Abstract
Background
Understanding why people take part in health research is critical to improve research efficiency and generalisability. The aim of this overview of systematic reviews was to identify psychosocial determinants of research participation and map them to psychological theory and empirical recruitment research, to identify effective strategies to increase research participation.
Methods
Qualitative and quantitative systematic reviews were systematically identified. No date or language limits were applied. Two reviewers independently selected reviews. Methodological quality was rated using AMSTAR, and poor-quality reviews (scoring 0–3) were excluded. Barriers and facilitators were coded to psychological theory (Theoretical Domains Framework) and empirical recruitment research (recruitment interventions that had been subjected to randomised controlled trial evaluation).
Results
We included 26 systematic reviews (429 unique primary studies), covering a wide range of patient populations and health settings. We identified five groups of facilitators, of which three were dominant (potential for personal benefit, altruism, trust) and appear to be relevant across research setting and design. We identified nine groups of barriers, which were more dependent on the particular study (context, population, design). Two determinants (participant information, social influences) were found to be both barriers and facilitators. Barriers and facilitators could be coded to the Motivation and Opportunity components of the Theoretical Domains Framework; only one was coded to a Capability component. There was some overlap between psychosocial determinants and empirical recruitment research, but some barriers and facilitators had not been tested at all.
Conclusions
Identifying effective recruitment strategies could increase the efficiency and generalisability of primary research. We identified a number of barriers and facilitators that could be addressed by researchers. There is a need for more research to identify effective recruitment strategies that draw on the psychosocial facilitators and barriers identified in this overview.
Similar content being viewed by others
Background
Research is essential to the development of improved health care; however, the recruitment of participants remains low [1,2,3]. This is a particular problem for randomised trials, which test the effectiveness of interventions aimed at prevention, diagnosis, screening or treatment [4]. Suboptimal recruitment can result in underpowered and inconclusive studies, increased research costs and delays as well as unrepresentative sampling [1, 5]. There is a need to better understand the influences on participation in health research, particularly trials, and to identify areas and strategies for intervention. Accordingly, the PRioRiTy study, a James Lind Alliance Priority Setting Partnership, recently concluded that one of the most pressing recruitment questions was to determine what motivates participation [6].
Research participation is determined by psychosocial factors (that is, the interrelationship of social factors and individual cognitions and behaviours) acting as barriers or facilitators to impede or increase individuals’ willingness to take part. These determinants will vary conceptually, including instrumental (e.g. receiving financial compensation), attitudinal (e.g. being motivated by the potential for societal benefit), cognitive (e.g. believing that health care practitioners are virtuous) and emotional (e.g. fearing treatment change) components. Important social influences are likely to include the opinions of family and others whose views are valued. A number of systematic reviews have been undertaken to collate barriers and facilitators reported in primary studies, with most reviews focusing on specific conditions or patient groups.
The challenges experienced in recruitment have stimulated the production of a wide range of interventions to increase recruitment rates. Often these have been evaluated within SWATs (Studies Within A Trial), using trial methods to provide rigorous evidence of impact. Regarding the topic of recruitment [7, 8], for recruitment to trials, SWATs have been meta-analysed by Treweek et al. (2018) [8]; for recruitment to health research more generally, a systematic review was last undertaken in 2007 [7]. Notable in the Treweek review was that, despite a significant number of embedded trials (n = 68) and a range of intervention types (n = 72), in many cases there was no clear link between the tested intervention and reasons underpinning decisions to take part [8]. Therefore, there was an opportunity to review and collate a substantial evidence base on psychosocial determinants of research participation, and to look for features in the evidence that are generic or more context-specific. Making links between determinants and theory and recruitment interventions could strengthen the potency of interventions and, as a corollary, reduce levels of ‘research waste’ created by the evaluation of interventions without a clear rationale for possible effect.
The aims of this research therefore were to:
-
Undertake an overview of systematic reviews of psychosocial determinants of research participation amongst patients and the public
-
Summarise the reported determinants thematically and as barriers or facilitators
-
Map these determinants to a behaviour change theoretical framework
-
Map these determinants to interventions intended to increase participation in research.
Methods
The review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [9]. The review was registered in PROSPERO: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017062738.
Data sources and searches
The search aimed to systematically identify reviews of psychosocial determinants of patient and public decisions on health research participation. The strategy was developed from one used in cancer trials [10] and was developed in MEDLINE (Ovid) before adaptation for other databases. No language, time or geographical limits were applied. Searches were limited to systematic reviews, using Database of Abstracts of Reviews of Effects (DARE) search strategies [11].
The following databases were searched 7th- 8th June 2016: MEDLINE, MEDLINE In-Process, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, Cochrane Database of Systematic Reviews (CDSR), Cochrane Methodology Register (CMR), DARE, Embase, Health Technology Assessment (HTA) database. PROSPERO was also searched for ongoing reviews. Results were imported into EndNote × 7 and de-duplicated. Reference lists of included articles were scanned, and forward citation searching was completed in Google Scholar. Searches were updated 4th December 2017 and 20th September 2019, retrieving a further 1197 and 1775 results, respectively (total 2972). (See Additional file 1 for the MEDLINE search strategy.)
Inclusion and exclusion criteria
We included quantitative, qualitative or mixed methods systematic reviews reporting findings from studies exploring patient or public psychosocial determinants of health research participation. The focus of this review was on real research scenarios and not hypothetical research: work in this area often has mixed content, and so at least two thirds of primary studies within a review needed to involve actual research scenarios for inclusion. No language or publication status restrictions were applied. Systematic reviews were excluded if they only reported the characteristics of research participants, or if they were limited to health care practitioners’ views on the determinants of participation.
Screening
Titles and abstracts were screened independently by two authors (RS and PK) using pre-defined criteria. All potentially relevant articles were retrieved and independently screened by RS and PK. Disagreements were resolved through discussion.
Quality assessment
Two authors (RS and PK) used the assessment of multiple systematic reviews (AMSTAR) tool to assess the quality of reviews, and as an entry criterion [12]. Ratings were undertaken independently, and then an agreed score was reached through discussion. Items were scored 1 if the criterion was met and 0 if not met or unclear. One small modification to the recommended scoring was that, for criterion 5, articles only had to list included studies and not excluded studies (most reviews did not report excluded studies). A total AMSTAR score was calculated with review articles categorised as low (0–3), moderate (4–7) or high quality (8–11); low quality reviews (scoring 0–3) were excluded [13].
Data extraction and analysis
Data extraction was undertaken using a pre-designed form. Extracted data included review aims, study design, participant details and key findings. Information was extracted by one reviewer (RS) and checked for accuracy by PK, except for key findings, which were independently extracted by both and reconciled by consensus. It was anticipated that the systematic reviews identified would include a variety of study designs, and thus a narrative reporting method was used. RS first identified psychosocial themes reported in included reviews and then grouped the data within these categories, in consultation with PK; themes were considered to facilitate participation or act as a barrier, or to do both. We adhered to behaviour change guidance by inductively coding barriers and facilitators to research participation (RS, PK, JH), which were then considered in relation to two theories of behaviour change: (1) the Theoretical Domains Framework (TDF) and COM-B model, described below [14,15,16], and (2) empirical research on interventions intended to increase rates of trial participation [8].
The TDF provides a comprehensive account of 14 domains which influence a person’s behaviour; it is used here because research participation is a behaviour. These 14 domains have been shown to cluster into three overarching constructs: capability, opportunity and motivation, which are defined in the behavioural science literature as the COM-B model [15, 17]. The capability construct recognises how psychological and physical capabilities influence behaviour. It includes the following TDF constructs: knowledge; skills; memory, attention and decision processes; and behavioural regulation. The opportunity construct outlines how the social and physical environment shapes behaviour. It includes the following TDF constructs: social influences; and environmental context and resources. The motivation construct considers conscious and unconscious cognitive processes that influence behaviour. It includes the following TDF domains: social or professional role and identity; beliefs about capabilities; optimism; beliefs about consequences; reinforcement; intentions; goals; and emotion.
In order to map barriers and facilitators to research participation against empirical interventions intended to increase recruitment research, we drew on a relevant Cochrane review [8]. The review included 68 trials, organised under six categories: trial design; trial conduct; consent process; modification to information; recruiter or recruitment site interventions; and incentives. The available evidence for the six categories varies considerably, and the lack of evidence for some means there is considerable uncertainty about effectiveness. Of note, whilst the included studies assessed 72 different recruitment strategies, only seven were assessed by more than one embedded study.
Results
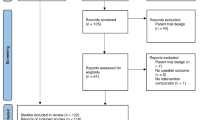
We identified 6374 records and an additional eight through citation searching; 2972 further records were identified via the search updates, resulting in a total of 9354 articles. We retrieved 156 articles for full text review and finally included 26 articles. Exclusions are detailed in Fig. 1.
Quality of the evidence
Six reviews (23.1%) were rated as high quality (AMSTAR score 8–11), and 20 reviews (72.9%) were of moderate quality (AMSTAR score 4–7) (see Table 1). Most involved a comprehensive literature search, employed duplicate study selection and data extraction and provided a list of included studies alongside their characteristics. The results of reviews were largely synthesised appropriately, and most authors stated whether there were any conflicts of interest. Just over half of the reviews assessed the scientific quality of the included studies, but only two assessed publication bias. The majority of reviews did not provide any evidence of a priori design, such as a published protocol.
Characteristics of included studies
The 26 reviews incorporated a total of 489 relevant primary studies, of which 179 (36.6%) had been undertaken in the USA; 80 (16.4%) in the UK; 19 (3.9%) in Australia; 17 (3.5%) in Canada; 12 (2.4%) in South Africa; 10 (2.0%) in Thailand; whilst 28 (5.7%) had been undertaken in more than one country (see Table 1). Country of origin was not reported in the source review for 82 (16.8%) studies, and the remaining 62 studies had been undertaken in one of 23 countries. Of the 489 primary studies, 56 (11.5%) were included in more than one review, leaving a total of 429 unique studies.This degree of overlap in the primary studies is low, incorporating a covered area of 4.4% and a corrected covered area of 0.5% [43]. Six (23.1%) reviews [10, 21, 26, 27, 34, 35] explicitly stated that they included only qualitative studies; the remainder included both quantitative and qualitative research. The focus of reviews varied in terms of health setting and types of research participation. Sixteen (61.5%) reviews were limited to studies of trial participation [10, 18,19,20, 22,23,24,25,26,27, 29, 32, 33, 35, 38, 39], and the remaining ten either included a mix of primary research designs or the design was unclear [21, 28, 30, 31, 34, 36, 37, 40,41,42]. Fifteen (57.7%) reviews were related to specific health conditions or settings: cancer (n = 6), HIV (n = 3), mental health (n = 2), chronic obstructive pulmonary disease (COPD), emergency medicine, pregnancy and bio-banking (each n = 1). Four studies focused on child or adolescent participants and their parents/caregivers [18, 21, 22, 40]; one study focused on ‘hard to reach’ older patients [19]; and four reviews focused on ethnic minority groups [24, 28, 29, 39]. Fifteen reviews (57.7%) only included real research scenarios [19,20,21, 23, 24, 26, 28,29,30, 32,33,34,35,36,37]; whereas 11 (42.3%) included both real and hypothetical scenarios. Most reviews (19; 73.1% considered both facilitators and barriers to research participation; three (11.5%) were limited to facilitators and four (15.4%) to barriers. The reviews were published during 1999–2019; their included primary studies were published during 1982–2016. Characteristics are further detailed in Table 1.
Identified psychosocial themes
Facilitators of research participation
A number of themes were identified which reported facilitators of research participation (see Table 2). The most commonly reported was perceived personal benefits, including the perception of therapeutic benefits, closer monitoring and access to new treatments [10, 20,21,22, 25,26,27, 29,30,31,32,33,34,35,36,37, 39,40,41].
Whilst altruism was the second most commonly reported factor, discussed in terms of benefitting science [10, 20, 29,30,31, 34, 36, 37, 39,40,41], helping others [10, 20, 21, 26, 27, 30, 31, 34, 35, 37, 40, 41] or altruism more generally [22], this was sometimes linked to personal benefit [27, 31, 33]. For example, patients with depression were less likely to participate if it might risk their own mental health, despite wanting to help others [27]. Further, two reviews highlighted that the desire to help others was not always concerned with helping all people, but specifically benefitting people who were personally important [32, 37]. Finally, a review involving research with children and adolescents concluded that the importance of altruism depended on the child’s health state; altruistic motives were given as a primary reason for participation by parents with healthy children, but for parents whose children had life-threatening conditions, altruism was secondary [21].
The influence of others was also important. Potential participants’ confidence in the physician and/or the research was motivating [18, 23, 25,26,27,28, 30,31,32,33, 35,36,37]. Having a positive, trusting relationship with the doctor was commonly cited as a facilitator; for example, the idea that the ‘doctor knows best’ was expressed [38]. The opinions of family and friends also facilitated participation [27,28,29, 35, 40, 41].
The impact of the potential participant’s knowledge of trials and the quality of the study information was mixed. For example, knowing you could leave the trial increased participation [10], but one review highlighted that enhanced knowledge and understanding could decrease participation [32]. A study with children and parents highlighted the need for age-appropriate information [18], whilst another highlighted the need for cultural appropriateness [24]. However, knowledge could act as a barrier when too much complex information was provided [10, 22, 30] or when information was vague [36]. Gaining knowledge of their health condition was a participation facilitator for children [40] and those invited to biobank studies [37].
Financial benefits were discussed in three reviews, but did not appear to be a primary determinant [29, 34, 40]; rather, financial benefits were seen as an added bonus [34]. However financial constraints and costs could inhibit participation [28, 39, 40].
Barriers to research participation
Fear was identified as a barrier in a large number of reviews, often related to perceived risks of treatments or interventions being tested and possible side effects [19, 21, 22, 25, 27, 31, 33,34,35,36, 39,40,41,42]. Assessment of risk varied with the severity of the patient’s illness [21]; for example, patients with a life-limiting diagnosis were more tolerant of research risk, potentially because of the access that participation granted them to new medication [21]. This was also linked to a perceived lack of choice imposed by the terminal diagnoses: patients stated the view that there seemed no option but to participate [21, 26, 35, 40]. More specific fears regarding the safety of interventions were common in reviews of HIV vaccine trials [19, 29]: potential trial participants were concerned about vaccine efficacy, or whether it could increase their susceptibility to HIV [29]. Other fears included discovering their HIV status [34] or being reported to immigration [39].
Distrust in research was common across patient groups [24, 27, 29, 30, 32, 34, 39,40,41,42], but was particularly prominent amongst minority ethnic groups [27, 39], minority indigenous populations [24] and people in sub-Saharan Africa [34]. In one review, distrust was linked to a lack of knowledge and understanding [29]. Specific distrust concerns included potential breaching of privacy or confidentiality [24, 29, 42], being a ‘guinea pig’ [30, 40] and a general mistrust of researchers’ intentions [34]. Nevertheless, trust in the safety of research was also reported as a motivating factor [21, 40].
Treatment preference, either for or against a specific treatment, was a reported barrier in several reviews [10, 25, 32, 38, 40]. Preferences included not wanting to change medication or not wanting to receive a placebo or experimental treatment [38]. However, preference for a specific treatment could also be a facilitator; in one mental health systematic review participants wanted access to the non-pharmaceutical, talking therapies on offer [27].
Perceived stigma was a commonly reported barrier to recruitment to trials in HIV [19, 34] or mental health [27, 42]. People did not want others to know their HIV status or to assume it as a result of trial participation [19, 34]. In mental health studies, stigma was largely due to people not wanting to be perceived as ‘crazy’, ‘weak’ or ‘vulnerable’ [27].
Practical difficulties were highlighted including the perceived inconvenience of trial participation (for example, additional procedures and appointments) [19, 22, 27, 32, 38, 40], a lack of time [19, 28, 32, 38, 41], travel or transport issues [10, 19, 24, 25, 28, 31, 32, 38, 39, 41, 42], costs [10, 25, 28, 38,39,40], as well as employment [10, 39] or childcare responsibilities [10, 28].
Concerns about trial methods were highlighted as barriers, including the inherent uncertainty [10, 21, 36, 38] and randomisation [22, 27, 32, 33, 36, 40, 41]. Potential participants also stated concerns about possible unknown side effects [10] and uncertain treatment effectiveness [21, 38]. There was some evidence of confusion about the meaning of randomisation [32], whilst other reviews noted that patients understood the concept but felt that randomisation signified a loss of control [25, 38] or that the doctor should choose treatments based on clinical expertise. In contrast to the inhibiting effects of concern about trial methods and the practical implications of research, the perception of a trial as low burden or convenient tended to facilitate participation [29, 37, 40].
Whilst knowledge could facilitate research participation, a lack of knowledge and understanding of clinical research could have a negative effect [24, 29, 30, 40], and participants identified a need for more information [38]. This lack of knowledge was sometimes linked to limitations of the informed consent process [30].
Finally, the patient’s health state at the time of invitation to participate was important in some reviews. Some patients felt too ill to participate [27, 28, 42]; others who were happy with their current health were less likely to participate for fear of disrupting this [27]. However, adverse health could favour research participation. One review of trials in acute conditions found that patients in pain said they were willing to agree to anything [30].
The thematic pattern of barriers and facilitators
It is notable that the identified barriers and facilitators include cognitive, emotional, social, practical and instrumental factors.
We identified a smaller number of facilitators than barriers, and three facilitating factors were dominant: the potential for personal benefit; altruism; and trust. Each of these was identified in a majority of the 26 included systematic reviews. These three factors were evidenced across different health settings and different research designs: they appear to be generic factors in being potentially important influences on individuals’ decisions about research participation whatever the context.
Barriers to participation were larger in number and more disparate. Their influence also appears to relate to the research design and to individual circumstances. For example, patients had stated treatment preferences or a current stable state of health, both of which might be disrupted by research involving a change to treatment. In patients with HIV or mental illness, research participation could be seen as threatening to self-identity or other’s perception of them. Distrust of research was reported and was often culturally specific, being reported most often in minority and ‘low power’ population groups. Practical difficulties associated with research were related to individuals’ circumstances, such as the impact of research on transport costs, childcare or paid work: the impact of these factors on participation will vary considerably across the population. Many stated barriers were specific to trial-based research, with expressed dislike of randomisation, uncertainty and possible treatment change.
Determinants and their links to the Theoretical Domains Framework
The identified barriers and facilitators from the 26 systematic reviews each link to at least one TDF domain, although there is a clustering on knowledge, social influences, optimism (or pessimism), goals and beliefs about consequences (see Table 2). Each of these domains was then mapped to the overarching constructs outlined in the COM-B model. Amongst the inductively identified facilitators of research participation, the three most commonly included were personal benefit; altruism; and trust. All the facilitators including the three most common ones map to different facets of the Motivation component of the COM-B model.
Amongst the 11 inductively identified barriers, all are linked to Motivation facets (both reflective and automatic), with two also linked to Opportunities. One barrier was linked to Physical Capabilities. The two factors that could operate either as facilitators or barriers (other people’s influence; information quality and participant knowledge) were mapped to Motivation and Opportunity components, respectively.
Reported reasons for/against research participation and links to empirical recruitment research
There is a lack of overlap between the barriers and facilitators we identified and the interventions tested, both in terms of the distribution of studied strategies and their impact. Whilst treatment preference was an important barrier to participation, only one study tested a strategy (patient preference trial design) which could be mapped to this theme. For a number of identified barriers, including condition stigma and distrust, we identified no related interventions. Similarly, no identified studies appeared to analyse strategies which may improve recruitment by impacting on altruistic motives. Additionally, there were no tested interventions linked to the patient’s confidence in the physician and the influence of family and/or friends, although the influence of recruitment via the Church or endorsements by previous participants has been studied.
Three tested recruitment strategies (phone reminders, recruitment primer letters, increased contact during recruitment in person or by phone) were not linked to any identified psychosocial determinants. Phone reminders act as a prompt to memory, whilst primer letters act by raising awareness, with neither cited as a barrier to participation. Increased contact during recruitment could potentially act on knowledge, although its intended action is not made clear. A fifth strategy not linked to the identified psychosocial determinants (strategies aimed at recruiters or recruitment sites) is intended to change the behaviour of recruiters, not participants. Also of note is that our overview identified three systematic reviews that investigated barriers or facilitators in relation to recruitment to paediatric research, and yet only one of the intervention studies included in the Treweek review [8] assessed recruitment to paediatric trials.
Discussion
Statement of principal findings
This overview found that a small number of psychosocial facilitators were evident, spanning settings and demographic groups. Psychosocial barriers were larger in number and more sensitive to research context and individual circumstances. When psychosocial determinants were mapped to the TDF and COM-B model, there was clustering on the opportunity and motivation domains. When determinants were mapped to recruitment strategies, there was incomplete overlap, and a number of determinants had no clear link to any evaluated recruitment interventions.
Strengths and weaknesses of the study
Overviews offer the potential for clarity in areas of significant systematic review activity [44] and may also identify consistencies and inconsistencies in primary evidence, and relative levels of importance [45]. This overview has clarified the psychosocial determinants of research participation and also identified clear opportunities to develop recruitment science by drawing on theory and empirical evidence. Some strengths of this overview are the searching of multiple databases, the use of dual independent assessors throughout and the inclusion of reviews not published in English. Excluding low-quality reviews (n = 4) increased rigour but reduced the number of included reviews and primary studies. We also excluded four reviews when we could not separate findings derived from patients and practitioners, and 22 reviews because less than two thirds of their included studies reported real research scenarios; in both cases this potentially reduced the total evidence base. In none of these cases do we think the exclusions have introduced bias or significantly limited findings. For example, AMSTAR has recently been shown to identify low-quality reviews for exclusion from an overview, without introducing bias [46]. Although overviews have been published for more than a decade, they continue to be subject to methodological debate, particularly around primary study duplication [43, 47]. Fifty-six (11.6%) of the primary studies in this overview were included in more than one review, and we did not adjust the findings to take account of this; our rationale was that we were reporting findings thematically and not undertaking pooling of quantitative data. However, primary study duplication may have led to overstatement of some determinants. The overview focused on barriers and facilitators of participation in health research broadly, but it mapped them against recruitment interventions to trials. We acknowledge the mismatch, but there is no recently published systematic review of interventions to increase participation in non-trial health research.
Strengths and weaknesses in relation to other studies, highlighting important differences in results
For the first time this overview has brought together evidence on the determinants of health research participation from a wide range of settings and methods. One of its key contributions is to clarify the relatively small number of psychosocial factors that have a consistent, positive influence on patients’ decisions. That these three factors (potential for personal benefit, altruism and trust) are key determinants is an important insight, as is the finding that their influence spans health setting and type of research design. They are evident both in qualitative and quantitative primary studies. In itself this speaks to the value of an overview; this pattern would not be evident in an individual systematic review. The barriers identified by the overview are more context-specific, such as the stigma associated with certain conditions, the practical demands that some research can place on participants and also the suspicions felt by some minority ethnic groups about some clinical research. Again, this finding of context-specificity could not be derived from a single-setting systematic review. Mapping identified psychosocial determinants onto a theoretical framework and assessing the overlap of psychosocial determinants with recruitment interventions has provided novel insights.
Meaning of the study: possible explanations and implications for clinicians and policymakers
Clarification of the main themes in psychosocial determination is itself useful knowledge for a number of stakeholders, including clinicians, researchers and research ethics organisations. It should be possible for researchers to use this knowledge to enhance recruitment, for example by drawing on the power of trust by using personal endorsements or role models from the same cultural background, or by acknowledging the credibility of the people and organisations involved in the research. However, interventions emphasising altruism (the potential for others to benefit from one’s actions) or the potential for personal benefit (when that could be uncertain in research, particularly in a controlled trial) could raise ethical challenges. Furthermore, it was notable that social influences—the effects of family, the doctor and other people seen as important—could act either as a barrier or a facilitator for a person deciding whether to participate in health research.
Identifying barriers to research should make it possible for adjustments to be made to the design and operationalisation of research, particularly if barriers are specific to study design and setting. Participant information, participant knowledge and social influences were found to act both as barriers and facilitators, and this perhaps presents a problem. First, researchers are unlikely to be able to control for social influences; second, information to inform research participation is mostly universal in provision and highly regulated [48], and yet amongst patients there can be strong, individual preferences for the quantity and complexity of information [49,50,51,52]. The opposing forces of universalism and individual preferences can be hard to reconcile, although digital provision does increase the potential for information to be tailored or personalised, whether by the originator or recipient.
Mapping identified determinants to theory offers the potential for greater understanding of individuals’ decisions and opportunities for linkage. Carey et al. (2019) systematically mapped the evidence for behaviour change techniques to mechanisms of action from hundreds of research studies [16]. They showed that the mechanism of action ‘beliefs about consequences’, which we linked to a number of participation determinants, had strong empirical links to the following: information about health consequences; information about social and environmental consequences; pros and cons; information about emotional consequences; and comparative imagining for future outcomes. Any of these could guide recruitment interventions.
The lack of complete overlap between psychosocial determinants and empirical recruitment research also offers the potential to guide intervention development. Recruitment interventions could focus on altruism (noting potential ethical concerns); the stigma associated with the health condition; or distrust of research or researchers—none of which has been tested in recruitment interventions. Furthermore many other determinants, such as treatment preferences; fear and perceived risk; confidence or trust in the physician or research; and desire for choice, have had little or no evaluation in recruitment research.
Unanswered questions and future research
Mapping of the psychosocial determinants onto recruitment interventions offers the potential for new research, as outlined above, and the possibility of applying an empirical framework to explain and predict the actions of recruitment interventions. Whilst the application of the TDF and COM-B models to the identified psychosocial determinants has produced new insights, it assumes that research participation is an explainable behaviour, and this assumption would benefit from empirical and theoretical evaluation. Very many published recruitment interventions have been atheoretical and lack clarity about possible mechanisms of action; thus, there is an opportunity in future recruitment research to incorporate the growing science of behaviour change.
This overview included 26 systematic reviews reporting more than 400 primary studies, but areas for development remain. For example, almost two thirds (59.9%) of primary studies in the reviews had been undertaken in just four countries, all of them English-speaking (although a proportion of reviews did not report country of origin). It was surprising that separate reviews had not been conducted in primary care settings or healthy person screening, since the experience of research participation may be very different from emergency care or long-term health conditions, for example. We excluded much research using hypothetical scenarios and, given the volume of real scenario research that we did include, the value of new hypothetical scenario research is questionable. Finally a significant proportion of the included reviews and primary studies used qualitative methods, and it is possible that narrative synthesis, an area of rapid methodological development, has potential to offer new insights into the determinants of research participation.
Conclusions
We identified a number of psychosocial barriers and facilitators to research participation, of which several spanned patient groups and settings, whilst the effect of others was more context-specific. These could be addressed by researchers when planning and implementing recruitment to studies. There is a need for more research to identify effective recruitment strategies that draw on theory and the psychosocial facilitators and barriers identified in this overview.
Availability of data and materials
Data are available from the corresponding author on reasonable request.
Change history
08 October 2020
An amendment to this paper has been published and can be accessed via the original article.
References
McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7(1):9.
Briel M, Olu KK, von Elm E, Kasenda B, Alturki R, Agarwal A, et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol. 2016;1(80):8–15.
Walters SJ, Bonacho dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7(3):e015276.
Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrom M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360.
Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, Sullivan F, Wilson S, Jackson C, Jones R. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010;(1):MR000013. https://doi.org/10.1002/14651858.MR000013.pub4.
Healy P, Galvin S, Williamson PR, Treweek S, Whiting C, Maeso B, et al. Identifying trial recruitment uncertainties using a James Lind Alliance Priority Setting Partnership–the PRioRiTy (Prioritising Recruitment in Randomised Trials) study. Trials. 2018;19(1):147.
Mapstone J, Elbourne D, Roberts I. Strategies to improve recruitment to research studies. Cochrane Database Syst Rev. 2007;2:Mr000013.
Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2:Mr000013.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;151(4):264–9.
Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol. 2007;60(10):990.e1–e33.
Centre for Reviews and Dissemination. Search strategies for DARE. 2015. http://www.crd.york.ac.uk/crdweb/searchstrategies.asp. Accessed 01 June 2016.
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10.
Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621–38.
Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7(1):37.
Atkins L, Francis J, Islam R, O'Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12(1):77.
Carey RN, Connell LE, Johnston M, Rothman AJ, de Bruin M, Kelly MP, et al. Behavior change techniques and their mechanisms of action: a synthesis of links described in published intervention literature. Ann Behav Med. 2019;53(8):693–707.
Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. ImplementSci. 2011;6(1):42.
Crane S, Broome ME. Understanding ethical issues of research participation from the perspective of participating children and adolescents: a systematic review. Worldviews Evid-Based Nurs. 2017;14(3):200–9.
Dhalla S, Poole G. Barriers to participation in actual HIV vaccine trials. Curr HIV Res. 2013;11(3):238–45.
Dhalla S, Poole G. Motivators to participation in actual HIV vaccine trials. AIDS Behav. 2014;18(2):263–77.
Fisher HR, McKevitt C, Boaz A. Why do parents enroll their children in research: a narrative synthesis. J Med Ethics. 2011;37(9):544–51.
Forcina V, Vakeesan B, Paulo C, Mitchell L, Bell JA, Tam S, et al. Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: a systematic review. Adolesc Health Med Ther. 2018;9:87–94.
Gad KT, Lassen U, Mau-Soerensen M, Hoybye MT, Johansen C. Patient information in phase 1 trials: a systematic review. Psychooncology. 2018;27(3):768–80.
Glover M, Kira A, Johnston V, Walker N, Thomas D, Chang AB, et al. A systematic review of barriers and facilitators to participation in randomized controlled trials by Indigenous people from New Zealand, Australia, Canada and the United States. Glob Health Promot. 2015;22(1):21–31.
Grand MM, O'Brien PC. Obstacles to participation in randomised cancer clinical trials: a systematic review of the literature. J Med Imaging Radiat Oncol. 2012;56(1):31–9.
Gregersen TA, Steffensen KD, Birkelund R, Wolderslund M, Ammentorp J, Netsey-Afedo ML. What matters in clinical trial decision-making: a systematic review of interviews exploring cancer patients’ experiences. Scand J Caring Sci. 2019;33(2):266–78.
Hughes-Morley A, Young B, Waheed W, Small N, Bower P. Factors affecting recruitment into depression trials: systematic review, meta-synthesis and conceptual framework. J Affect Disord. 2015;172:274–90.
Liljas AEM, Walters K, Jovicic A, Iliffe S, Manthorpe J, Goodman C, et al. Strategies to improve engagement of ‘hard to reach’ older people in research on health promotion: a systematic review. BMC Public Health. 2017;17(1):349.
Limkakeng A, Phadtare A, Shah J, Vaghasia M, Wei DY, Shah A, et al. Willingness to participate in clinical trials among patients of Chinese heritage: a meta-synthesis. PLOS One. 2013a;8(1):e51328.
Limkakeng AT, de Oliveira LLH, Moreira T, Phadtare A, Rodrigues CG, Hocker MB, et al. Systematic review and metasummary of attitudes toward research in emergency medical conditions. J Med Ethics. 2013b;40(6):401–8.
Martinsen EMH, Leiten EO, Bakke PS, Eagan TML, Grønseth R. Participation in research bronchoscopy: a literature review. Eur Clin Respir J. 2016;3(1):29511.
McCann S. Patients’ perspectives on participation in randomised controlled trials. Aberdeen: University of Aberdeen; 2007.
McCann S, Campbell M, Entwistle V. Recruitment to clinical trials: a meta-ethnographic synthesis of studies of reasons for participation. J Health Serv Res Policy. 2013;18(4):233–41.
Nalubega S, Evans C. Participant views and experiences of participating in HIV research in sub-Saharan Africa: a qualitative systematic review. JBI Database System Rev Implement Rep. 2015;13(5):330–420.
Nielsen ZE, Berthelsen CB. Cancer patients’ perceptions of factors influencing their decisions on participation in clinical drug trials: a qualitative meta-synthesis. J Clin Nurs. 2019;28(13–14):2443–61.
Nievaard M, De Vos R, de Haes J, Levi M. Reasons why patients do or do not participate in clinical trials; a systemic review of the literature. Ned Tijdschr Geneeskd. 2004;148(4):186–90.
Nobile H, Vermeulen E, Thys K, Bergmann MM, Borry P. Why do participants enroll in population biobank studies? A systematic literature review. Expert Rev Mol Diagn. 2013;13(1):35–47.
Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess. 1999;3(20):1–143.
Quay TA, Frimer L, Janssen PA, Lamers Y. Barriers and facilitators to recruitment of South Asians to health research: a scoping review. BMJ Open. 2017;7(5):e014889.
Tromp K, Zwaan CM, van de Vathorst S. Motivations of children and their parents to participate in drug research: a systematic review. Eur J Pediatr. 2016;175(5):599–612.
van der Zande ISE, van der Graaf R, Hooft L, van Delden JJM. Facilitators and barriers to pregnant women’s participation in research: a systematic review. Women Birth. 2018;31(5):350–61.
Woodall A, Morgan C, Sloan C, Howard L. Barriers to participation in mental health research: are there specific gender, ethnicity and age related barriers? BMC Psychiatry. 2010;10(1):103.
Pieper D, Antoine S-L, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39.
McKenzie JE, Brennan SE. Overviews of systematic reviews: great promise, greater challenge. Syst Rev. 2017;6(1):185.
Pollock A, Campbell P, Brunton G, Hunt H, Estcourt L. Selecting and implementing overview methods: implications from five exemplar overviews. Syst Rev. 2017;6(1):145.
Ballard M, Montgomery P. Risk of bias in overviews of reviews: a scoping review of methodological guidance and four-item checklist. Res Synth Methods. 2017;8(1):92–108.
Health Research Authority. Informing participants and seeking consent. 2019. https://www.hra.nhs.uk/planning-and-improving-research/best-practice/informing-participants-and-seeking-consent/. Accessed 01 April 2019.
Woolfall K, Shilling V, Hickey H, Smyth RL, Sowden E, Williamson PR, et al. Parents’ agendas in paediatric clinical trial recruitment are different from researchers’ and often remain unvoiced: a qualitative study. PLOS One. 2013;8(7):e67352.
Martin-Kerry JM, Knapp P, Atkin K, Bower P, Watt I, Stones C, et al. Supporting children and young people when making decisions about joining clinical trials: qualitative study to inform multimedia website development. BMJ Open. 2019;9:1.
Sheridan R, Martin-Kerry J, Watt I, Higgins S, Stones S, Horton Taylor D, et al. User testing digital, multimedia information to inform children, adolescents and their parents about healthcare trials. J Child Health Care. 2018;23(3):468–82.
Bower P, King M, Nazareth I, Lampe F, Sibbald B. Patient preferences in randomised controlled trials: conceptual framework and implications for research. Soc Sci Med. 2005;61(3):685–95.
Acknowledgements
We thank Jo Rick for her contributions to the early discussions about this overview. We thank Melissa Harden (University of York) for developing the search strategy and undertaking the database searches.
Funding
Access to information scientist expertise to inform the search strategy was funded through the Department of Health Sciences, University of York. The study received no other specific funding.
Author information
Authors and Affiliations
Contributions
The original research ideas were conceived by JH, AP and PB, and then adapted and enhanced by RS, JMK and PK. RS and PK undertook the main SR work (search development, sifting decisions, entry decisions, quality appraisal, data extraction, thematic generation). RS, PK and JH mapped the findings to theory and recruitment interventions. All authors contributed to the writing of the paper. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Adwoa Parker and Peter Bower are authors on one of the included systematic reviews. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
MEDLINE search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sheridan, R., Martin-Kerry, J., Hudson, J. et al. Why do patients take part in research? An overview of systematic reviews of psychosocial barriers and facilitators. Trials 21, 259 (2020). https://doi.org/10.1186/s13063-020-4197-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-020-4197-3