Abstract
Background
The one anastomosis gastric bypass (OAGB) is being performed by an increasing number of bariatric centers over the world. However, the optimal length of the biliopancreatic (BP) limb remains a topic of discussion. Retrospective studies suggest the benefit of tailoring BP-limb length; however, randomized trials are lacking. The aim of this study is to investigate whether tailoring the length of the BP-limb based on total small bowel length (TSBL) leads to better results in terms of weight loss, vitamin deficiencies, and bowel movements compared to a fixed BP-limb length.
Methods
The TAILOR study is a double-blind single-center randomized controlled trial. Patients scheduled for primary OAGB surgery will be randomly allocated either to a standard BP-limb of 150 cm or to a BP-limb length based on their TSBL: TSBL < 500 cm, BP-limb 150 cm; TSBL 500–700 cm, BP-limb 180 cm; TSBL > 700 cm, BP-limb 210 cm. The primary outcome is to compare the percent total weight loss (%TWL) at 5 years between the two groups. Secondary outcomes include nutritional deficiencies, remission of comorbidities, symptoms of dumping, quality of life, and daily bowel movements. The study includes a total of 212 patients and is designed to detect a 5% difference in the primary endpoint.
Discussion
The TAILOR study will provide new insights into the effect of different BP-limb lengths and the role of the TSBL in the OAGB. The study is designed to provide guidance for bariatric surgeons to determine the optimal BP-limb length in the OAGB.
Trial registration
Dutch Trial Register NL7945. Prospectively registered on 08 September 2019. NTR (trialregister.nl)
Similar content being viewed by others
Administrative information
Trial registration | Dutch trial register: NL7945 |
Date registration | 09-08-2019 |
Trial status | We started participant recruitment in September 2020 and we expect to complete the recruitment in November 2022. |
Version | 2 |
Sponsor | Medical Center Leeuwarden 8934 AD Leeuwarden The Netherlands |
Phone: +31 58 286 6969 | |
Principal investigator(s) | L.J.M. de Heide, MD, Internist-Endocrinologist, Center for Obesity Northern Netherlands (CON)/Medical Center Leeuwarden |
Roles and responsibilities | Daily support and coordination of the study is provided by: Principle investigator: supervision of the study, annual safety reports, and trial registration. Study coordinator: coordinates study, ensures follow-up according to protocol, reporting Serious Adverse Events (SAE), takes informed consent, organizes data capture and collection, and follow-up of study patients. Surgeons: identifies potential recruits, explanation of the study, and applying the intervention. Study physician/physician assistant: explanation of the study, takes informed consent, data collection, and follow-up of study patients. |
Introduction
The one anastomosis gastric bypass (OAGB) is currently one of the most effective treatment options for morbid obesity. It is a technically simpler procedure compared to the gold standard, the Roux-en-Y gastric bypass (RYGB), and has proven to have equivalent outcomes in terms of weight loss and reduction of comorbidities [1,2,3]. Currently, there is no standard guideline for the optimal biliopancreatic (BP) limb length for the OAGB. The aim of a suitable BP-limb is to achieve optimum weight loss in combination with a minimum of side effects as nutritional deficiencies and diarrhea [4, 5]. There is substantial variation in the determination of the BP-limb length, as some surgeons use a fixed length and some tailor the length based on body mass index (BMI), age, gender, or comorbidities [1, 2, 4, 6,7,8]. Furthermore, the lengths of the applied BP-limb vary between less than 150 cm and more than 250 cm.
Evidence exists in RYGB surgery that the length of the BP-limb influences weight loss. Nergaard et al. performed a randomized controlled trial (RCT) comparing a RYGB with a BP-limb of 200 cm with an alimentary limb of 60 cm to a RYGB with a BP-limb of 60 cm with an alimentary limb of 150 cm. The longer BP-limb length led not only to more weight loss, but also to more bowel movements and micronutrient deficiencies [9]. Zorrilla et al. performed a systematic review on limb length in RYGB and found weight loss on the whole was better in patients with longer BP-limbs [10].
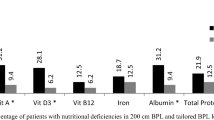
Compared to the RYGB, there is less research about the effect of different limb lengths in the OAGB. A study of Ahuja et al. adjusted the BP-limb length in OAGB from 150 to 250 cm depending on age, sex, BMI, comorbidities, and diet. They found an increasing number of deficiencies of micronutrients with a BP-limb length of 250 cm [5]. In our own retrospective study, we found that tailoring BP-limb length based on baseline BMI did not abrogate the BMI differences at 3 years with also no differences in the resolution of comorbidities [11].
The total length of the small bowel can vary considerably with measured values between 350 and more than 1000 cm. In a study of 443 patients, the median total small bowel length (TSBL) was 690 cm with a wide SD of 94 cm [12]. The effect of a certain BP-limb length may be influenced by the high variance in TSBL. Two recent retrospective studies showed promising results of less nutritional deficiencies when adjusting the BP-limb based on the TSBL [13, 14]. These retrospective studies emphasize the need for prospective randomized studies.
The aim of this RCT is to investigate if tailoring the BP-limb length based on TSBL is superior compared to a fixed BP-limb length in terms of weight loss, nutritional deficiencies, bowel movements, and resolution of comorbidities.
Methods
Study design
This is a double-blind single-center RCT with two arms and a follow-up period of 5 years. The study is being conducted in a non-academic teaching hospital in the Northern Netherlands. Patients are eligible to participate if they are scheduled for primary OAGB surgery in the Center for Obesity Northern Netherlands (CON) and are willing to participate. All patients comply with the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) guidelines and undergo a multidisciplinary screening. The enrolment of patients started in July 2020 and the first patients underwent surgery in September 2020.
Study population
Inclusion criteria
In order to be eligible to participate in this study, a subject must meet the following criteria:
-
Scheduled for primary OAGB
-
Age between 18 and 65 years
-
BMI > 40 kg/m2 or BMI > 35 kg/m2 with comorbidity: diabetes mellitus type 2 (T2D), hypertension, obstructive sleep apnea (OSA), cardiac disease, or arthrosis
-
No preoperative deficiencies of vitamin B12, D, and iron (measured as ferritin)
-
No use of extra (multi-)vitamin supplements with exception of vitamin D max 800 IU/day
-
Willing to participate with written informed consent before the start of the surgery
-
Able to swallow the multivitamin Fit For Me (FFM) weight loss surgery (WLS) primo (tested before surgery)
-
A completely measured TSBL during laparoscopic surgery
Exclusion criteria
A potential subject who meets any of the following criteria will be excluded from participation in this study:
-
BMI > 50 kg/m2
-
Known gastro-intestinal disease or history of gastro-intestinal disease, e.g., celiac disease and inflammatory bowel disease
-
Known addiction behavior
-
Intolerance to FFM WLS primo multivitamin
-
Pregnancy planning within the first 2 years after surgery
-
Renal or hepatic insufficiency
-
Former abdominal surgery and therefore not able to measure the small bowel length during laparoscopic surgery
-
Parenteral use of vitamin B12
Intervention
During the surgery, patients will be allocated to one of the two surgical treatment arms after complete measurement of the TSBL. Eligible patients will be randomized in equal proportions in both groups.
-
1.
A standard BP-limb length of 150 cm
-
2.
A BP-limb length depending on TSBL measured during the OAGB procedure:
-
TSBL < 500 cm: BP-limb 150 cm
-
TSBL 500–700 cm: BP-limb 180 cm
-
TSBL > 700 cm: BP-limb 210 cm
-
Patients with a TSBL that could not be measured during surgery will be treated according to the current daily practice and will not enter the study. Currently, in our bariatric center, a fixed BP-limb length of 150 cm is performed in daily practice.
Non-investigational product
After bariatric surgery, a lifelong daily intake of WLS multivitamin is recommended. In order to make vitamin supplementation uniform during the study, patients will be provided with daily multivitamin FFM WSL primo during the 5-year follow-up. FFM WSL primo is a multivitamin and mineral containing product with a content that is especially developed for patients after OAGB.
Study parameters
Primary outcome
To compare the percent total weight loss (%TWL) at 5 years between the group with the standard BP-limb length and the group with an adjusted BP-limb length.
Secondary outcome
As shown in Table 1, secondary outcomes are %TWL at the other follow-up moments; percent excess weight loss (%EWL); percent excess BMI loss (%EBMIL); proportion of patients with 22 ≤ BMI ≤ 30 kg/m2; daily bowel movements; total and partial remission of T2D, hypertension, and OSA; nutritional deficiencies; quality of life measured by the OBESI-Q questionnaire [15]; and percentage of patients experiencing dumping symptoms defined by the Dumping Severity Score (DSS) [16]. All secondary outcome parameters will be compared between the groups with the standard BP-limb length and the adjusted BP-limb length. Furthermore, analysis in the subgroups with different TSBL will be performed. Total remission of T2D is defined as an HbA1c less than 48 mmol/mol without diabetes medication in the last 6 months. Improvement of diabetes is defined as a reduction of HbA1c of 10 mmol/mol or more but not reaching remission criteria and/or less anti-diabetic medication. The definition of total remission of hypertension is being able to stop all antihypertensive medication. Improvement of hypertension is defined as less antihypertensive medication. Resolution of OSA is defined by cessation of continuous positive airway pressure (CPAP) or other device use, documented by their own pulmonologist.
Other study parameters
Measurements of CRP, hemoglobin, WBC, thrombocytes, sodium, potassium, creatinine, BUN, ASAT, ALAT, alkaline phosphatase, yGT, and HbA1c are part of the routine investigation in the follow-up and will also be part of the study. The serum will be stored for analysis in the later stage in case of new developments or insights relevant for the study become evident.
Study procedure
Recruitment
Patients will be asked to participate by the bariatric surgeon during their preoperative visit. After a verbal explanation of the study, they will be provided with written patient information and a consent form. After 2–4 weeks, they will be approached by a physician or a physician assistant of the research team. Any questions still not answered will be addressed and patients will be asked to sign the informed consent form. The patient will be asked to swallow one FFM WLS primo capsule in order to see whether they are able to swallow the capsule.
Randomization, blinding, and treatment allocation
Participants will be randomly assigned to either the control or the experimental group with a simple randomization with 1:1 allocation (Fig. 1). Randomization is performed using a table of random figures delivered by an independent party (clinical pharmacist), using sealed envelopes with the treatment allocation. The sealed envelope will be opened by the surgery assisting nurse after the surgeon has succeeded in measuring the TSBL. Only the operating surgeon is aware of the allocation.
An employee of the CON outside the research team will document TSBL, treatment allocation, and BP-limb length in a secured coded database containing only study numbers. The length of the BP-limb and treatment allocation will not be documented in the patient file, and the research team, other CON care providers, and the patient will not be informed.
Preoperative assessment and surgery
Patients will be asked to fill in the OBESI-Q and DSS questionnaires before the surgery. A standard operation protocol for OAGB will be used in all patients and applied by four skilled bariatric surgeons. The surgical technique has been described before by Apers et al. [17]. After the creation of the gastric pouch, the TSBL is measured. The TSBL is measured performing the stepwise hand-over-hand technique using two marked graspers to pass the small bowel in estimated steps of 5 cm. This is the same technique performed in daily practice to measure BP-limb length. Based on the randomization outcome, the standard or adjusted BP-limb length is applied. After the surgery, baseline data of the patients as preoperative weight, blood pressure, comorbidities, perioperative data, and complications will be recorded in the database. On leave of the hospital, the patients will be provided with FFM WSL primo multivitamin capsules for the first half year. All patients will also be treated with 800 IU vitamin D and 500 mg calcium as part of standard care.
Follow-up assessment
Follow-up will take place in accordance with usual care, i.e., after 6 and 18 months and yearly up till 5 years. Extra visits will be at 30, 42, and 54 months. At each follow-up visit, standard care includes inquiry after well-being and complaints, daily bowel movements, current medication use, evaluation of comorbidities, and measurement of body weight, blood pressure, and pulse. Patients will be asked to fill in the OBESI-Q and DSS questionnaires before the follow-up appointment. For counting of compliance, patients will be asked to bring back all used and non-used blisters of the FFM WLS primo multivitamins. Blood samples will be taken in accordance with the standard protocol of follow-up after bariatric surgery, added with 20 ml extra blood draw for selenium and copper measurements at 1-, 3-, and 5-year visits. These samples will be stored in a freezer at a laboratory in the hospital and will be used in the current study and also in the future for ancillary studies. In case of deficiencies of vitamins or micronutrients, they will be substituted according to standard care. Patients receive a package with 6 months of FFM multivitamins at each follow-up visit to improve adherence to follow-up protocol.
Potential harm
Measuring TSBL induces a slight increase in the risk of damaging the bowel compared to the standard procedure of solely measuring the length of the BP-limb. In addition, this measurement will increase the time of surgery with on average 10 min.
In OAGB surgery, surgeons use BP-limb lengths varying between 150 and 250 cm based on personal experience. The different lengths in our study are within these measures. Potential risks are too much or too little weight loss and more than expected deficiencies of vitamins and micronutrients. Potential benefits are a better outcome of weight reduction compared to current usual care without an increase in vitamin and/or micronutrient deficiencies.
Statistics and data management
Sample size calculation
The hypothesis of the study is that the mean %TWL in the experimental group is 5% more than the standard group (40% and 35%, respectively). A sample size of 2 × 92 will achieve 80% power to detect a difference of 5% with estimated group standard deviations of 12 and with a significance level (alpha) of 0.05. With an expected maximum drop-out due to conversion of OAGB to RYGB of 15%, the sample size is 106 patients in each group, leading to a total of 212 participants in the study.
Data management
Data will be recorded on an electronic case report form (CRF) using Gemstracker (Generic Medical Survey Tracker, Erasmus MC Rotterdam). The questionnaires of OBESI-Q and DSS will be sent automatically to the patients by email using the questionnaire function of LimeSurvey (LimeSurvey Project, Hamburg, Germany) in Gemstracker. An automatic reminder will be sent after 7 days. Extracting the data from Gemstracker, data will be coded using a unique numerical code. Data concerning participants will be kept confidential and will only be accessible for study team members. Treatment allocation will be recorded on a password secured hard drive in a database containing only study numbers. Any data required to support the protocol can be supplied on request.
Statistical analysis
The full analysis set will include all randomized patients with at least one post-baseline measurement in accordance with the intention-to-treat principle. The primary outcome and continuous secondary outcomes of the two treatment strategy groups will be compared using a two-sample T-test or Mann-Whitney U-test, for normal (mean ± SD) or skewedly distributed (median [IQR]) data, respectively. For categorical secondary outcomes, a Fisher exact test or a chi-square test will be used. In addition to the abovementioned analyses, a multivariable model will be used to formally correct for the effect modification of potentially relevant factors on the treatment effect. Missing data will remain as missing, and no attempt will be made to estimate or replace missing values. A p-value of ≤ 0.05 will be considered to indicate statistical significance.
Monitoring and quality assurance
Based on the current experience of the surgeons with the procedures, a data safety monitoring board (DSMB) is not considered necessary. An independent physician with experience in trials will be asked to monitor the quality of data acquisition and documentation. The principal investigator will report all serious adverse events (SAE) to the sponsor after obtaining knowledge of the events without undue delay. All adverse events (AE) reported spontaneously by the subject or observed by the research team members will be recorded. The deblinding of the allocated treatment will be performed by an independent person, who is not part of the investigation team. Reasons for premature termination of the study are an unexpectedly high rate of surgical complications due to measuring TSBL or adjusting the BP-limb length as to the discretion of the investigator. Interim analyses will be performed after all patients have completed the visits at 1, 2, 3, and 4 years. A data safety monitoring board was not instituted according to Dutch legislation as this is expected to be a low-risk study.
Withdrawal
Patients who develop complications needing revisional surgery during the first 30 days after the surgery will be withdrawn and replaced. Patients who are not able to tolerate the FFM WSL primo capsule after the surgery, patients who need a conversion from the OAGB to a RYGB, and patients not meeting study compliance will be withdrawn from the study and will not be replaced. They will be analyzed on an intention-to-treat principle and all data up to the exclusion will be used in the study.
Ethics and dissemination
Ethics committee
Ethical approval has been obtained from the medical ethical committee (METC): RTPO, Leeuwarden (NR 1082). In addition, the principal investigator will submit a summary of the progress of the trial to the accredited METC once a year. In the case of any modifications of the study protocol that may impact the conduct of the study, the medical ethical committee will be notified. In accordance with the legal requirement in the Netherlands, the sponsor has an insurance to compensate for injury during and after the study (Article 7 Medical Research Involving Human Subjects Act (WMO).
Public disclosure and publication policy
The results of the study will be published in a peer-reviewed medical journal after agreement of all participating authors. FFM will not be involved in the decision to publish nor in the content of the publication. The Central Committee on Research Involving Human Subjects (CCMO) statement concerning publication policy will be followed.
Discussion
The TAILOR study is the first RCT to investigate the effect of tailoring limb length based on TSBL compared to a fixed BP-limb length in terms of weight loss, nutritional deficiencies, and resolution of comorbidities. This study is designed to provide guidance for bariatric surgeons to determine the optimal BP-limb length in the OAGB.
The available literature exists on retrospective studies with subjective variations, limiting the possibility to compare studies and therefore hinder consensus on the optimal BP-limb length. Furthermore, the impact of the size of the TSBL on bypassing a certain part of the small bowel remains unclear. A short TSBL may result in a short residual bowel with the risk of serious nutritional deficiencies and a long TSBL might result in a long residual bowel with the consequence of poor weight loss. We expect that the outcomes of the TAILOR study will provide knowledge about the effect of different BP-limb lengths and the role of the TSBL in the OAGB. We hypothesize that adjusting the length of the BP-limb based on measured TSBL leads to more weight loss with less development of micronutrient deficiencies and bowel movements compared to a fixed limb length.
This study has some limitations. First, the TSBL is measured using a stepwise hand-over-hand technique using marked graspers to pass the small bowel in estimated steps of 5 cm. As the steps are estimated by the bariatric surgeon, this is not an objective measurement technique. Therefore, it probably results in the variation of the measured lengths of the BP-limb and TSBL. However, this is currently the daily practice. Furthermore, the applied BP-limb lengths of 150, 180, and 210 cm may show little differences, where larger differences might provide more clarity of the effect. Nevertheless, some studies found increasing numbers of micronutrient deficiencies with a BP-limb length of 250 cm, and therefore, we choose all BP-limb lengths below the 250 cm [5].
Availability of data and materials
The datasets analyzed during the current study and statistical code are available from the corresponding author on reasonable request, as is the full protocol.
Abbreviations
- AE:
-
Adverse event
- BMI:
-
Body mass index
- BP:
-
Biliopancreatic
- CCMO:
-
Central Committee on Research Involving Human Subjects
- CON:
-
Center for Obesity Northern Netherlands
- CPAP:
-
Continuous positive airway pressure
- CRF:
-
Case report form
- DSMB:
-
Data safety monitoring board
- DSS:
-
Dumping Severity Score
- EBMIL:
-
Excess BMI loss
- EWL:
-
Excess weight loss
- FFM:
-
Fit For Me
- IFSO:
-
International Federation for the Surgery of Obesity and Metabolic Disorders
- METC:
-
Medical ethical committee
- OAGB :
-
One anastomosis gastric bypass
- OSA:
-
Obstructive sleep apnea
- RCT:
-
Randomized controlled trial
- RYGB:
-
Roux-en-Y gastric bypass
- SAE:
-
Serious adverse events
- T2D:
-
Diabetes mellitus type 2
- TSBL :
-
Total small bowel length
- TWL:
-
Total weight loss
- WLS:
-
Weight loss surgery
- %TWL:
-
(Initial weight − postoperative weight)/initial weight × 100
- %EWL:
-
(Initial weight − postoperative weight)/(initial weight − ideal weight) × 100
- Ideal weight:
-
Weight corresponding to a BMI of 25 kg/m2
- %EBMIL:
-
ΔBMI/(initial BMI − 25) × 100
References
Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15:1304–8.
Carbajo MA, Luque-de-León E, Jiménez JM, Ortiz-de-Solórzano J, Pérez-Miranda M, Castro-Alija MJ. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg. 2017;27:1153–67.
Lee WJ, Ser KH, Lee YC, Tsou JJ, Chen SC, Chen JC. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012;22:1827–34.
Mahawar KK, Parmar C, Carr WRJ, Jennings N, Schroeder N, Small PK. Impact of biliopancreatic limb length on severe protein-calorie malnutrition requiring revisional surgery after one anastomosis (mini) gastric bypass. J Minim Access Surg. 2018;14:37–43 Medknow Publications.
Ahuja A, Tantia O, Goyal G, Chaudhuri T, Khanna S, Poddar A. MGB-OAGB: effect of biliopancreatic limb length on nutritional deficiency, weight loss, and comorbidity resolution. Obes Surg. 2018:28:3439–45.
Chevallier JM, Arman GA, Guenzi M, Rau C, Bruzzi M, Beaupel N, et al. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: outcomes show few complications and good efficacy. Obes Surg. 2015;25:951–8.
Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses - first study from Indian subcontinent. Obes Surg. 2014;24:1430–5.
Lee WJ, Wang W, Lee YC, te Huang M, Ser KH, Chen JC. Laparoscopic mini-gastric bypass: experience with tailored bypass limb according to body weight. Obes Surg. 2008;18:294–9.
Nergaard BJ, Leifsson BG, Hedenbro J, Gislason H. Gastric bypass with long alimentary limb or long pancreato-biliary limb—long-term results on weight loss, resolution of co-morbidities and metabolic parameters. Obes Surg. 2014;24:1595–602.
Zorrilla-Nunez LF, Campbell A, Giambartolomei G, Lo Menzo E, Szomstein S, Rosenthal RJ. The importance of the biliopancreatic limb length in gastric bypass: a systematic review. Surg Obes Relat Dis. 2019;15:43–9. https://doi.org/10.1016/j.soard.2018.10.013 Elsevier Inc.
Slagter N, de Heide LJM, Jutte EH, Kaijser MA, Damen SL. Outcomes of the one anastomosis gastric bypass with various biliopancreatic limb lengths : a retrospective single-center cohort study. Obes Surg. 2021;31:4236–42.
Tacchino RM. Bowel length: measurement, predictors, and impact on bariatric and metabolic surgery. Surg Obes Relat Dis. 2015;11:328–34. https://doi.org/10.1016/j.soard.2014.09.016 Elsevier.
Komaei I, Sarra F, Lazzara C, Ammendola M, Memeo R, Sammarco G, et al. One anastomosis gastric bypass–mini gastric bypass with tailored biliopancreatic limb length formula relative to small bowel length: preliminary results. Obes Surg. 2019;29:3062–70.
Soong TC, Almalki OM, Lee WJ, Ser KH, Chen JC, Wu CC, et al. Measuring the small bowel length may decrease the incidence of malnutrition after laparoscopic one-anastomosis gastric bypass with tailored bypass limb. Surg Obes Relat Dis. 2019;15:1712–8. https://doi.org/10.1016/j.soard.2019.08.010 Elsevier Inc.
Dutch Institute for Clinical Auditing (DICA). Patiënt gerapporteerde uitkomsten (PROMS) bij behandeling van obesitas. 2020. Available from: https://www.obesitaskliniek.nl/wp-content/uploads/2020/07/2020-06-08-Eindrapportage-PROMs-in-de-bariatrische-chirurgie-definitief.pdf
Emous M, Wolffenbuttel BHR, Totté E, van Beek AP. The short- to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life. Surg Obes Relat Dis. 2017;13:1489–500. https://doi.org/10.1016/j.soard.2017.04.028 Elsevier Inc.
Apers J, Wijkmans R, Totte E, Emous M. Implementation of mini gastric bypass in the Netherlands: early and midterm results from a high-volume unit. Surg Endosc. 2018;32:3949–55. https://doi.org/10.1007/s00464-018-6136-x Springer US.
Acknowledgements
N/A
Funding
Research Fund Medical Center Leeuwarden is funding the costs of the study. FitForMe BV, Rotterdam, will provide the funding for the standardized multivitamin supplements (FitForMe Primo) for each study participant during 5 years of follow-up. All funding sources had no role in the design of this study and will not have any role during its analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception and trial design. LJMH obtained funding and ethical approval for the study. NS is the coordinator of the study and has written the manuscript of the study. LJMH, ME, and APvB were involved in the critical revision of the article. All the authors contributed to the refinement of the study protocol and the final approval of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the medical ethical committee, RTPO Leeuwarden with study number NL7106409919. Written informed consent will be obtained from all individual participants included in the study.
Consent for publication
No identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. Informed consent materials are attached as supplementary materials.
Competing interests
FitForMe BV, Rotterdam, will provide the funding for the standardized multivitamin supplements.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Informed consent
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Slagter, N., de Heide, L.J.M., Jutte, E.H. et al. Tailoring limb length based on total small bowel length in one anastomosis gastric bypass surgery (TAILOR study): study protocol for a randomized controlled trial. Trials 23, 526 (2022). https://doi.org/10.1186/s13063-022-06456-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06456-w