Abstract
Background
One of the most popular techniques for cancer detection is the nuclear medicine technique. The present research focuses on Platelet-12-lipoxygenase (P-12-LOX) as a promising target for treating and radio-imaging tumor tissues. Curcumin was reported to inhibit this enzyme via binding to its active site.
Results
A novel curcumin derivative was successfully synthesized and characterized with yield of 74%. It was radiolabeled with the diagnostic radioisotope technetium-99m with 84% radiochemical yield and in vitro stability up to 6 h. The biodistribution studies in tumor bearing mice confirmed the high affinity predicted by the docking results with a free binding energy value of (ΔG −50.10 kcal/mol) and affinity (13.64 pki) showing high accumulation in solid tumor with target/non-target ratio >6.
Conclusion
The newly synthesized curcumin derivative, as a result of a computational study on platelet-12 lipoxygenase, showed its excellent free binding energy (∆G −50.10 kcal/mol) and high affinity (13.64 pKi). It could be an excellent radio-imaging agent that targeting tumor cells via targeting of P-12-LOX.

This novel curcumin derivative was successfully synthesized and radiolabeled with technetium-99m and biologically evaluated in tumor bearing mice that showed high accumulation in solid tumor with target/non-target ratio >6 confirming the affinity predicted by the docking results. Predicted binding mode of a new curcumin derivative in complex with 12-LOX active site. b Curcumin itself in the 12-LOX active site biological distribution of 99mTc-curcumin derivative complex in solid tumor bearing Albino mice
Similar content being viewed by others
Background
Cancer is the main cause of mortality worldwide and the number of cancer patients is increasing at an alarming rate. Early detection of cancer greatly increases the chances of saving the patient’s life [1–3]. One of the most popular techniques for cancer detection is the nuclear medicine technique that is a breakthrough in the cancer imaging procedures [4, 5]. Technetium-99m is the most popular gamma emitting radionuclides used in nuclear medicine due to its perfect characteristics (6.02 h half-life and 140 keV γ-ray energy) and to its low cost and good availability [3–7]. In order to develop a successful radioactive tracer for cancer targeting, a selective organic compound that can differentiate between tumor and normal cells is extensively needed [8]. Curcumin is a natural product isolated from the rhizomes of the Indian Curcuma longa plant [9]. The Indian culture used curcumin as a food-flavoring agent, coloring agent and also in medicine as antiseptic, analgesic and antimalarial agent [10]. Researches proved that curcumin possesses other diverse of biological activities including antiviral [11], antibacterial [12], antifungal [13], anti-inflammatory [14], and antioxidant activities [15]. Recently, curcumin has drawn much attention due to its powerful anti-proliferative effect and anticancer activity in multiple cancers including ovarian, pancreatic, breast, melanoma, neck, colon, prostate, and head cancers [16–20]. The anticancer effect is manifested through the induction of apoptosis, growth arrest, and inhibition of the tubulin polymerization [21–23]. Furthermore, studies have shown that curcumin appeared as cytotoxic to cancer cells and cytoprotective to normal cells indicating that curcumin could be used as a selective safe radiotracer [24]. Specific enzyme targeting that is overexpressed in cancer, by using selective radiolabeled inhibitor of this enzyme, could be a great approach for treatment, imaging and diagnosis of cancers. The targeting process is a highly selective step that can be achieved by the computational approach [25]. The high over-expression of the Platelet-12 Lipoxygenase (P-12-LOX) was reported in different cancer tissues [26, 27]. Inhibition of such enzyme is considered to be a promising target for cancer treatment. To date few P-12-LOX inhibitors are known. Curcumin was reported to inhibit P-12-LOX via binding to its active site [28, 29]. The development of a novel curcumin derivative, which possesses higher free binding energy and good affinity to P-12-LOX, was one of the main objectives of this work. This is to select the highly predicted selective inhibitor of P-12-LOX to be synthesized then radiolabeled with technetium-99m followed by its in vivo evaluation as a novel target agent to P-12-LOX receptor in cancer cells.
Experimental
Chemicals
Curcumin and 2,4,6-trimethylbenzoyl chloride were purchased from Sigma-Aldrich, Steinheim, Germany. Analytical grade chemicals were directly used without further purification. All solutions were prepared using deionized water. Technetium-99m was eluted as 99mTcO4 − from 99Mo/99m Tc generator, Elutec Brussels, Belgium.
Instruments
Mettler FP 80 melting point apparatus was used to determine the melting points that were uncorrected. Ultrospec-2100 Pro UV visible spectrophotometer was used to record ultraviolet (UV) spectrum. Infrared (IR) spectra were recorded on FT/IR Shimadzu, Fourier transform, Infrared spectrometer/cm scale using KBr disc technique. 1H NMR and 13C NMR were carried out on Burker AC 500 MHz Spectrometer; chemical shifts are expressed in δ (ppm) downfield from TMS as an internal standard. Accela U-HPLC system coupled to a TSQ Quantum Access MAX triple stage quadrupole mass spectrometer carried out the LC–MS analysis (Thermo Scientific Corporation, USA) that was controlled with Xcalibur software version 2.2. Radioactivity measurements were done using Sodium Iodide (Tl) γ-ray scintillation counter (Scaler Ratemeter SR7, Nuclear Enterprises, Edinburgh, England). Ascending thin layer chromatography (TLC) run on pre-coated (0.25 mm) (GF 254) silica gel plates were used to follow up the reaction and the homogeneity of the compound. Routinely, used developing solvents system was C6H6:EtOAc:CHCl3 (5:1:5) (in ratio v/v). UV lamp at 254 nm was used to visualize the spots. Silica gel (60-230 mesh E. Merck) was used after heating at 110 °C for 1 h and was used for column chromatography separations. Silica gel 60 GF254 for TLC was used for coating 20 × 20 cm glass plates for preparative TLC.
Animals
The biological distribution was evaluated in 20–25 g Albino mice.
Software programs
Molecular Operating Environment (MOE) package license was purchased from Chemical Computing Group Inc, Sherbooke St, Montreal, QC, Canada [30].
Molecular docking
All compounds were built and saved as MOE. Rigid receptor was used as a docking approach. Receptor and solvent were kept as a “receptor’’. Triangle matcher was used as a placement method with timeout of 300 s. Two rescoring were computed; rescoring 1 was selected as London dG while rescoring 2 was selected as affinity. Force field was used as a refinement. The best conformation for each compound was kept inside the docking pocket and the affinity (pKi) was computed. The free energy of binding ∆G (kcal/mol) for the proposed derivatives were also recorded.
Synthesis of curcumin derivative (1,7-bis[((4′-(2″,4″,6″-trimethylbenzoyl)oxy)-3′-methoxyphenyl]-1,6-heptandiene-3,5-dione)
To an ice-cold curcumin solution (1) (3.68 g, 0.01 mol) in 50 ml dry acetone, 2 g of sodium carbonate was added with stirring for 15 min. The 2,4,6-trimethylbenzoyl chloride (0.025 mol) was added dropwise to the mixture over a period of 30 min. The reaction was then refluxed for 12 h [31]. The reaction mixture was filtered, evaporated then extracted using EtOAc (3 × 30 ml). The combined organic layers were dried using anhydrous MgSO4 and the solvent volume was reduced under reduced pressure to give a yellow precipitate, which was recrystallized from ethanol to give compound (2) (Scheme 1) with yield 74%, mp 210–212 °C, molecular formula C41H40O8 and M.Wt. 660.
Preparation of 99mTc-curcumin derivative complex
Curcumin (150 μg) was dissolved in 1 ml DMSO in 10 ml penicillin vials. Then about 10 mg of NaBH4 was added to each vial with pH adjustment in a range of 6–10 using of 0.1 N sodium hydroxide or 0.1 N Hydrochloric Acid. Followed by the addition of 100 μl of freshly eluted 99mTcO4 − (~200 MBq) to each vial. These reactions were performed for 15 min at room temperature. These procedures were repeated to evaluate the radiochemical yields with varying NaBH4 amounts (5–30 μg), varying curcumin amounts (50–300 μg), on different time scale (5–60 min).
Radiochemical yield assay of 99mTc-curcumin derivative complex
99mTc-curcumin derivative complex radiochemical yield and in vitro stability were evaluated by using strips of ascending Whatman paper chromatography (PC). Two strips were used per experiment, on which two drops of the reaction product were placed on origin line at distance of 2 cm from the bottom.
For the determination of the ratio of free 99mTcO4 − radio-contaminant, acetone was used as a developing solvent for one PC strip, where free 99mTcO4 − Rf was 1 while 99mTc-curcumin derivative complex and reduced hydrolyzed technetium colloid species Rf was zero.
Another strip was developed in C2H5OH:H2O:NH4OH mixture (2:5:1, v/v/v) to determine the ratio of the hydrolyzed 99mTc radio-contaminant, where reduced hydrolyzed technetium colloid Rf is zero while free 99mTcO4 − and 99mTc-curcumin derivative complex species Rf is 1.
At the end of the developing process, the strips were dried, cut into 1 cm pieces and counted using the sodium iodide (Tl) γ-ray scintillation counter. Each experiment was repeated three times.
The radiochemical yield percent of 99mTc-curcumin derivative complex was calculated according to the following equation:
Biological distribution
The animal ethics committee guided this study was following the guidelines of the Egyptian Atomic Energy Authority. The biological distribution was evaluated in mice bearing solid tumor.
Solid tumor induction in mice
The solid tumor induction was done using Ehrlich ascites carcinoma (EAC) that was derived from a murine mammary carcinoma [32, 33]. The parent tumor line EAC had been derived from 7 days old donor female Swiss Albino mice and diluted with sterile physiological saline solution. To induce a solid tumor, about 0.2 ml solution was I.M. injected in the female Albino mice right thigh for 4–6 days [3, 5, 34].
Biodistribution assay of 99mTc-curcumin derivative complex
The biodistribution study of 99mTc-curcumin derivative complex was evaluated at time intervals of 5, 15, 30, 60, 120 and 180 min post injection (p. i) in solid tumor bearing Albino mice (n = 5 mice/time point). Mice were separated in groups and supplied with food and water. 99mTc-curcumin derivative complex was I.V. injected in the mice tail vein.
Firstly, animals were anaesthetized using chloroform, then weighted and sacrificed at different time intervals. All body organs and tissues were separated, collected and washed with saline then weighted. Blood, bone and muscle samples were collected and weighted then were assumed to be 7, 10 and 40% of the total body weight, respectively [3, 35]. The organs radioactivities as well as the background were measured in a well type γ-counter NaI(Tl). In a population of five, the percent injected dose/gram organ or tissue (%ID/g) were calculated. Target (solid tumor) to non-target (normal muscle) ratio (%T/NT) was calculated from %ID/g for solid tumor and normal muscle.
Statistical analysis
Graph Pad Prism version 6.0 software was used to do all the statistical analyses. Statistical analysis was conducted using one-way ANOVA followed by multiple Tukey–Kranes post hoc test at ʋ < 0.05 considered for statistical significance.
Results and discussion
Computational selection of the best curcumin derivative
Selectivity plays a major role in drug targeting process. It can help in identifying the most suitable ligand (key) for a specific enzyme (lock). Computational approaches are widely used nowadays to compute the free energy of binding, affinity and other parameters like LogP that have an indication of good fitting and predictive high selectivity. P-12-LOX is overexpressed in many tumor tissues [36]. Arachidonic acid is metabolized by P-12-LOX to produce a hydroxyeicosatetraenoic acid that has been reported to be a main cause of cancer development [37, 38]. Thus, inhibition of P-12-LOX can decrease both cell proliferation and metastasis [39]. Curcumin was reported to inhibit P-12-LOX (66 µmol/l) [28, 29]. Also, a number of synthetic curcuminoids were reported to have a promising P-12-LOX inhibitory activity [40]. Compound E26C, a curcumin derivative with benzofuran moiety, had P-12-LOX inhibitory activity IC50 = 17 µmol/l and showed the best fitting distance 3.3 Å among all the reported curcuminoids (Fig. 1). The discovery of a curcumin derivative that can possess high affinity toward P-12-LOX and can be radiolabeled for tumor imaging was the main objective of this study. Radiolabeling of a highly selective P-12-LOX inhibitor will also ensure high accuracy of tumor cells imaging, as this enzyme is overexpressed in tumor tissues as mentioned before.
The study aimed to propose different curcumin derivatives that possess different substitution on the aromatic ring and that simulate the lead structure (Fig. 1).
A number of curcumin derivatives with a substituted phenyl ring at the same position of the lead derivative were designed (Fig. 2). The computational affinity (pKi), cLogP and free binding energy (ΔG) were computed and compared to those of both curcumin itself and the reported benzofuran derivative (Table 1).
It was clear that E26C had more inhibitory activity than curcumin, it showed higher affinity (pKi) 10.40 and higher cLogP (7.9) as well. Besides, its free binding energy was very less and favorable (−51.65 kcal/mol) than that of curcumin (−30.85 kcal/mol).
As a result, from all the proposed structures, we were looking for the one with higher affinity, less free binding energy and higher cLogP than curcumin and comparing it with E26C as well. The 2,4,6-trimethyl phenyl derivative showed almost the same cLogP (7.7) to that of E26C and with high affinity (13.64) that was higher than both of E26C and curcumin. The 2,4,6-trimethyl phenyl derivative also showed less free binding energy (−50.10 kcal/mol) when compared to other proposed derivatives.
The new curcumin derivative showed better free binding energy that was reflected upon the total potential energy, which was lower toward the most stable state in case of the new derivative when compared to that of curcumin complex. In addition, it had a higher affinity (13.64). The binding of the new compound showed good coordination with the iron metal in the active site that was not achieved by the curcumin itself (Fig. 3).
In accordance, the curcumin derivative with (1,7-Bis[((4′-(2″,4,6″-trimethylbenzoyl)oxy)-3′-methoxyphenyl]-1,6-heptandiene-3,5-dione) was selected to be synthesized and radiolabeled with techniethium-99m.
The proposed chemical complex that may be formed between technetium 99m and the top ranked selected curcumin derivative compound with (1,7-Bis[((4′-(2″,4″,6″-trimethylbenzoyl)oxy)-3′-methoxyphenyl]-1,6-heptandiene-3,5-dione) was compared to curcumin-technetium-99m complex. It was proposed that two molecules of curcumin were complexed with one technetium-99m. While, (1,7-Bis[((4′-(2″,4″,6″-trimethylbenzoyl)oxy)-3′-methoxyphenyl]-1,6-heptandiene-3,5-dione) was complexed with by 1:1 ratio in which the technetium-99m formed a complex with the two carbonyl of the benzoyl moieties in addition to that at the 3,5-dione. In the first case the two curcumin molecules formed a long and wide complex while the 2,4,6-trimethyl benzoyl derivative showed a conformation that illustrated a well-fitting. When both complexes were docked, curcumin showed an affinity pki of (33.54) that was lower than that of the selected curcumin derivative that achieved an affinity pki of (45.20) to prove that it has more stability than that of curcumin in enzyme binding in the technetium-99m complex form (Fig. 4).
Chemical synthesis of curcumin derivative
The chemical synthesis was achieved in one-step reaction through reacting 2,4,6-trimethylbenzoyl chloride with curcumin according to the method mentioned previously.
IR (KBr) ʋ (cm−1): 3008 (CH-Ar), 2922, 2854 (CH-aliphatic), 1741 (C=O of ester), 1631 (C=O) 1600 (C=C aromatic), and 1122 (C–O). 1H NMR (CDCl3) δ ppm (500 MHz): 2.35–2.49 (s, 18H, 6 × CH3), 3.94 (s, 6H, 2 × OCH3), 5.91 (s, 1H, H-4), 6.62 (d, 2H, H-2 & H-6, J = 15.5 Hz), 6.96 (s, 4H, H-3″ & H-5″), 7.21–7.29 (m, 6H, Ar–H) and 7.68 (d, 2H, H-1 & H-7, J = 16 Hz). 13C NMR (DMSO-d 6) δ ppm (125 MHz): 20.0 (4 × CH3), 21.2 (2 × CH3), 55.8 (2 × OCH3), 101.9 (C-4), 111.6 (2 × C-2′), 121.2 (2 × C-6′), 123.3 (2 × C-5′), 124.4 (2 × C-1′), 128.7 (C-2, C-6, 2 × C-3″ & 2 × C-5″), 129.6 (2 × C-1″), 134.1 (2 × C-4″), 136.0 (2 × C-2″ & 2 × C-6″), 140.0 (C-1 & C-7), 141.3 (2 × C-3′), 151.7 (2 × C-4′), 167.6 (2 × C=O of ester) and 183.1 (C-3 & C-5). MS: m/z (%) for C41H40O8: 661 [M+ + 1] (26%).
Factors affecting on radiochemical yield of 99mTc-curcumin derivative
Effect of reducing agent (NaBH4) amount
The effect of NaBH4 on the % radiochemical yield of 99mTc-curcumin derivative complex was shown in (Fig. 5). At 5 mg NaBH4, the %99mTcO4 − was equal to 5.3 ± 0.8% that may be due to insufficient amount of NaBH4 to complete the reduction of 99mTcO4 − to form 99mTc-complex. Increasing the amount of NaBH4 to 10 mg, the maximum radiochemical yield (84 ± 1.4%) was revealed. Increasing the NaBH4 greater than 10 mg the radiochemical yield was decreased to 75 ± 1.11% at 30 μg NaBH4.
Effect of pH
As shown in Fig. 6, the radiochemical yield of 99mTc-curcumin derivative complex was affected by pH change. At pH 6, the radiochemical yield was relatively low (72.1 ± 1.2%). While, the maximum radiochemical yield (84 ± 1.4%) was observed at pH 8, where the curcumin combined all the reduced technetium. When the pH increased above 8, the percent radiochemical yield was slightly decreased.
Effect of curcumin derivative amount
The correlation between the radiochemical yield and the amount of curcumin derivative is shown in Fig. 7. The maximum radiochemical yield of 99mTc-curcumin derivative complex (84 ± 1.4%) was obtained at 150 µg curcumin. At low curcumin amount (50 µg), the radiochemical yield was low (74.6 ± 1.3%), where the amount of curcumin was insufficient for forming complex with the reduced technetium. The radiochemical yield was increased by increasing the curcumin amount where a maximum radiochemical yield of 84 ± 1.4% was obtained at 150 µg curcumin.
Effect of reaction time
The formation of 99mTc-curcumin derivative complex was started relatively slowly as the radiochemical yield was 70 ± 1.1% at 5 min reaction time as shown in Fig. 8. The maximum radiochemical yield of 99mTc-curcumin derivative complex was obtained at 30 min. Also, it remained constant up to 1 h.
In vitro stability study
The radiochemical yield of the 99mTc-curcumin derivative complex showed stability for up to 6 h that confirmed its suitability for use during this time period.
Biology study
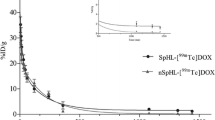
The distribution of 99mTc-curcumin derivative complex was studied in solid tumor-bearing mice (%ID/g) at 5, 15, 30, 60, 120 and 180 min post injection. The accumulation of the %ID/g of 99mTc-curcumin derivative in different body organs and fluids is illustrated in Fig. 9. It was clear that 99mTc-curcumin derivative didn’t accumulate in a specific body organ and was mainly excreted via both the urinary tract and hepatobiliary pathways. The kidneys showed 15.72 ± 3.1% ID/g at 15 min and intestine showed 8.49 ± 1.8% ID/g at 30 min.
The tumor tissue (mouse right leg muscle)/normal tissue (mouse left leg muscle) ratio represents the key factor in the evaluation of the selectivity and sensitivity of 99mTc-curcumin derivative complex to solid tumor. As shown from Fig. 10, the T/NT ratio of 99mTc-curcumin derivative complex in solid tumor-bearing mice was ~1.7 at 15 min post injection and increases to its highest value of ~6.01 at 120 min post injection (p.i.) that clearly prove its high selectivity for the tumor cells.
This high preclinical T/NT ratio presents 99mTc-curcumin derivative complex as a non-invasive probe for solid tumor imaging when compared with many other agents such as: 99mTc-meropenem (3.5 at 1 h p.i.) [7], 99mTc-sunitinib (3 at 1 h p.i.) [3], 99mTc-PyDA (3 at 1 h p.i.) [5], Radioiodinated anastrozole (4.7 ± 0.06 at 2 h p.i.) [41], radioiodinated epirubicin (5.2 ± 0.09 at 1 h p.i.) [42], 99mTc-BnAO-NI (2.59, 2 h) [43], [99mTc(CO)3(IDA–PEG3–CB)]− (3.45, 3 h) [44], 99mTc(CO)3-labeled chlorambucil analog (3.2 at 3 h p.i) [45], 99mTc-nitride-pyrazolo [1,5-a] pyrimidine (2.2 at 1 h p.i.) [46], 99mTc-DETA (2.47 at 4 h p.i.) [32], 99mTc-TETA (2.45 at 4 h p.i.) [32], 99mTc-TEPA (2.91 at 4 h p.i.) [32], 99mTc-citro-folate (4.3 at 4 h p.i.) [47] and 99mTc-gemcitabine (4.9 at 2 h p.i.) [48]. All of these present 99mTc-curcumin derivative complex as a promising solid tumor imaging agent.
Conclusion
A promising curcumin derivative with high affinity 13.64 (pKi) and excellent free binding energy (−50.10 kcal/mol) was selected for chemical synthesis and radiolabeling to target P-12-LOX. It showed a high radiochemical yield of 84% and in vitro stability up to 6 h. Its high accumulation in solid tumor with target/non-target ratio >6 indicated that it could be an excellent radio-imaging agent that targets tumor cells via selectivity of P-12-LOX.
References
Badenhorst J, Todd A, Lindsey L, Husband A (2015) Widening the scope for early cancer detection: identification of alarm symptoms by community pharmacies. Int J Clin Pharm 37(3):465–470
Ma L, Ji L, Yu Y, Wang J (2015) Novel molecular targets for diagnosis and treatment of hepatocellular carcinoma. Discov Med 19(102):7–14
Sakr TM, El-Safoury DM, Awad GAS, Motaleb MA (2013) Biodistribution of 99mTc-sunitinib as a potential radiotracer for tumor hypoxia imaging. J Label Compd Radiopharm 56:392–395
Ibrahim AB, Sakr TM, Khoweysa OMA, Motaleb MA, Abd El-Bary A, El-Kolaly MT (2014) Formulation and preclinical evaluation of 99mTc-gemcitabine as a novel radiopharmaceutical for solid tumor imaging. J Radioanal Nucl Chem 302(1):179–186
Sakr TM, Essa BM, El-Essawy FA, El Mohty AA (2014) Synthesis and biodistribution of 99mTc-PyDA as a potential marker for tumor hypoxia imaging. Radiochemistry 56(1):76–80
Dilworth JR, Parrott SJ (1998) The biomedical chemistry of technetium and rhenium. Chem Soc Rev 27:43–55
Sakr TM, Motaleb MA, Ibrahim IT (2012) 99mTc-meropenem as a potential SPECT imaging probe for tumor hypoxia. J Radioanal Nucl Chem 292(2):705–710
Du C, Ying H, Zhou J, Jiang J, Liu C, Chen J, Wang X, Hu C (2014) Prediction of the response to docetaxel-based chemotherapy for locoregionally advanced nasopharyngeal carcinoma: the role of double-phase (99m)Tc-MIBI SPECT/CT. Med Oncol 31:833–842
Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK (2004) Turmeric and curcumin biological actions and medicinal applications. Curr Sci 87:44–50
Ammon HP, Wahl MA (1991) Pharmacology of Curcuma longa. Planta Med 57:1–7
Chen DY, Shien JH, Tiley L, Chiou SS, Wang SY, Chang TJ, Lee YJ, Chan KW, Hsu WL (2010) Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem 119:1346–1351
Lal J, Gupta SK, Thavaselvamc D, Agarwal DD (2013) Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides. Eur J Med Chem 64:579–588
Chen J, He ZM, Wang FL, Zhang ZS, Liu X, Zhai DD, Chen WC (2016) Curcumin and its promise as an anticancer drug: an analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol 772:33–42
Liu W, Li Y, Yue Y, Zhang K, Chen Q, Wang H, Lu Y, Huang M, Zheng X, Du Z (2015) Synthesis and biological evaluation of curcumin derivatives containing NSAIDs for their anti-inflammatory activity. Bioorg Med Chem Lett 25:3044–3051
Sribalan R, Kirubavathi M, Banuppriya G, Padmini V (2015) Synthesis and biological evaluation of new symmetric curcumin derivatives. Bioorg Med Chem Lett 25:4282–4286
Raghavan S, Manogaran P, Narasimha KKG, Kuppusami BK, Mariyappan P, Gopalakrishnan A, Venkatraman G (2015) Synthesis and anticancer activity of novel curcumin-quinolone hybrids. Bioorg Med Chem Lett 25:3601–3605
Chen QH, Yu K, Zhang X, Chen G, Hoover A, Leon F, Wang R, Subrahmanyam N, Mekuria EA, Rakotondraibe LHA (2015) A new class of hybrid anticancer agents inspired by the synergistic effects of curcumin and genistein: design, synthesis, and anti-proliferative evaluation. Bioorg Med Chem Lett 25:4553–4556
Wang R, Zhang X, Chen C, Chen G, Zhong Q, Zhang Q, Zheng S, Wang G, Chen QH (2016) Synthesis evaluation of 1, 7-diheteroarylhepta-1, 4, 6-trien-3-ones as curcumin-based anticancer agents. Eur J Med Chem 110:164–180
LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang ES (2005) Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 11(19):6994–7002
Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R (2005) Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of I kappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer 104(4):879–890
Magalska A, Sliwinska M, Szczepanowska J, Salvioli S, Franceschi C, Sikora E (2006) Resistance to apoptosis of HCW-2 cells can be overcome by curcumin-orvincristine-induced mitotic catastrophe. Int J Cancer 119:1811–1818
Mosieniak G, Adamowicz M, Alster O, Jaskowiak H, Szczepankiewicz AA, Wilczynski GM, Ciechomska IA, Sikora E (2012) Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech Ageing Dev 133:444–455
Sharm S, Gupta MK, Saxena AK, Bedi PM (2015) Triazole linked monocarbonyl curcumin-isatin bifunctional hybrids as novel antitublin agents: design, synthesis, biological evaluation and molecular modeling studies. Bioorg Med Chem 23:7165–7180
Sood A, Mathew R, Trachtman H (2001) Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to shiga toxin. Biochem Biophys Res Commun 283(1):36–41
Essa BM, Sakr TM, Khedr MA, El-Essawy FA, El-Mohty AA (2015) 99mTc-Amitrole as a novel selective imaging probe for solid tumor: in silico and preclinical pharmacological study. Eur J Pharm Sci 76:102–109
Tang K, Cai Y, Joshi S, Tovar E, Tucker SC, Maddipati KR, Crissman JD, Repaskey WT, Honn KV (2015) Convergence of eicosanoid and integrin biology: 12-lipoxygenase seeks a partner. Mol Cancer 14:111
Gondek T, Szajewski M, Szefel J, Aleksandrowicz-Wrona E, Skrzypczak-Jankun E, Jankun J (2014) Evaluation of 12-lipoxygenase (12-LOX) and plasminogrn activator inhibitor 1 (PAI-1) as prognostic markers in prostate cancer. Biomed Res Int 2014:102478
Cuendet M, Pezzuto JM (2000) The role of cyclooxygenase and lipoxygenase in cancer chemoprevention. Drug Metabol Drug Interact. 17:109–157
Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS (2004) Modulation of arachidonic acid metabolism by curcumin and related {beta}-diketone derivatives: effects on cytosolic phospholipase A2, cyclooxygenases, and 5-lipoxygenase. Carcinogenesis 25:1671–1679
Molecular Operating Environment (MOE) (2014) 9th edition. Montreal: Chemical Computing Group Inc
Al-Wabli R, AboulWafa OM, Youssef K (2012) Synthesis of curcumin and ethyl curcumin bioconjugates as potential antitumor agents. Med Chem Res 21:874–890
Sheeja KR, Kuttan G, Kuttan R (1997) Cytotoxic and antitumour activity of Berberin. Amala Res Bull 17:73–76
Abu-Zeid M, Hori H, Nagasawa H, Uto Y, Inayama S (2000) Studies of methyl 2-nitroimidazole-1-acetohydroxamate (KIN-804) 2: effect on certain antioxidant enzyme systems in mice bearing ehrlich ascites carcinoma. Biol Pharm Bull 23(2):195–198
Wan W, Yang M, Pan S, Yu C, Wu N (2008) [99mTc]polyamine analogs as potential tumor imaging agent. Drug Dev Res 69(8):520–525
Rhodes BA (1974) Considerations in the radiolabeling of albumin. Semin Nucl Med 4(3):281–293
Timar J, Raso E, Dome B, Li L, Grignon D, Nie D, Honn KV, Hagmann W (2000) Expression subcellular localization and putative function of platelet-type 12-lipoxygenase in human prostate cancer cell lines of different metastatic potential. Int J Cancer 87:37–43
Eling TE, Glasgow WC (1994) Cellular proliferation and lipid metabolism: importance of lipoxygenases in modulating epidermal growth factor dependent mitogenesis. Cancer Metastasis Rev 13:397–410
Honn KV, Timar J, Rozhin J, Bazaz R, Sameni M, Ziegler G, Sloane BFA (1994) A lipoxygenase metabolite, 12-(S)- HETE, stimulates protein kinase C-mediated release of cathepsin B from malignant cells. Exp Cell Res 214:120–130
Connolly JM, Rose DP (1998) Enhanced angiogenesis and growth of 12- lipoxygenase gene-transfected MCF-7 human breast cancer cells in athymic nude mice. Cancer Lett 132:107–112
Jankun J, Aleem AM, Malgorzewicz M, Szkudlarek M, Zavodszky MI, Dewitti DL, Feig M, Selman SH, Skrzypczak-Jankun E (2006) Synthetic curcuminoids modulate the arachidonic acid metabolism of human platelet 12-lipoxygenase and reduce sprout formation of human endothelial cells. Mol Cancer Ther 5(5):1371–1382
Badr AB, Sakr TM, Khoweysa OMA, Motaleb MA, Abd El-Bary A, El-Kolaly MT (2015) Radioiodinated anastrozole and epirubicin as potential targeting radiopharmaceuticals for solid tumor imaging. J Radioanal Nucl Chem 303(1):967–975
Hsia CC, Huang FL, Hung GU, Shen LH, Chen CL, Wang HE (2010) The biological characterization of 99mTc-BnAO-NI as a SPECT probe for imaging hypoxia in a sarcoma-bearing mouse model. Appl Radiat Isot 69(4):649–655
Wang J, Yang J, Yan Z, Duan X, Tan C, Shen Y, Wu W (2011) Synthesis and preliminary biological evaluation of [99mTc(CO)3(IDA–PEG3–CB)]− for tumor imaging. J Radioanal Nucl Chem 287(2):465–469
Satpati D, Korde A, Venkatesh M, Banerjee S (2009) Preparation and bioevaluation of a 99mTc-labeled chlorambucil analog as a tumor targeting agent. Appl Radiat Isot 67(9):1644–1649
Ding R, He Y, Xu J, Liu H, Wang X, Feng M, Qi C, Zhang J, Peng C (2012) Preparation and bioevaluation of 99mTc nitrido radiopharmaceuticals with pyrazolo[1,5-a]pyrimidine as tumor imaging agents. Med Chem Res 21:523–530
Altiparmak B, Lambrecht FY, Bayrak E, Durkan K (2010) Design and synthesis of 99mTc-citro-folate for use as a tumor targeted radiopharmaceuticals. Int J Pharm 400(1–2):8–14
Machac J, Krynyckyi B, Kim C (2002) Peptide antibody imaging in lung cancer. Semin Nucl Med 32(4):276–292
Badr AB, Sakr TM, Khoweysa MA, Motaleb OMA, Abd El-Bary A, El-Kolaly MT (2014) Formulation and preclinical evaluation of 99mTc-gemcitabine as a novel radiopharmaceutical for solid tumor imaging. J Radioanal Nucl Chem 302(1):179–186
Authors’ contributions
RIA, TMS & MAK had formulated the research idea and results interpretation. RIA prepared the title compound and wrote the organic synthesis part. TMS & MAM and AAS carried out the radiolabeling and biological distribution experimental work. MAK undertook and wrote the molecular modeling studies. TMS has formulated the discussion and wrote the manuscript. WAZ participated in revising and preparation of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Al-Wabli, R.I., Sakr, T.M.M.H., Khedr, M.A. et al. Platelet-12 lipoxygenase targeting via a newly synthesized curcumin derivative radiolabeled with technetium-99m. Chemistry Central Journal 10, 73 (2016). https://doi.org/10.1186/s13065-016-0220-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-016-0220-x