-glucosidase binding site.
Results and discussions
Chemistry
The synthetic route was described in Scheme 1.
To a solution of aniline derivatives in DMF, chloroacetylchloride was added and the mixture was stirred at room temperature for 5 h to get the desired product, 3a–r. The reaction of 4-hydro-3-methoxy benzaldehyde (4) compound 3a–r in DMF in the presence of potassium carbonate gave compound 5a–r. Finally, 2-aminobenzamide 6 reacted with different aldehydes, 5a–r in DMF in the presence of Na2S2O5 at room temperature to prepare desired derivative 7a–r. The molecular structure of all compounds was deduced by spectroscopic techniques including EI-MS, CNHOS, 1H, and 13C-NMR.
α-glucosidase inhibitory activity
Derivatives 7a–r were synthesized to evaluate their potency as α-glucosidase inhibitors. Results are summarized in Table 1 in terms of IC50s.
7a as unsubstituted derivative exhibited an IC50 value of 363.4 µM. Compounds 7b–g were solely substituted with halogen groups including F, Cl, and Br, at a different position of the phenyl ring. Mostly, these analogs displayed improved inhibition potential against the α-glucosidase enzyme. Amongst, compound 7b with ortho fluorine substitution was found to be the most potent α-glucosidase inhibitor with IC50 values of 14.4 ± 0.2 µM. A comparison of compound 7b with compound 7c showed the positive effect of ortho-fluorine on inhibitory potential vs para-fluorine counterparts. Vice versa trends were seen in chlorine substitutions so that potencies changed in the following order: para-chlorine (7f, IC50 = 25.6 µM) > meta-chlorine (7e, IC50 = 114.3 µM) > ortho-chlorine (7d IC50 = 692.5 µM).
In the case of halogen substitutions, it can be understood that the position of the halogen played the most dominant role in the potency so that ortho-fluorine followed by para-chlorine group demonstrated high inhibitory potential.
Compounds 7h–j bearing methyl substitutions at different positions of phenyl part were found to have fewer inhibitory potential compared to 7a as unsubstituted counterpart. The exception in this trend came back to 7i with almost similar potency as 7a. Less inhibitory potential of compounds 7j than compounds 7h and 7i might be due to the steric effect by the second methyl-substituted position. Again as can be seen in 7k bearing ethyl substitution, the increased bulkiness at the para position and inferior to the potency.
Compounds 7l, 7m, and 7n containing methoxy, hydroxy, and nitro phenyl substitutions did not show any activity against α-glucosidase under the tested concentration. In these cases, it seems that even the presence of heteroatom with potency to participate in various form of interactions were unable to improve the inhibition.
Ring replacement of phenyl with naphthyl (7o) due to bigger size was found to completely inferior the inhibition. As can be seen in 7p, 7q, and 7r the elongation of the linker (= 1) showed a deteriorated involvement of length in the inhibitory potential compared to their computer parts, 7a, 7b, and 7i. This comparison showed that the shorter chain is playing an imperative character in the inhibitory potential. Also mostly para-substitutions reduced the potency, the only exception in this trend came back to 7f.
Enzyme kinetic studies
According to Fig. 2a, the Lineweaver–Burk plot showed that the Km gradually increased and Vmax remained unchanged with increasing inhibitor concentration indicating a competitive inhibition. The results show sample 7b binds to the active site on the enzyme and competes with the substrate for binding to the active site. Furthermore, the plot of the Km versus different concentrations of inhibitor gave an estimate of the inhibition constant, Ki of 14.0 µM (Fig. 2b).
Molecular docking
In order to clarify the interactions of the 7b in the active site of α-glucosidase and explain the related inhibitory activities, molecular docking studies were performed. First, the molecular docking validation was executed on acarbose as a native ligand against the α-glucosidase and the alignment of the best pose of acarbose in the active site of the enzyme and crystallographic ligand recorded an RMSD value less than 2 Å which confirms the accuracy of docking. As observed in Fig. 3, quinazolin-4(3H)-one ring stabilized through participation in one H-bound with Leu677 plus three pi-pi stacking interactions with Trp346 and Trp481. On the other side of the molecule, another H-bound interaction was seen between acetamide moiety and Ash616. 2-methoxyphenoxy made two H-bound interactions with Arg600 and Ash606 as well as one hydrophobic interaction with Trp481.
Conclusion
In summary, quinazolin-4(3H)-one derivatives (7a–r) were synthesized and evaluated for inhibitory activities against α-glucosidase. The synthesized derivatives showed a diverse range of inhibitory activities against α-glucosidase with IC50 values in the range of 14.4 ± 0.2 to > 750 µM. Compound 7b recorded the highest inhibitory activity against α-glucosidase with around ~ 53 times better potencies than acarbose. The enzyme inhibitory kinetics and mode of binding for the most active inhibitor 7b was performed which showed that the compound is a competitive inhibitor and effectively inhibits the target enzyme by binding to its active site. Docking results of the most active compound 7b showed a good protein−ligand interaction profile against the corresponding target.
In summary, we believe that the search for new modifications of quinazolin-4(3H)-one will contribute to the development of potent anti-DM agents.
Experimental section
General
All chemicals and reagents were purchased from Merck and Aldrich. The IR spectra were obtained on a Nicolet Magna FTIR 550 spectrometer (potassium bromide disks). Melting points were determined using Kofler hot stage apparatus and are uncorrected. NMR spectra were recorded on a Bruker 300 MHz.
Synthesis of compound 7a–r
To a solution of aniline derivatives (1 mmol) in DMF (4 mL), chloroacetylchloride (1.2 mmol) was added at 0 °C. The mixture was stirred at room temperature for 5 h and poured into water and then filtered to get the desired products (3a–r). The obtained solids were then filtered, dried, and recrystallized from ethanol. A 25 mL round bottom flask was charged with 4-hydro-3-methoxy benzaldehyde (1 mmol) and DMF (5 mL). 2-chloro-N-phenyl acetamide derivatives (1.1 mmol) were added, followed by potassium carbonate (1.2 mmol). The reaction mixture was stirred at room temperature for 5 h and then poured into ice water (25 ml). The products, 5a–r, was collected by filtration and rinsed with water. 5a–r derivatives (1 mmol) and 2-aminobenzamide (6, 1.2 mmol) were dissolved in 2 mL DMF and under stirring at room temperature, 1 mmol of sodium metabisulfite was added and allowed to react at 120 °C for about 4 h. After completion of the reaction, the mixture was precipitated in ice water, filtered, and dried at room temperature (Additional file 1).
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-phenylacetamide (7a)
Cream solid; Yield: 77%; MP = 192–194 °C; IR (KBr, vmax) 3252 (NH), 3025 (CH Aromatic), 2970(CH Aliphatic), 1660 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 10.14 (s, 1H, NHAmide), 8.14 (d, J = 8.20 Hz, 1H, H5), 7.62 (s, 1H, H2′) 7.84–7.73 (m, 2H, H6″ H7), 7.70 (d, J = 8.00 Hz, 1H, H8), 7.65 (d, J = 8.00 Hz, 2H, H2″, H6″), 7.45 (t, J = 7.60 Hz, 1H, H6), 7.32 (t, J = 7.80 Hz, 2H, H3″, H5″), 7.13–7.06 (m, 2H, H5′, H4″), 4.82 (s, 2H, CH2), 3.92 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.2, 162.3, 151.7, 150.1, 148.8, 148.7, 138.3, 134.3, 128.8, 127.4, 126.1, 125.8, 123.6, 121.0, 120.7, 119.5, 113.1, 111.0, 67.9, 55.7; ESI–MS (C23H19N3O4): calculated m/z 401.14 [M + H]+, observed m/z 401.21 [M + H]+; Anal. Calcd:C23H19N3O4; C, 68.82; H, 4.77; N, 10.47; Found; C, 68.98; H, 4.92; N, 10.65.
N-(2-fluorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7b)
Brown solid; Yield: 69%; MP = 222–225 °C; IR (KBr, vmax) 3376 (NH), 3045 (CH Aromatic), 2995 (CH Aliphatic), 1674 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.46 (s, 1H, NH Quinazolinone), 9.84 (s, 1H, NHAmide), 8.13 (d, J = 7.80 Hz, 1H, H5), 7.98 (d, J = 6.60 Hz, 1H, H6″), 7.90–7.74 (m, 3H, H7, H2′, H6′), 7.70 (d, J = 8.0 1H, H8), 7.47 (t, J = 7.4 Hz, 1H, H6), 7.33–7.24 (m, 1H, H5′), 7.22–7.04 (m, 3H, H3″, H4″, H5″), 4.89 (s, 2H, CH2), 3.93 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.6, 162.3, 153.4 (d, 1JCF = 243.7 Hz), 151.6, 149.9, 148.8, 148.7, 134.3, 127.5, 125.8, 125.5, 125.3, 124.5, 123.4, 120.7, 115.6, 113.3, 111.2, 110.9, 67.5, 55.4; ESI–MS (C23H18FN3O4): calculated m/z 419.13 [M + H]+, observed m/z 419.18 [M + H]+; Anal. Calcd: C23H18FN3O4; C, 65.87; H, 4.33; N, 10.02; Found; C, 66.05; H, 4.58; N, 10.19.
N-(4-fluorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7c)
Brown solid; Yield: 67%; MP = 216–218 °C; IR (KBr, vmax) 3369 (NH), 3030 (CH Aromatic), 2980 (CH Aliphatic), 1680 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.45 (s, 1H, NH Quinazolinone), 10.21 (s, 1H, NHAmide), 8.13 (d, J = 7.70 Hz, 1H, H5), 7.87–7.74 (m, 3H, H7, H2′, H6′), 7.73–7.60 (m, 3H, H8, H2″, H6″), 7.24 (t, J = 7.5 Hz, 1H, H6), 7.20–7.11 (m, 2H, H3″, H5″), 7.10–7.05 (m, 1H, H3″, H5″), 4.87 (s, 2H, CH2), 3.93 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.1, 162.3, 158.2 (d, 1JCF = 239.2 Hz), 151.7, 150.1, 148.8, 148.7, 134.7, 127.5, 125.8, 121.4, 121.0, 120.7, 115.5, 113.3, 111.3, 111.0, 67.8, 55.4; ESI–MS (C23H18FN3O4): calculated m/z 419.13 [M + H]+, observed m/z 419.24 [M + H]+; Anal. Calcd: C23H18FN3O4; C, 65.87; H, 4.33; N, 10.02; Found; C, 66.11; H, 4.54; N, 10.25.
N-(2-chlorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7d)
Brown solid; Yield: 78%; MP = 207–209 °C; IR (KBr, vmax) 3357 (NH), 3070 (CH Aromatic), 2980 (CH Aliphatic), 1677 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.40 (s, 1H, NH Quinazolinone), 9.53 (s, 1H, NHAmide), 8.13 (d, J = 7.80 Hz, 1H, H5), 8.06 (d, J = 8.60 Hz, 1H, H6″), 7.87 (s, 1H, H2′), 7.86–7.77 (m, 2H, H6′, H7), 7.71 (d, J = 8.10 Hz, 1H, H8), 7.59–7.42 (m, 2H, H3″, H5″), 7.36 (t, J = 7.70 Hz, 1H, H6), 7.22–7.11 (m, 2H, H5′, H4″), 4.87 (s, 2H, CH2), 3.94 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.4, 162.3, 151.7, 151.6, 149.4, 149.2, 148.7, 134.7, 134.7, 134.0, 132.5, 129.5, 127.7, 126.1, 125.8, 124.6, 122.4, 121.0, 10.7, 67.6, 55.6; Anal. Calcd: C23H18ClN3O4; C, 63.38; H, 4.16; N, 9.64; Found; C, 63.59; H, 4.41; N, 9.87.
N-(3-chlorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7e)
Brown solid; Yield: 74%; MP = 203–205 °C; IR (KBr, vmax) 3363 (NH), 3070 (CH Aromatic), 2980 (CH Aliphatic), 1681 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.47 (s, 1H, NH Quinazolinone), 10.28 (s, 1H, NHAmide), 8.13 (d, J = 7.90 Hz, 1H, H5), 7.89–7.79 (m, 4H, H7, H2′, H6′, H2″), 7.70 (d, J = 8.1 Hz, 1H, H8), 7.75–7.41 (m, 2H, H6″, H5″), 7.35 (t, J = 8.10 Hz, 1H, H6), 7.16–7.06 (m, 2H, H4″, H5′), 4.83 (s, 2H, CH2), 3.93 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.6, 162.3, 151.7, 150.0, 148.8, 148.7, 139.8, 133.1, 130.5, 125.7, 123.3, 120.7, 118.6, 113.2, 111.3, 110.9, 67.7, 55.4; ESI–MS (C23H18ClN3O4): calculated m/z 435.10 [M + H]+, observed m/z 435.19[M + H]+; Anal. Calcd: C23H18ClN3O4; C, 63.38; H, 4.16; N, 9.64; Found; C, 63.57; H, 4.32; N, 9.81.
N-(4-chlorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7f)
Brown solid; Yield: 79%; MP = 211–213 °C; IR (KBr, vmax) 3378 (NH), 3065 (CH Aromatic), 2990 (CH Aliphatic), 1703 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.42 (s, 1H, NH Quinazolinone), 10.29 (s, 1H, NHAmide), 8.13 (d, J = 7.90 Hz, 1H, H5), 7.85 (s, 1H, H2′) 7.82–7.75 (m, 2H, H6′, H7), 7.74–7.62 (m, 3H, H2″, H6″, H8), 7.47 (t, J = 7.50 Hz, 1H, H6), 7.37 (d, J = 8.30 Hz, 2H, H3″, H5″), 7.09 (d, J = 8.40 Hz, 1H, H5′), 4.81 (s, 2H, CH2), 3.92 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.3, 162.3, 151.7, 150.0, 148.8, 148.7, 137.3, 134.3, 128.7, 128.3, 127.5, 127.2, 125.8, 121.1, 120.7, 113.0, 111.0, 67.8, 55.4; ESI–MS (C23H18ClN3O4): calculated m/z 435.10 [M + H]+, observed m/z 435.23[M + H]+; Anal. Calcd: C23H18ClN3O4; C, 63.38; H, 4.16; N, 9.64; Found; C, 63.54; H, 4.31; N, 9.91.
N-(4-bromophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7g)
Brown solid; Yield: 84%; MP = 203–205 °C; IR (KBr, vmax) 3378 (NH), 3020 (CH Aromatic), 2885 (CH Aliphatic), 1661 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.42 (s, 1H, NH Quinazolinone), 10.27 (s, 1H, NHAmide), 8.13 (d, J = 7.90 Hz, 1H, H5), 7.85 (s, 1H, H2′) 7.83–7.73 (m, 2H, H6′, H7), 7.69 (d, J = 7.90 Hz, 1H, H8), 7.61 (d, J = 8.50 Hz, 2H, H2″, H6″), 7.53–7.41 (m, 3H, H3″, H5″, H6), 7.09 (d, J = 8.40 Hz, 1H, H5′), 4.81 (s, 2H, CH2), 3.93 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.4, 162.3, 151.6, 150.0, 148.8, 148.7, 137.7, 134.3, 131.6, 127.2, 126.1, 125.8, 121.4, 120.7, 115.3, 113.2, 111.1, 67.9, 55.7; ESI–MS (C23H18BrN3O4): calculated m/z 479.05 [M + H]+, observed m/z 479.13 [M + H]+; Anal. Calcd: C23H18BrN3O4; C, 57.51; H, 3.78; N, 8.75; Found; C, 57.64; H, 3.96; N, 8.91.
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(o-tolyl)acetamide (7h)
Cream solid; Yield: 76%; MP = 196–198 °C; IR (KBr, vmax) 3238 (NH), 3035 (CH Aromatic), 2965 (CH Aliphatic), 1682 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 9.37 (s, 1H, NHAmide), 8.14 (d, J = 7.70 Hz, 1H, H5), 7.86 (s, 1H, H2′) 7.85–7.75 (m, 2H, H6′, H7), 7.71 (d, J = 8.00 Hz, 1H, H8), 7.59 (d, J = 7.90 Hz, 1H, H3″), 7.48 (t, J = 7.50 Hz, 1H, H6), 7.27–7.05 (m, 4H, H5′, H6″, H5″, H4″), 4.83 (s, 2H, CH2), 3.93 (s, 3H, OCH3), 2.23 (s, 3H, CH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.1, 162.3, 151.6, 149.8, 148.8, 148.7, 135.5, 134.6, 130.7, 130.3, 127.5, 126.1, 125.8, 125.1, 123.9, 121.0, 120.7, 113.1, 11.0, 67.7, 55.5, 17.4; Anal. Calcd: C24H21N3O4; C, 69.39; H, 5.10; N, 10.11; Found; C, 69.54; H, 5.28; N, 10.24.
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(p-tolyl)acetamide (7i)
Cream solid; Yield: 76%; MP = 199–201 °C; IR (KBr, vmax) 3400 (NH), 3020 (CH Aromatic), 2975 (CH Aliphatic) 1701 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.47 (s, 1H, NH Quinazolinone), 10.07 (s, 1H, NHAmide), 8.13 (d, J = 7.70 Hz, 1H, H5), 7.84 (s, 1H, H2′) 7.83–7.74 (m, 2H, H6′, H7), 7.70 (d, J = 8.00 Hz, 1H, H8), 7.53–7.43 (m, 3H, H2″, H6″, H6), 7.16–7.03 (m, 3H, H3″, H5″, H5′), 4.78 (s, 2H, CH2), 3.92 (s, 3H, OCH3), 2.72 (s, 3H, CH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 165.9, 162.3, 151.7, 150.1, 148.8, 148.7, 135.8, 134.3, 132.6, 129.2, 129.1, 127.1, 125.9, 125.8, 121.0, 120.7, 119.4, 113.1, 11.2, 67.8, 55.4, 20.4; ESI–MS (C24H21N3O4): calculated m/z 415.15 [M + H]+, observed m/z 415.19 [M + H]+; Anal. Calcd: C24H21N3O4; C, 69.39; H, 5.10; N, 10.11; Found; C, 69.62; H, 5.27; N, 10.30.
N-(2,6-dimethylphenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7j)
Cream solid; Yield: 68%; MP = 215–217 °C; IR (KBr, vmax) 3235 (NH), 3030 (CH Aromatic), 2970 (CH Aliphatic), 1684 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 9.46 (s, 1H, NHAmide), 8.18–8.08 (m, 1H, H5), 7.86 (s, 1H, H2′) 7.85–7.66 (m, 2H, H6′, H7), 7.55–7.41 (m, 1H, H8), 7.29–6.98 (m, 5H, H3″, H6, H5′, H6″, H5″, H4″), 4.85 (s, 2H, CH2), 3.92 (s, 3H, OCH3), 2.14 (s, 6H, 2 × CH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 164.4, 162.0, 151.6, 150.3, 149.8, 148.8, 138.9, 135.5, 133.1, 130.6, 127.6, 126.0, 120.3, 118.9, 116.9, 113.1, 110.4, 67.2, 54.6, 17.4; Anal. Calcd: C25H23N3O4; C, 69.92; H, 5.40; N, 9.78; Found; C, 70.12; H, 5.67; N, 9.91.
N-(4-ethylphenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7k)
Cream solid; Yield: 81%;MP = 195–197 °C; IR (KBr, vmax) 3401 (NH), 3065 (CH Aromatic), 2950(CH Aliphatic), 1670 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 10.03 (s, 1H, NHAmide), 8.13 (d, J = 7.70 Hz, 1H, H5), 7.85 (s, 1H, H2′) 7.83–7.75 (m, 2H, H6′, H7), 7.70 (d, J = 8.00 Hz, 1H, H8), 7.53 (d, J = 8.10 Hz, 2H, H2″, H6″), 7.47 (t, J = 7.60 Hz, 1H, H6), 7.14 (d, J = 8.10 Hz, 2H, H3″, H5″), 7.09 (d, J = 8.40 Hz, 1H, H5′), 4.79 (s, 2H, CH2), 3.93 (s, 3H, OCH3), 2.53 (d, J = 7.50 Hz, 2H, CH2 Ethyl), 1.13 (t, J = 7.50 Hz, 3H, CH3 Ethyl) ppm. 13C NMR (75 MHz, DMSO-d6): δ 165.9, 162.3, 151.7, 150.1, 148.8, 148.7, 139.1, 136.0, 134.6, 127.9, 127.4, 126.1, 125.7, 120.7, 119.5, 119.4, 113.2, 113.1, 111.3, 111.0, 67.9, 55.8, 27.6, 15.7, 15.5; Anal. Calcd: C25H23N3O4; C, 69.92; H, 5.40; N, 9.78; Found; C, 70.11; H, 5.57; N, 9.94.
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(4-methoxyphenyl)acetamide (7l)
Cream solid; Yield: 88%;MP = 205–207 °C; IR (KBr, vmax) 3253 (NH), 3030 (CH Aromatic), 2975 (CH Aliphatic), 1661(C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 9.93 (s, 1H, NHAmide), 8.13 (d, J = 7.80 Hz, 1H, H5), 7.85 (s, 1H, H2′) 7.77–7.82 (m, 2H, H6′, H7), 7.75 (d, J = 8.10 Hz, 1H, H8), 7.54 (d, J = 8.50 Hz, 2H, H2″, H6″), 7.47 (t, J = 7.50 Hz, 1H, H6), 7.09 (d, J = 8.10 Hz, 1H, H5′), 6.89 (d, J = 8.55 Hz, 2H, H3″, H5″), 4.77 (s, 2H, CH2), 3.93 (s, 3H, OCH3), 3.71 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 165.6, 162.3, 155.5, 151.7, 150.1, 148.8, 148.7, 134.3, 131.4, 127.5, 126.1, 125.7, 121.0, 120.9, 120.7, 114.0, 113.8, 113.1, 111.0, 67.9, 55.2, 55.0; Anal. Calcd: C24H21N3O5; C, 66.81; H, 4.91; N, 9.74; Found; C, 66.97; H, 5.03; N, 9.87.
N-(4-hydroxyphenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7m)
Cream solid; Yield: 67%; MP = 219–221 °C;; IR (KBr, vmax) 3321(NH), 3035 (CH Aromatic), 2960 (CH Aliphatic), 1683 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.46 (s, 1H, NH Quinazolinone), 9.13 (s, 1H, NHAmide), 8.13 (d, J = 7.3 Hz, 1H, H5), 7.94–7.77 (m, 3H, H7, H2′, H6’) 7.70 (d, J = 7.7 Hz, 1H, H8), 7.47 (t, J = 7.7 Hz, 1H, H6), 7.40 (d, J = 8.1 Hz, 2H, H2″, H6″), 7.07 (t, J = 8.1 Hz, 1H, H5′),6.71 (d, J = 8.3, 2H, H3″, H5″), 4.75 (s, 2H, CH2), 3.92 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 165.4, 162.3, 153.6, 151.7, 150.1, 148.7, 139.1, 134.7, 129.9, 125.6, 120.6, 115.2, 114.9, 113.1, 110.8, 67.9, 55.4; Anal. Calcd: C24H21N3O4; C, 66.18; H, 4.59; N, 10.07; Found; C, 66.39; H, 4.76; N, 10.28.
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(4-nitrophenyl)acetamide (7n)
Brown solid; Yield :81%; MP = 216–218 °C; IR (KBr, vmax) 3343(NH), 3040(CH Aromatic), 2980(CH Aliphatic), 1674(C=O), 1560–1355(NO2) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.49 (s, 1H, NH Quinazolinone), 9.57 (s, 1H, NHAmide), 8.26–8.19 (m, 2H, H3″, H5″), 8.12 (d, J = 7.2 Hz, 1H, H5), 7.92–7.76 (m, 3H, H2′, H2″, H6″), 7.69 (d, J = 7.2 Hz, 1H, H7), 7.55 (d, J = 8.10 Hz, 1H, H6’), 7.46 (d, J = 7.5 Hz, 1H, H8), 7.18 (t, J = 7.80 Hz, 1H, H6), 7.04–6.95 (m, 1H, H5′), 5.00 (s, 2H, CH2), 4.00 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.7, 165.9, 162.7, 162.2, 151.5, 148.7, 148.2, 141.2, 134.3, 132.3, 129.5, 128.2, 128.0, 126.0, 120.8, 115.2, 113.1, 111.0, 67.3, 56.2; Anal. Calcd: C23H18ClN3O4; C, 61.88; H, 4.06; N, 12.55; Found; C, 62.07; H, 4.31; N, 12.72.
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(naphthalen-2-yl)acetamide (7o)
Cream solid; Yield: 65%; MP = 217–219 °C; IR (KBr, vmax) 3291 (NH), 3065 (CH Aromatic), 2980(CH Aliphatic), 1675 (C=O) Cm−1; 1H NMR (300 MHz, DMSO-d6) δ 12.47 (s, 1H, NH Quinazolinone), 10.10 (s, 1H, NHAmide), 8.14 (d, J = 7.9 Hz, 1H, H5), 8.08 (d, J = 7.9 Hz, 1H, H8″) 7.96 (d, J = 7.8 Hz, 1H, H3″), 7.91–7.84 (m, 2H, H2″, H5″), 7.80–7.75 (m, 3H, H2′, H6′, H7), 7.72 (d, J = 8.1 Hz, 1H, H8), 7.61–7.43 (m, 4H, H6, H4″, H6″, H7″), 7.21 (d, J = 8.7 Hz, 1H, H5′), 4.99 (s, 2H, CH2), 3.91 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 166.9, 162.3, 151.7, 150.0, 148.8, 148.7, 134.4, 133.6, 132.6, 127.4, 125.8, 120.7, 113.2, 111.3, 111.0, 67.8, 59.8; Anal. Calcd: C27H21N3O4; C, 71.83; H, 4.69; N, 9.31;Found; C, 71.98; H, 4.87; N, 9.56.
2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(4-methylbenzyl)acetamide (7p)
Cream solid; Yield: 87%; MP = 191–193 °C; IR (KBr, vmax) 3258 (NH), 3030(CH Aromatic), 2910 (CHAliphatic), 1666(C=O)Cm−1; 1H NMR(300 MHz, DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 8.47 (t, J = 6.10 Hz, 1H, NHAmide), 8.13 (d, J = 7.80 Hz, 1H, H5), 7.83 (s, 1H, H2′) 7.82–7.75 (m, 2H, H6′, H7), 7.71 (d, J = 8.00 Hz, 1H, H8), 7.48 (t, J = 7.40 Hz, 1H, H6), 7.16–7.06 (m, 4H, H Phenyl), 7.05 (d, J = 8.10 Hz, 1H, H5′), 4.65 (s, 2H, CH2), 4.30 (d, J = 5.90 Hz, 2H, CH2), 3.89 (s, 3H, OCH3), 2.26 (s, 3H, CH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 167.3, 162.3, 151.7, 150.0, 148.8, 136.0, 135.8, 134.5, 128.8, 127.2, 126.0, 125.8, 121.0, 120.7, 113.3, 11.3, 67.9, 55.4, 41.6, 20.6; Anal. Calcd: C25H23N3O4; C, 69.92; H, 5.40; N, 9.78; Found; C, 70.13; H, 5.69; N, 9.97.
N-(4-fluorobenzyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7q)
Cream solid; Yield: 76%; MP = 224–226 °C; IR (KBr, vmax) 3314 (NH), 3050(CH Aromatic), 2960 (CHAliphatic),1671(C=O) Cm−1; 1H NMR(300 MHz,DMSO-d6) δ 12.44 (s, 1H, NH Quinazolinone), 8.55 (t, J = 6.10 Hz, 1H, NHAmide), 8.13 (d, J = 7.90 Hz, 1H, H5), 7.89–7.75 (m, 3H, H2′, H6′, H7) 7.70 (d, J = 7.8 Hz, 1H, H8), 7.46 (t, J = 7.4 Hz, 1H, H6), 7.34–7.27 (m, 2H, H2″, H6″), 7.18–7.08 (m, 2H, H3″, H5″), 7.05 (d, J = 8.10 Hz, 1H, H5′), 4.66 (s, 2H, CH2), 4.34 (d, J = 5.90 Hz, 2H, CH2 Benzyl), 3.89 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 167.5, 162.7, 160.9(d, 1JCF = 208.5), 151.6, 149.9, 148.8, 135.3, 129.2, 126.0, 125.8, 121.0, 120.7, 115.0, 113.3, 111.2, 110.9, 67.8, 55.9, 41.2; Anal. Calcd: C24H20FN3O4; C, 66.51; H, 4.65; N, 9.69; Found; C, 66.72; H, 4.84; N, 9.91.
N-benzyl-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7r)
Cream solid; Yield: 81%; MP = 194–196 °C; IR (KBr, vmax) 3264 (NH), 3025(CH Aromatic), 2960 (CH Aliphatic),1662 (C=O) Cm−1; 1H NMR (300 MHz,DMSO-d6) δ 12.45 (s, 1H, NH Quinazolinone), 8.53 (t, J = 6.10 Hz, 1H, NHAmide), 8.13 (d, J = 7.90 Hz, 1H, H5), 7.84 (s, 1H, H2′) 7.83–7.75 (m, 2H, H6′, H7), 7.71 (d, J = 8.10 Hz, 1H, H8), 7.48 (t, J = 7.50 Hz, 1H, H6), 7.35–7.20 (m, 5H, H Phenyl), 7.06 (d, J = 8.10 Hz, 1H, H5′), 4.67 (s, 2H, CH2), 4.35 (d, J = 6.00 Hz, 2H, CH2), 3.89 (s, 3H, OCH3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 167.4, 162.3, 151.7, 150.0, 148.8, 139.1, 134.6, 128.2, 127.2, 126.8, 126.8, 126.19, 15.8, 120.7, 113.4, 111.0, 67.9, 55.4, 41.9; Anal. Calcd: C24H21N3O4; C, 69.39; H, 5.10; N, 10.11; Found; C, 69.57; H, 5.28; N, 10.26.
α-glucosidase inhibition assay
The anti-α-glucosidase effects of synthesized compounds, 7a–r were screened according to the previously reported method [7, 12, 25].
Enzyme kinetic studies
The mode of inhibition of the most active compound 7b, identified with the lowest IC50, was investigated against an α-glucosidase activity with different concentrations of p-nitrophenyl α-d-glucopyranoside (1–10 mM) as substrate in the absence and presence of 7b at different concentrations (0, 3.6, 7.2, and 14.4 µM). A Lineweaver–Burk plot was generated to identify the type of inhibition and the Michaelis–Menten constant (Km) value was determined from the plot between the reciprocal of the substrate concentration (1/[S]) and reciprocal of enzyme rate (1/V) over various inhibitor concentrations. The experimental inhibitor constant (Ki) value was constructed by secondary plots of the inhibitor concentration [I] versus Km [3, 7].
Molecular docking
The molecular docking studies were performed using the Maestro Molecular Modeling platform (version 10.5) by Schrödinger, LLC. The X-ray crystal structure of the receptor (PDB ID: 5NN8) was extracted from the PDB database. The protein is then prepared using a protein preparation wizard so that co-crystallized ligands and all water molecules were removed, the missing side chains and loops were filled using the prime tool, and PROPKA assigned H-bonds at pH: 7.4. To prepare the ligands, the 2D structures of the ligands were drawn in ChemDraw and converted into SDF files and subjected to ligprep module. Ligands were prepared by OPLS_2005 force field using EPIK. The grid box was generated for each binding site using entries with a box size of 25 A, the derivative was docked on binding sites using induced-fit docking, reporting 10 poses per ligand to form the final complex [14, 26].
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Worldwide Protein Data Bank (wwPDB) repository. (http://www.rcsb.org).
References
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan P-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790.
Sohrabi M, Binaeizadeh MR, Iraji A, Larijani B, Saeedi M, Mahdavi M. A review on α-glucosidase inhibitory activity of first row transition metal complexes: a futuristic strategy for treatment of type 2 diabetes. RSC Adv. 2022;12(19):12011–52.
Noori M, Davoodi A, Iraji A, Dastyafteh N, Khalili M, Asadi M, Mohammadi Khanaposhtani M, Mojtabavi S, Dianatpour M, Faramarzi MA, Larijani B, Amanlou M, Mahdavi M. Design, synthesis, and in silico studies of quinoline-based-benzo[d]imidazole bearing different acetamide derivatives as potent α-glucosidase inhibitors. Sci Rep. 2022;12(1):14019.
Srisongkram T, Waithong S, Thitimetharoch T, Weerapreeyakul N. Machine learning and in vitro chemical screening of potential α-amylase and α-glucosidase inhibitors from Thai indigenous plants. Nutrients. 2022;14(2):267.
Huneif MA, Alshehri DB, Alshaibari KS, Dammaj MZ, Mahnashi MH, Majid SU, Javed MA, Ahmad S, Rashid U, Sadiq A. Design, synthesis and bioevaluation of new vanillin hybrid as multitarget inhibitor of α-glucosidase, α-amylase, PTP-1B and DPP4 for the treatment of type-II diabetes. Biomed Pharmacother. 2022;150: 113038.
Lari ZN, Hajimonfarednejad M, Riasatian M, Abolhassanzadeh Z, Iraji A, Vojoud M, Heydari M, Shams M. Efficacy of inhaled Lavandula angustifolia Mill Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251: 112560.
Iraji A, Shareghi-Brojeni D, Mojtabavi S, Faramarzi MA, Akbarzadeh T, Saeedi M. Cyanoacetohydrazide linked to 1,2,3-triazole derivatives: a new class of α-glucosidase inhibitors. Sci Rep. 2022;12(1):8647.
Kam A, Li KM, Razmovski-Naumovski V, Nammi S, Shi J, Chan K, Li GQ. A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate on α-amylase and α-glucosidase. Phytother Res. 2013;27(11):1614–20.
Nasli Esfahani A, Iraji A, Alamir A, Moradi S, Asgari MS, Hosseini S, Mojtabavi S, Nasli-Esfahani E, Faramarzi MA, Bandarian F, Larijani B, Hamedifar H, Hajimiri MH, Mahdavi M. Design and synthesis of phenoxymethybenzoimidazole incorporating different aryl thiazole-triazole acetamide derivatives as α-glycosidase inhibitors. Mol Divers. 2022;26(4):1995–2009.
Li X, Bai Y, Jin Z, Svensson B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT. 2022;153: 112455.
Karami M, Hasaninejad A. One-pot multi-component synthesis of novel chromeno[4,3-b]pyrrol-3-yl derivatives as alpha-glucosidase inhibitors. Mol Divers. 2021. https://doi.org/10.1007/s11030-021-10337-w.
Shareghi-Boroujeni D, Iraji A, Mojtabavi S, Faramarzi MA, Akbarzadeh T, Saeedi M. Synthesis, in vitro evaluation, and molecular docking studies of novel hydrazineylideneindolinone linked to phenoxymethyl-1,2,3-triazole derivatives as potential α-glucosidase inhibitors. Bioorg Chem. 2021;111: 104869.
Zarenezhad E, Farjam M, Iraji A. Synthesis and biological activity of pyrimidines-containing hybrids: focusing on pharmacological application. J Mol Struct. 2021;1230: 129833.
Shayegan N, Iraji A, Bakhshi N, Moazzam A, Faramarzi MA, Mojtabavi S, Pour SMM, Tehrani MB, Larijani B, Rezaei Z, Yousefi P, Khoshneviszadeh M, Mahdavi M. Design, synthesis, and in silico studies of benzimidazole bearing phenoxyacetamide derivatives as α-glucosidase and α-amylase inhibitors. J Mol Struct. 2022;1268: 133650.
Auti PS, George G, Paul AT. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020;10(68):41353–92.
Sepehri N, Iraji A, Yavari A, Asgari MS, Zamani S, Hosseini S, Bahadorikhalili S, Pirhadi S, Larijani B, Khoshneviszadeh M, Hamedifar H, Mahdavi M, Khoshneviszadeh M. The natural-based optimization of kojic acid conjugated to different thio-quinazolinones as potential anti-melanogenesis agents with tyrosinase inhibitory activity. Bioorg Med Chem. 2021;36: 116044.
Khalifa MM, Sakr HM, Ibrahim A, Mansour AM, Ayyad RR. Design and synthesis of new benzylidene-quinazolinone hybrids as potential anti-diabetic agents: in vitro α-glucosidase inhibition, and docking studies. J Mol Struct. 2022;1250: 131768.
Sherafati M, Mirzazadeh R, Barzegari E, Mohammadi-Khanaposhtani M, Azizian H, Sadegh Asgari M, Hosseini S, Zabihi E, Mojtabavi S, Ali Faramarzi M, Mahdavi M, Larijani B, Rastegar H, Hamedifar H, Hamed Hajimiri M. Quinazolinone-dihydropyrano[3,2-b]pyran hybrids as new α-glucosidase inhibitors: design, synthesis, enzymatic inhibition, docking study and prediction of pharmacokinetic. Bioorg Chem. 2021;109: 104703.
Menteşe E, Karaali N, Akyüz G, Yılmaz F, Ülker S, Kahveci B. Synthesis and evaluation of α-glucosidase and pancreatic lipase inhibition by quinazolinone-coumarin hybrids. Chem Heterocycl Compd. 2016;52(12):1017–24.
Saeedi M, Mohammadi-Khanaposhtani M, Pourrabia P, Razzaghi N, Ghadimi R, Imanparast S, Faramarzi MA, Bandarian F, Esfahani EN, Safavi M, Rastegar H, Larijani B, Mahdavi M, Akbarzadeh T. Design and synthesis of novel quinazolinone-1,2,3-triazole hybrids as new anti-diabetic agents: in vitro α-glucosidase inhibition, kinetic, and docking study. Bioorg Chem. 2019;83:161–9.
Yavari A, Mohammadi-Khanaposhtani M, Moradi S, Bahadorikhalili S, Pourbagher R, Jafari N, Faramarzi MA, Zabihi E, Mahdavi M, Biglar M, Larijani B, Hamedifar H, Hajimiri MH. α-Glucosidase and α-amylase inhibition, molecular modeling and pharmacokinetic studies of new quinazolinone-1,2,3-triazole-acetamide derivatives. Med Chem Res. 2021;30(3):702–11.
Mehreen S, Zia M, Khan A, Hussain J, Ullah S, Anwar MU, Al-Harrasi A, Naseer MM. Phenoxy pendant isatins as potent α-glucosidase inhibitors: reciprocal carbonyl⋯carbonyl interactions, antiparallel π⋯π stacking driven solid state self-assembly and biological evaluation. RSC Adv. 2022;12(32):20919–28.
Sepehri N, Azizian H, Ghadimi R, Abedinifar F, Mojtabavi S, Faramarzi MA, Moghadamnia AA, Zabihi E, Mohebbi G, Larijani B, Hamedifar H, Mohammadi-Khanaposhtani M, Mahdavi M. New 4,5-diphenylimidazole-acetamide-1,2,3-triazole hybrids as potent α-glucosidase inhibitors: synthesis, in vitro and in silico enzymatic and toxicity evaluations. Monatsh Chem. 2021;152(6):679–93.
Ansari S, Azizian H, Pedrood K, Yavari A, Mojtabavi S, Faramarzi MA, Golshani S, Hosseini S, Biglar M, Larijani B, Rastegar H, Hamedifar H, Mohammadi-Khanaposhtani M, Mahdavi M. Design, synthesis, and α-glucosidase-inhibitory activity of phenoxy-biscoumarin–N-phenylacetamide hybrids. Arch Pharm. 2021;354(12):2100179.
Pedrood K, Rezaei Z, Khavaninzadeh K, Larijani B, Iraji A, Hosseini S, Mojtabavi S, Dianatpour M, Rastegar H, Faramarzi MA, Hamedifar H, Hajimiri MH, Mahdavi M. Design, synthesis, and molecular docking studies of diphenylquinoxaline-6-carbohydrazide hybrids as potent α-glucosidase inhibitors. BMC Chemistry. 2022;16(1):57.
Zarenezhad E, Montazer MN, Tabatabaee M, Irajie C, Iraji A. New solid phase methodology for the synthesis of biscoumarin derivatives: experimental and in silico approaches. BMC Chemistry. 2022;16(1):53.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MM, ND, MKG, and MN synthesized compounds and contributed to the design and characterization of compounds. AI and FR performed in silico study and contributed to the preparation of the manuscript. SM, KZ performed the biological assay. MAF, and BL supervised the biological tests. SESE and MM supervised all phases of the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figure S1. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-phenylacetamide (7a). Figure S2. N-(2-fluorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7b). Figure S3. N-(4-fluorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7c). Figure S4. N-(2-chlorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7d). Figure S5. N-(3-chlorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7e). Figure S6. N-(4-chlorophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7f). Figure S7. N-(4-bromophenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7g). Figure S8. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(o-tolyl)acetamide (7 h). Figure S9. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(p-tolyl)acetamide (7i). Figure S10. N-(2,6-dimethylphenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7j). Figure S11. N-(4-ethylphenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7k). Figure S12. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(4-methoxyphenyl)acetamide (7 l). Figure S13. N-(4-hydroxyphenyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7m). Figure S14. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(4-nitrophenyl)acetamide (7n). Figure S15. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(naphthalen-2-yl)acetamide (7o). Figure S16. 2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)-N-(4-methylbenzyl)acetamide (7p). Figure S17. N-(4-fluorobenzyl)-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7q). Figure S18. N-benzyl-2-(2-methoxy-4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenoxy)acetamide (7r).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moheb, M., Iraji, A., Dastyafteh, N. et al. Synthesis and bioactivities evaluation of quinazolin-4(3H)-one derivatives as α-glucosidase inhibitors. BMC Chemistry 16, 97 (2022). https://doi.org/10.1186/s13065-022-00885-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-022-00885-z