Abstract
Elagolix (ELX) is an orally administered non-peptidic GnRH antagonist that has been approved by the Food and Drug Administration in 2018 for the treatment of endometriosis pain. A sensitive and selective method for estimating elagolix (ELX) in human plasma and content uniformity was developed and validated. The spectrofluorimetric technique was used to investigate ELX utilizing boron-doped carbon quantum dots (B@CQDs). After gradually adding ELX, the quantum dots fluorescence was enhanced with LOQ of 1.74 ng mL−1, the calibration curve between ELX and corresponding fluorescence intensity was found over a range of 4–100 ng mL−1. The method was successfully applied in real human plasma with pharmacokinetic study and content uniformity test. The pharmacokinetic parameters as Cmax were found to be 570 ± 5.32 ng. mL−1 after 1 h, t1/2 was found to be 6.50 h, and AUC was found to be 1290 ± 30.33 ng. h. mL−1. B@CQDs were characterized using variety of instruments. The strategy is simple to implement in clinical labs and therapeutic drug monitoring systems.
Similar content being viewed by others
Introduction
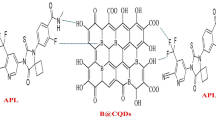
ELX (Fig. 1a) is 4-[[(1R)-2-[5-(2-Fluoro-3-methoxyphenyl) -3-[[2-fluoro -6- (trifluoromethyl) phenyl] methyl]-4-methyl-2, 6-dioxopyrimidin-1-yl]-1- phenylethyl]amino] butanoic acid. Endometriosis is the most common gynecological disorder affecting women of reproductive age, except for postmenopausal women [1]. Endometriosis is a condition that develops outside of the uterus because of tissues within it, causing pelvic pain and infertility [2,3,4,5,6]. Pain relievers (nonsteroidal anti-inflammatory drugs) are the first-line treatment for endometriosis pain [7]. ELX is an oral first-generation and short-acting gonadotropin-releasing hormone (GnRH) antagonist drug that was approved by the FDA in 2018 for the treatment of endometriosis pain [8]. ELX is used to relieve pelvic pain by inhibiting GnRH signals by binding competitively to GnRH receptors [8].
Only three methods were reported for the determination of ELX [9,10,11]. The reported method has many disadvantages as using expensive and sophisticated equipment, expensive solvents, and difficulty of handling. The presented study provides varying advantages other than reported methods as the method is environmentally friendly, time saving, very simple and sensitive with a quantitative range from 4 to 100 ng mL−1. Quantum dots is smart materials that have many applications as in industries, science, technology, and pharmaceutical analysis [12,13,14]. As a result, carbon quantum dots were used to analyze ELX in human plasma (pharmacokinetic assay), content uniformity, and then heteroatoms were introduced into carbon dots synthesis to fine-tune the conduction band position of doped CQDs, which led to the adjustment of the functions used to treat impurities and fluorescence of doped carbon dots. This study describes the practical implementation of a selective and cheap B@CQDs to estimation of ELX in pharmaceutical formulations and human plasma without matrix interference as a proof-of-concept to utilize B@CQDs in clinical laboratories.
Experimental part
Apparatus
the fluorescence was measured using Jasco, FP spectrofluorimetric instrument with a 1 cm quartz cell and slit width 5 nm (USA). The dynamic light scattering measurements (DLS) were scanned by Zetasizer Red badge instrument of ZEN 3600 Nano ZS model (Malvern, UK). Transmission electron microscope (TEM) images were captured on TEM Assembly Parts Power of JEOL JEM-100CX II unit tungsten EM filament 120 (USA). Fourier-transform infrared (FTIR) spectrometer was utilized to determine function groups of B@CQDs (Germany). Ultrasonic Cleaner (USA). Hana pH-meter (China). The powder X-ray diffraction (PXRD) was scanned by Philips X-ray diffractometer. UV–Vis spectra of carbon dots was recorded by Shimadzu 1601PC UV–Vis. The elemental composition of B@CQDs was determined by NEX QC + QuantEZ (50 kV X-ray tube and SDD detector) energy dispersive X-ray spectrometer.
Materials and reagents
Elagolix (ELX, 99.99%) was kindly supplied by Hekma pharmaceutical industries, Cairo, Egypt. Orlissa® (200 mg/tablet) was purchased from local pharmacies. FeCl3⋅6H2O, FeCl2⋅4H2O, boric acid and glucose, SDS, starch and mannitol were obtained from El-Gomhoria, Egypt. Methanol, ethanol, ethyl acetate and acetonitrile were obtained from El-Nasr Co, Egypt.
A stock solution of ELX was prepared in ultra-pure distilled water by dissolving 10 mg of the drug in a 100-mL volumetric flask to reach a concentration of 100 µg mL−1. Stock standard solution was stored for 14 days in the refrigerator at 4 °C. A series of working standard solutions with concentration range between 40 and 1000 ng mL−1 were prepared in volumetric flasks using the same solvent.
Preparation of human plasma
Human plasma samples were achieved from healthy human volunteers following ethical standards of the responsible committee on human experimentation (institutional and national) and with Helsinki Declaration of 1975, as revised in 2008.
Human plasma was obtained from healthy human volunteers (aged from 25 to 40 years old). Plasma samples was spiked with various concentrations of ELX in centrifugation tubes, 2 mL of acetonitrile [15, 16] was added as protein precipitating agent, and the mixture was centrifuged at 4000 rpm for 15 min. After that, the supernatant completed to 10 mL with ultra-pure water and the procedure was followed.
Six healthy female volunteers were administered Orlissa® tablets (200 mg/tablet) as a single oral dose as part of a pharmacokinetic study. After collecting blood samples at predetermined intervals, the plasma was separated by centrifugation at 4000 rpm for 30 min. The plasma samples were treated as spiked human plasma without the addition of the mentioned drug.
Boron doped carbon dots (B@CQDs)
Were synthesized using hydrothermal method [17], 0.3 g boric acid and 0.5 g glucose were dissolved into 50 mL of ultra-pure water via sonication for 20 min. The electric oven was adjusted at 300 ◦C and the mixture was heated for 4 h. After cooling, the resultant mixture was centrifuged at 5000 rpm for 30 min. The material was collected and then dried, 100.0 mg of B@CQDs were transferred into 100.0 mL ultra-pure water. After that, the colloid was sonicated for 1 h and filtered to remove the large particles.
Procedure for ELX using B@CQDs
Into 5 mL volumetric flasks, 0.5 mL B@CQDs solution, 1.0 mL of B.R. buffer (pH 6.7), and 1 mL of different concentrations of ELX were added. the mixture was diluted to the mark with DDW and mixed. Then, the mixture was measured at 435 nm (excitation at 370 nm) after 10 min.
Analysis of ELX pharmaceutical dosage form and content uniformity test
Pharmaceutical tablets were prepared as the following procedure, 10 tablets (orlissa®) were weighed and finely powdered. An amount of the powdered tablets equivalent to 10 mg of ELX was transferred into 100-mL volumetric flask followed by addition of 50 mL of ultra-pure water. After that, the solution mixture was sonicated for 5 min followed by filtration and dilution with double distilled water to reach final concentration of 100 µg mL−1.
The content uniformity testing was assessed and performed using USP [18, 19]. Each tablet was individually weighed, crushed, and analyzed as the previously mentioned procedure of pharmaceutical tablets.
Results and discussion
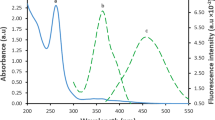
ELX is an orally administered non-peptidic GnRH antagonist used to treat endometriosis pain [8, 9]. The fluorometric technique, as is well known, is a highly selective, quick, and sensitive technique widely employed for pharmaceutical substances [15, 16]. After excitation at 370 nm, the fluorescence of synthesized B@CQDs was seen at 435 nm. After the addition of varying concentrations of ELX to B@CQDs, enhancement of the fluorescence was observed Fig. 1b.
The enhancing reaction mechanism of ELX with B@CQDs, which is based on hydrogen bonding, energy/electron transfer, and electrostatic contact, resulted in an increase in the quantum dots' fluorescence. Because of the hydrogen bonding and electron-donor–acceptor complex between B@CQDs and ELX, as well as the abundance of carboxyl, hydroxyl, and trivalent boron moieties, the B@CQDs' fluorescence was enhanced by combining with ELX. The carboxyl or hydroxyl groups of B@CQDs and the fluorine of ELX form active and strong nearby hydrogen bonds [20, 21].
Furthermore, the electron-accepting representative of trivalent boron stabilized by the carbon skeleton of B@CQDs and the electron-donating character of ELX, which may promote the conjugation of C = C bonds [22], as well as the merging effect of hydrogen bonding and electron-donor–acceptor complex effect, led to an increase in the initiation of massive chromophores and fluorophores [22].
Characterizations of doped boron carbon quantum dots (B@CQDs)
Varying morphological characters of the quantum dots were studied, firstly TEM image was carried out to study their particle size. The size of the quantum dots was found to be 3 nm and conformed with DLS spectrum Fig. 2a.
The FTIR for B@CQDs, the bands that emerge at 3412, 1690, 1622, 1095, and 1236 cm−1 correspond to (OH), (C = O), (C = C), (C O C), and (CO) respectively [14, 23].
However, B@CQDs FTIR provides characteristic peaks at 2952, 1425, 1031, and 796 cm−1 are indicated to (B‒OH), (B‒C), (B‒O), and (B‒O‒B) respectively, which confirms the doping of boron in the carbonaceous structure of the synthesized B@CQDs compared to the undoped CQDs [24] Fig. 2b.
EDX spectrum provides three characteristic peaks for B@CQDs refer to B 20.13% at 0.18 keV, C 49.22% at 0.24 keV and O 30.65% at 0.51 keV as shown in Fig. 2c.
To characterize B@CQDs further, PXRD (Fig. 2d) was employed strong peak at 2θ = 23.9, which corresponds to the graphite phase (002) plane [25] and diffraction peaks at 11.4 (001) and 42.3 (100) for B@CQDs compared with undoped carbon dots Additional file 1: Fig. S1.
As shown in Fig. 3a, XPS was used for elemental analysis. The B 1 s high-resolution spectra revealed two peaks with binding energies of 192.09and 192.97 eV, which correspond to B–C and B–O, respectively (Fig. 3b). Carboxylic groups are present at 288.20, as well as C–O/C–N and C = C bonds at 285.27 and 284.47, respectively, in the high-resolution C 1 s image (Fig. 3c). The spectrum of N 1 s exhibits two distinct peaks at 399.20 and 400.10 eV, indicating two components for N–C and N–H [26, 27] Fig. 3d.
The quantum yield of B@CQDs was determined using the single point method [12, 17]:
where, Qst is quantum yield for standard solution (quinine sulphate), (I) is the integrated fluorescence intensity, \({\varvec{\eta}}\) is the refractive index of the water and A is absorption. B@CQDs have quantum yield 38.44%.Spectrophotometric and spectrofluorimetric equipment were used to analyses the quantum dots' spectrum properties. Two peaks at 209 and 312 nm seen in Fig. 4a. These peaks were referred to as the π-π* electronic transition of C = C and the n-π* electronic transition of C = O which are related to the synthesized B@CQDs.Furthermore, B@CQDs provides an emission peak at 435 nm (excitation at 370 nm), which indicates carbon optical properties. Fluorescence (FL) spectra of B@CQ-dots were studied with change wavelength excitation from 340 to 430 nm. Increasing excitation led to a red shift in the emission of B@CQ dots followed by a decrease in RFI, that validates carbon dots excitation-dependent emission [14] Fig. 4b.
Under various settings, the reaction between ELX and B@CQDs was optimized. The influence of pH on fluorescence enhancement was investigated in the range of 5.5 to 7.3, the highest fluorescence intensity was achieved using pH 6.4 ± 0.3 (Additional file 1: Fig. S2a) with a buffer concentration equal to 0.02 M Additional file 1: Fig. S2b.
Furthermore, the effect of B@CQD concentration was investigated from 0.005 to 0.035 mg mL−1, the greatest fluorescence obtained using 0.012 mg mL−1 and not affected by increasing B@CQD volume. As a result, the optimum concentration of B@CDs was determined to be 0.015 mg mL−1 (0.5 mL) Additional file 1: Fig. S2c.
At varied time intervals spanning from 0 to 20 min as in Additional file 1: Fig. S2d, the efficiency of fluorescence amplification in the presence of ELX was studied. The maximum fluorescence increase of B@CQDs was achieved after 8 min, so 10 min was used as the optimum reaction time.
A suggested reaction mechanism between ELX and B@CQDs
The hydrogen bonding and electron-donor–acceptor complex between B@CQDs and ELX could explain the fluorescence enhancement technique. Based on the two highlighted, active, and strong neighboring hydrogen bonds between the hydroxyl/carboxyl of B@CQDs and fluorine atoms of ELX [14, 17, 21], the existence of carboxyl, hydroxyl, and trivalent boron groups offers viability to conjugate with the referenced analyte. Strong intermolecular hydrogen bonds can form a center that connects two molecules that are next to each other. The types of interactions (OH.F and/or OH.N) and molecular symmetry are connected to the hydrogen bond strength and amount of resonance within the hydrogen-bonded system in all circumstances [14, 21].
Reaction of ELX with B@CQDs validation
The reaction was validated in accordance with ICH and FDA guidelines [28, 29]. Plotting different concentrations of ELX with B@CQDs against RFI was used to investigate the reaction's sensitivity. The calibrated range was found to be 4 – 100 ng mL−1 with the regression equation y = 10.0493x + 1585 as shown in Table 1, the lower limit of quantitation (LOQ) was found to be 1.74 ng mL−1 and the lower limit of detection (LOD) was determined to be 0.57 ng mL−1. The results show that the proposed approach has high sensitivity.
The accuracy of B@CQDs with ELX was investigated using five concentrations (10, 20, 50, 90, and 100 ng mL.−1) within the calibration range, the percent of recoveries were ranged from 99.86 to 100.65 and RSD values were ranged from 0.21 to 1.00. The results show that the proposed approach is high accurate. Table 2
While, the intra-day precision of the presented method was tested at three concentration levels (10, 50 and 100 ng mL−1) at three successive measurements. While the inter-day precision was investigated using three concentrations measured as three replicates for three consecutive days. The results obtained refer to excellent repeatability Table 2.
In order to evaluate the interference from plasma, the effect of matrix solution was established with ELX using three levels of quality control samples of the investigated drug. The percent of recovery ± RSD ranged from 94.05 ± 0.99 to 97.90 ± 1.44. The results indicated the absence of interference from the matrix with ELX under different conditions and referred to the high selectivity of the proposed method as shown in Additional file 1: Table S1.
Incurred sample reanalysis (ISR) is a very important parameter to evaluate accuracy and precision of incurred samples in bio-analytical validations using FDA guidelines. In the presented study, the percentage difference between the initial and incurred samples was found to be 3.40%. According to FDA guidelines, the results of incurred samples met the accepted criteria as shown in Additional file 1: Table S2.
Selectivity of the reaction
The selectivity of the proposed method was studied using external materials as (sucrose, glycine, urea, Ca2+, Cu2+ and cystine) and different analgesic as tenoxicam, diclofenac. No interference of the external materials was observed as in Additional file 1: Fig S3. It was observed that non-significant enhancement was observed with sucrose, glycine, and urea. However quenching effect was observed with Ca2+, Cu2+, tenoxicam and diclofenac due to the absence of fluorine atom that forming hydrogen bonding with B@CQDs [14, 21]. The results indicate to the high selectivity of this work.
Applications of ELX and B@CQDs method
The method was successfully applied in spiked, real human plasma and its formulation. The percent of recovery in spiked human plasma was observed to be 98.80 ± 0.92 as in Table 3.
Determination of ELX in real human plasma (pharmacokinetic study) was carried out about its therapeutic level, peak plasma level (C max) of cited drug was found to be 570 ± 5.32 ng mL−1 after administration of ELX 150 mg/ tablet as single oral dose which agrees with other reported one [30]. All the parameters of PK were recorded in Table 4.
Besides, B@CQDs was applied for determination of ELX in pharmaceutical dosage form, and the obtained results were found to be satisfactory with a good recovery (99.70 ± 0.69) with t value (1.40) and F value (2.86) compared with other reported method [10]. The evaluation of content uniformity test for ELX was performed by applying the general procedure according to USP guidelines [17, 18]. The content of individual dosage form was analyzed then percentage recoveries were calculated individually. The percent of recovery was recorded at Table 5.
Comparison of the presented method with the reported methods
Comparing the results in our work with other reported as in Table 6. It was found B@CQDs can serve as a probe for the detection of ELX in a low concentration with higher sensitivity and reliability than other reported methods.
Conclusion
This work presents a sensitive, selective, and low-cost spectrofluorometric method for determination of elagolix (ELX) using B@CQDs with LOQ equal to 1.74 ng mL−1. It was successfully applied for determination of ELX in pharmaceutical dosage form, content uniformity and pharmacokinetic study. Pharmacokinetic parameters were established as Cmax was found to be 570 ± 5.32 ng. mL−1 after 1 h, t ½ was found to be 6.50 h and AUC was found to be 1290 ± 30.33 ng. h. mL−1. B@CQDs were confirmed using transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (PXRD), dynamic light scattering (DLS), and Fourier-transform infrared spectroscopy (FTIR).
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its additional files].
Abbreviations
- ELX:
-
Elagolix
- B@CQDs:
-
Boron doped quantum dots
- RFI:
-
Relative fluorescence intensity
- Cmax :
-
Maximum plasma concentration
- AUC:
-
Area under curve
- XPS:
-
X-ray photoelectron spectroscopy
References
Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200.
Rolla E. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res. 2019;8:1–28. https://doi.org/10.12688/f1000research.14817.1.
Alimi Y, Iwanaga J, Loukas M, Tubbs RS. The clinical anatomy of endometriosis: a review. Cureus. 2018;10:336. https://doi.org/10.7759/cureus.3361.
Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–75. https://doi.org/10.1038/nrendo.2013.255.
Lund I, Lundeberg T. Is acupuncture effective in the treatment of pain in endometriosis? J Pain Res. 2016;9:157–65. https://doi.org/10.2147/JPR.S55580.
Mira TAA, Buen MM, Borges MG, Yela DA, Benetti-Pinto CL. Systematic review and meta-analysis of complementary treatments for women with symptomatic endometriosis. Int J Gynecol Obstet. 2018;143:2–9. https://doi.org/10.1002/ijgo.12576.
Morotti M, Vincent K, Becker CM. Mechanism of pain of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:8–13. https://doi.org/10.1016/j.ejogrb.2016.07.497.
Perricos A, Wenzl R. Efficacy of elagolix in the treatment of endometriosis. Expert Opin Pharmacother. 2017;18:1391–7. https://doi.org/10.1080/14656566.2017.1359258.
Todkar PP, Hamrapurkar PD. Development and validation of a stability-indicating reversed-phase high-performance liquid chromatography method for elagolix sodium using quality by design approach. IJPQA. 2021;12:1–12.
Ahmed RM. Greenness assessment of micellar spectrofluorometric approach for determination of Elagolix: application to dosage form, content uniformity and human plasma. Heliyon. 2021;7: e08521. https://doi.org/10.1016/j.heliyon.2021.e08521.
Wang Q, Gu E, Chen C, Xu R, Luo S. Analytical methodology and pharmacokinetic study of elagolix in plasma of rats using a newly developed UPLC-MS/MS assay. Arab J Chem. 2021;14: 103235. https://doi.org/10.1016/j.arabjc.2021.103235.
Salman BI, Hassan YF, Eltoukhi WE, Saraya RE. Quantification of tyramine in different types of food using novel green synthesis of ficus carica quantum dots as fluorescent probe. Luminescence. 2022. https://doi.org/10.1002/bio.4291.
Jafarieh MP, Akbarzadeh A, Salamat-Ahangari R, Moghaddam MP, Jamshidi-Ghaleh K. Solvent effect on the absorption and emission spectra of carbon dots: evaluation of ground and excited state dipole moment. BMC Chemistry. 2021;15:1–8. https://doi.org/10.1186/s13065-021-00779-6.
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM. Mannitol capped magnetic dispersive micro-solid-phase extraction of polar drugs sparfloxacin and orbifloxacin from milk and water samples followed by selective fluorescence sensing using boron-doped carbon quantum dots. J Enviro Chem Eng. 2021;9: 105078. https://doi.org/10.1016/j.jece.2021.105078.
Salman BI, Ali MFB, Marzouq MA, Hussein SA. Utility of the fluorogenic characters of benzofurazan for analysis of tigecycline using spectrometric technique; application to pharmacokinetic study, urine and pharmaceutical formulations. Luminescence. 2019;34:175–82. https://doi.org/10.1002/bio.3590.
Salman BI, Saraya RE. Bio-analytically fluorimetric method for estimation of ertapenem in real human plasma and commercial samples; application to pharmacokinetics study. Luminescence. 2022;35:796–802. https://doi.org/10.1002/bio.4223.
Salman BI, Ibrahim AE, El Deeb S, Saraya RE. Fabrication of novel quantum dots for the estimation of COVID-19 antiviral drug using green chemistry: application to real human plasma. RSC Adv. 2022;12:16624–31. https://doi.org/10.1039/D2RA02241A.
United States Pharmacopeial Convention, (905) Uniformity of dosage units. stage 6 harmonization. 2011;3:4–6.
Saraya RE, Hassan YF, Eltukhi WE, Salman BI. J Fluoresc. 2022. https://doi.org/10.1007/s10895-022-02979-2.
Samant V, Singh AK, Ramakrishna G, Ghosh HN, Ghanty TK, Palit DK. Ultrafast intermolecular hydrogen bond dynamics in the excited state of fluorenone. J Phys Chem A. 2005;109:8693–704. https://doi.org/10.1021/jp050848f.
Hua J, Jiao Y, Wang M, Yang Y. Determination of norfloxacin or ciprofloxacin by carbon dots fluorescence enhancement using magnetic nanoparticles as adsorbent. Microchim Acta. 2018;185:137. https://doi.org/10.1007/s00604-018-2685-x.
Liu ZQ, Fang Q, Wang D, Cao DX, Xue G, Yu W, Lei H. Trivalent boron as an acceptor in donor-pi-acceptor-type compounds for single- and two-photon excited fluorescence. Chem Eur J. 2003;9:5074–84. https://doi.org/10.1002/chem.200304833.
Hill S, Galan MC. Fluorescent carbon dots from mono- and polysaccharides: synthesis, properties and applications. Beilstein J Org Chem. 2017;13:675–93. https://doi.org/10.3762/bjoc.13.67.
Shen P, Xia Y. Synthesis-modification integration: one-step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Anal Chem. 2014;86:5323–9. https://doi.org/10.1021/ac5001338.
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM. Development of dual function polyamine-functionalized carbon dots derived from one step green synthesis for quantitation of Cu2+ and S2− ions in complicated matrices with high selectivity. Anal Bioanal Chem. 2020;412:1353–63. https://doi.org/10.1007/s00216-019-02362-4.
Cordes DB, Gamsey S, Singaram B. Fluorescent quantum dots with boronic acid substituted viologens to sense glucose in aqueous solution. Angew Chem Int Ed. 2006;45:3829–32. https://doi.org/10.1002/anie.200504390.
Lan M, Zhang J, Chui YS, Wang H, Yang Q, Zhu X, Wei H, Liu W, Ge J, Wang P. A recyclable carbon nanoparticle-based fluorescent probe for highly selective and sensitive detection of mercapto biomolecules. J Mater Chem B. 2015;3:127–34. https://doi.org/10.1039/C4TB01354A.
International Conference on Harmonization, Topic Q2 (R1) Validation of analytical procedures: text and methodology. 2005.
Zimmer D. New US-FDA draft guidance on bio-analytical method validation versus current FDA and EMA guidelines: chromatographic methods and ISR. Bioanalysis. 2014;6:13–9. https://doi.org/10.4155/bio.13.298.
Shebley M, Polepally AR, Nader A, Ng JW, Winzenborg I, Klein CE, Noertersheuser P, Gibbs MA, Mostafa NM. Clinical pharmacology of elagolix: an oral gonadotropinreleasing hormone receptor antagonist for endometriosis. Clin Pharmacokinetic. 2020;59:297–309. https://doi.org/10.1007/s40262-019-00840-7.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
BS and YH conceived and designed the experiments. AH and RS conducted the experiments and interpreted the results. All participated in analyzing the data and writing the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the method was carried out in accordance with relevant guidelines and regulations according to the Declaration of Helsinki 1975, as revised in 2008. The method and the study was approved by the Egyptian Network of Research Ethics Committees (ENREC). The authors confirmed informed consent was obtained from all subjects participating in the experiments.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
PXRD for undoped carbon quantum dots. Fig. S2. optimization of B-CDs reaction a effect of pH, b effect of volume of buffer, c volume of B-CDs using ELX (50 ng mL-1), d reaction time. Fig. S3. Selectivity of B@CQDs to ELX. Table S1. Stability and selectivity of ELX in human plasma using different stability conditions. Table S2. Incurred sample reanalysis data of ELX.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salman, B.I., Hassan, A.I., Hassan, Y.F. et al. Ultra-sensitive and selective fluorescence approach for estimation of elagolix in real human plasma and content uniformity using boron-doped carbon quantum dots. BMC Chemistry 16, 58 (2022). https://doi.org/10.1186/s13065-022-00849-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-022-00849-3