Abstract
Background
Transition immediately after birth is a complex physiological process. The neonate has to establish sufficient ventilation to ensure significant changes from intra-uterine to extra-uterine circulation. If hypoxia or bradycardia or both occur, as commonly happens during immediate transition in preterm neonates, cerebral hypoxia–ischemia may cause perinatal brain injury.
The primary objective of the COSGOD phase III trial is to investigate whether it is possible to increase survival without cerebral injury in preterm neonates of less than 32 weeks of gestation by targeting cerebral tissue oxygen saturation (crSO2) using specified clinical treatment guidelines during the immediate transition period after birth (the first 15 min) in addition to the routine monitoring of arterial oxygen saturation (SpO2) and heart rate (HR).
Methods/Design
COSGOD III is an investigator-initiated, randomized, multi-center, multi-national, phase III clinical trial. Inclusion criteria are neonates of less than 32 weeks of gestation, decision to provide full life support, and parental informed consent. Exclusion criteria are severe congenital malformations of brain, heart, lung, or prenatal cerebral injury or a combination of these.
The premature infants will be randomly assigned to study or control groups. Both groups will have a near-infrared spectroscopy (NIRS) device (left frontal), pulse oximeter (right palm/wrist), and electrocardiogram placed immediately after birth. In the study group, the crSO2, SpO2, and HR readings are visible, and the infant will receive treatment in accordance with defined treatment guidelines. In the control group, only SpO2 and HR will be visible, and the infant will receive routine treatment. The intervention period will last for the first 15 min after birth during the immediate transition period and resuscitation. Thereafter, each neonate will be followed up for primary outcome to term date or discharge. The primary outcome is mortality or cerebral injury (or both) defined as any intra-ventricular bleeding or cystic periventricular leukomalacia (or both). Secondary outcomes are neonatal morbidities.
Discussion
crSO2 monitoring during immediate transition has been proven to be feasible and improve cerebral oxygenation during immediate transition. The additional monitoring of crSO2 with dedicated interventions may improve outcome of preterm neonates as evidenced by increased survival without cerebral injury.
Trial registration
ClinicalTrials.gov Identifier: NCT03166722. Registered March 5, 2017.
Similar content being viewed by others
Background
The transition from the intra-uterine to extra-uterine environment is a complex physiological process characterized by major physiological changes in respiratory and hemodynamic functions, which are predominantly initiated by breathing at birth and clamping of the umbilical cord [1]. If hypoxia or bradycardia or both occur, as commonly happens during immediate transition in preterm neonates, cerebral hypoxia–ischemia may cause perinatal brain injury [2,3,4]. Therefore, protecting the brain from injury is of major importance since up to 10% of infants who survive very preterm birth will develop motor deficits such as cerebral palsy [3] and cognitive deficits [5].
Monitoring during immediate transition after birth
Routine non-invasive monitoring during immediate transition after birth might include arterial oxygen saturation (SpO2) and heart rate (HR) measurement with pulse oximetry/electrocardiogram (ECG), blood pressure, and temperature measurements. Special interest has grown in the use of pulse oximetry and ECG to monitor SpO2 and HR during this transitional period [6,7,8,9,10].
However, there is an ongoing debate about the use of supplemental oxygen during neonatal resuscitation. It is still unknown which oxygen concentration is appropriate for preterm infants during immediate transition after birth [10]. In addition, non-invasive monitoring of SpO2 does not provide information about adequate oxygen supply to the brain.
Recently, there has been increasing interest in continuous monitoring of cerebral tissue oxygen saturation (crSO2) using near-infrared spectroscopy (NIRS) during immediate fetal-to-neonatal transition [2, 11]. In 1992, Peebles et al. reported the first study of NIRS during immediate transition in term neonates and showed a rapid increase of oxygenated hemoglobin and decrease of deoxygenated hemoglobin with initiation of respiration [12]. Further studies showed that crSO2 is less affected by mode of delivery (e.g., vaginal delivery versus cesarean section) [13,14,15] than SpO2 and HR. Lower SpO2 and HR values have been reported in infants born via cesarean section compared with infants born vaginally [14]. The different behavior of crSO2 and SpO2 might be due to changes/decreases in cerebral blood flow (CBF) [16]. Differences in arterial oxygen content of the blood or shunting through the patent ductus arteriosus (or both) are most likely the reason for CBF changes/differences [16, 17]. In addition, there is increasing evidence that cerebral tissue oxygenation can be modified by resuscitation interventions in preterm infants [18,19,20,21]. It has been demonstrated that preterm infants who need respiratory support showed significantly lower crSO2 values compared with those who do not [19].
Therefore, as the brain is the most vulnerable organ of the infant, to monitor cerebral oxygenation in a non-invasive way is a potentially useful to guide supplemental oxygen and respiratory support in neonates. In a two-center prospective observational case control study, we demonstrated that neonates developing an intra-ventricular hemorrhage (IVH) during the first week after birth showed lower crSO2 values already during immediate transition compared with neonates without IVH [22].
COSGOD phase I/II trial
Based on the findings in the observational studies [18,19,20,21,22], preterm neonates of less than 34 weeks of gestation were enrolled in a prospective randomized controlled pilot feasibility study at two tertiary-level neonatal intensive care units (Graz, Austria and Edmonton, Canada) [23]. In an NIRS-visible group, crSO2 monitoring in addition to routine monitoring with pulse oximetry and ECG was used to guide respiratory and supplemental oxygen support during the first 15 min after birth. In the NIRS-not-visible group, routine monitoring which consisted of pulse oximetry and ECG was used. The primary outcomes were burden of cerebral hypoxia (<10th centile) or hyperoxia (>90th centile) measured in percentage minutes crSO2 during the first 15 min after birth. In the NIRS-visible group, the burden of cerebral hypoxia was halved with a relative reduction of 55.4% (95% confidence interval 37.6–73.2%; P = 0.028) [22].
Assessment of brain injury
Cerebral ultrasonography is a valuable screening tool to determine significant brain injury like IVH grade 1–3 (+ periventricular hemorrhage), cerebellar hemorrhage, and periventricular leukomalacia (PVL) grade 1–3 when conducted regularly over the first weeks of life in preterm infants [24]. Magnetic resonance imaging (MRI) scans of the brain offer even more precise information [25, 26]. However, ultrasound abnormalities in low-gestational-age neonates are already strongly associated with impaired psychomotor and mental development. Children without cranial ultrasound abnormality had the lowest probability of delayed psychomotor or mental development [27].
Trial objectives
Based on the findings in the observational studies and COSGOD phase I/II trial, the objective of the present clinical trial is to monitor crSO2 using NIRS INVOS 5100 (Medtronic, Minneapolis, MN, USA) in addition to routine monitoring of SpO2 and HR to guide supplemental oxygen delivery and respiratory/circulatory support in preterm neonates during the first 15 min after birth.
The primary aim is to increase survival without cerebral injury at discharge or term age. The secondary aim is to assess neonatal morbidity until discharge or term age.
We hypothesize that supplemental oxygen support and respiratory/circulatory support guided by crSO2 and SpO2/HR monitoring during the first 15 min after birth will increase survival without cerebral injury and reduce morbidities in preterm neonates of less than 32 weeks of gestation.
Methods
Design
The present trial is an investigator-initiated, randomized, multi-center, multi-national, clinical trial that will enroll 362 preterm neonates of less than 32 weeks of gestation in the study group and 362 preterm neonates of less than 32 weeks of gestation in the control group (Figs. 1 and 2). The trial is carried out in accordance with the Declaration of Helsinki in its latest form and the “International Conference of Harmonization – Good Clinical Practice” (ICH GCP) guidelines.
Patients
Preterm neonates of less than 32 weeks of gestation are eligible for the study. Further inclusion criteria are the decision to provide full life support, written informed consent, and the application of NIRS sensors within 3 min after birth. Exclusion criteria are decision not to provide full life support, no written informed consent, or severe congenital malformation of brain, heart, lung, or prenatal cerebral injury or a combination of these.
Sample size and statistical analyses
According to data of two European centers (Medical University of Graz and Erasmus Medical Center in Rotterdam) and one Canadian center (Royal Alexandra Hospital in Edmonton), the percentage of neonates who survive without cerebral injury ranges from 56% to 77% and the overall percentage is 65%. Given an increase of neonates who survive without cerebral injury from 65% to 75%, 329 neonates in each group are required to detect this difference with a two-group chi-squared test with a 0.05 two-sided significance level and a power of 80%. Given a dropout rate of 10%, a total of 724 neonates will be enrolled.
Demographic and baseline data for the infants will be compared between groups (study group versus control group) using the chi-squared test or Fisher’s exact test in the case of categorical variables and using the t test or Mann–Whitney U test in the case of continuous variables. To investigate the primary hypothesis whether the frequency of cerebral injury in preterm neonates of less than 32 weeks of gestation will be different in the two groups, a chi-squared test will be performed. Secondary outcome parameters including mortality and neonatal morbidities (cerebral injury, culture-proven early-onset infection/sepsis, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity, and persistent ductus arteriosus receiving intervention) will be compared between groups by using the chi-squared test or Fisher’s exact test. Exploratory outcomes (need for respiratory support, intubation, and medications during resuscitation, need for mechanical ventilation and treatment with catecholamines on the first day after birth) will be compared between groups by using the chi-squared test or Fisher’s exact test; trends of monitoring parameters (SpO2, HR, and NIRS parameters) during the first 15 min after birth will be analyzed by using linear mixed models. With these linear mixed models, differences in the course of the monitored parameters between groups (study group versus control group) will be analyzed. Baseline characteristics, which will show significant differences between groups, will also be included in the linear mixed models.
Randomization
Neonates will be randomly assigned before birth to either the study group or the control group by using the web-based randomization service (“Randomizer for Clinical Trials”), developed at the Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz (https://www.randomizer.at/random/). The ratio of allocation is 1:1. Patients will be stratified according to trial site. In case of multiple births, only the first infant will be randomly assigned.
Informed consent procedure
Parents of potential participants will be invited to enroll their preterm neonates before delivery or, if permitted by local regulations, after delivery and resuscitation (deferred consent).
Groups
In the study group, supplemental oxygen support and respiratory/circulatory support are guided by crSO2 and SpO2/HR monitoring. In the control group, supplemental oxygen support and respiratory/circulatory support are guided by SpO2/HR monitoring according routine resuscitation management; the resuscitation team is blinded to the crSO2 monitoring.
Duration of interventions
Monitoring and clinical interventions start within 3 min after birth and last for 15 min after birth.
Duration of follow-up
Each neonate will be followed until discharge or until term date (37–42 weeks of gestation).
Cerebral ultrasound will be performed at 2–24 h (optional), 2–5 days, 6–8 days, 12–16 days, before discharge/37–42 weeks of gestation and MRI (optional) before discharge, or at 37–42 weeks of gestation.
Monitoring
The antepartum medical history and birth history will be collected. Gestational age, birth weight, gender, pH of umbilical artery, and Apgar score will be documented for each neonate. Continuous positive airway pressure and positive pressure ventilation with a face mask, if necessary, will be performed.
Monitoring during the first 15 min after birth consists of (i) pulse oximetry for SpO2 and HR as routine non-invasive monitoring, (ii) ECG for HR as routine non-invasive monitoring, and (iii) NIRS measurements. NIRS measurements will be blinded in the control group.
For NIRS measurements, the Invos™ Cerebral/Somatic Oximeter monitor (Medtronic) with the neonatal sensor will be used.
Immediately after birth, when the neonate is brought to the resuscitation area, the cerebral NIRS sensor will be placed on the left forehead and fixed with a continuous positive airway pressure cap or elastic bandage on the left forehead. ECG electrodes will be applied to the chest for HR monitoring. One pulse-oximetry sensor will be applied to the right palm or wrist for monitoring pre-ductal SpO2 and HR.
Interventions
Resuscitation will be conducted in accordance with the “Consensus Guidelines” on the management of neonatal respiratory distress syndrome [28, 29], but cerebral oxygen saturation targeting and resuscitation will be at the discretion of the clinical team.
Control group
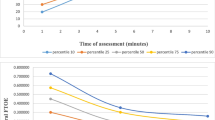
Depending on the infants’ breathing efforts and HR, the SpO2 targeting is conducted in accordance with “Local Guidelines” and “Resuscitation Guidelines” [28, 29] but at least between the 10th and 90th centiles [30] (Fig. 3). If SpO2 remains less than the 10th centile or below the local lower limit, respiratory support will be increased/started or fraction of inspired oxygen (FiO2) will be increased by 10–20% every 60 s. Respiratory support will be reduced/stopped or FiO2 will be reduced by 10–20% if SpO2 remains stable more than the 10th centile [30] or above the local lower limit for more than 60 s or if SpO2 is more than the 90th centile [30] or above the local upper limit. The clinical team will be blinded to the crSO2 monitoring.
Study group
In the study group, the crSO2 monitoring is visible to the clinical team (Fig. 4). If SpO2 is within local limits and at least between the 10th and 90th centiles, the crSO2 value will be considered. If crSO2 is less than the 10th centile (Fig. 5) [31], respiratory support will be started or increased or oxygen support will be increased every 60 s by 10–20%. In case of history of volume loss and clinical signs of volume loss, an administration of intravenous fluid (10 mL/kg) can be considered [28, 29]. Respiratory support will be reduced/stopped or FiO2 will be reduced by 10–20% if crSO2 remains stable more than the 10th centile [31] for more than 60 s or if crSO2 is more than the 90th centile [31].
10th and 90th centiles of cerebral tissue oxygen saturation (crSO2) in each minute after birth (according to Table 1 “All neonates” published in Pichler et al. [31])
Outcome measure
The primary outcome measure is survival without cerebral injury defined as any grade of IVH or cystic PVL. The secondary outcome measures include mortality and neonatal morbidities (cerebral injury, culture-proven early-onset infection/sepsis, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity, and persistent ductus arteriosus receiving intervention). The exploratory outcome measures are need for respiratory support, intubation, and medications during resuscitation; need for mechanical ventilation and treatment with catecholamines on the first day after birth; and monitoring parameters (SpO2, HR, and NIRS parameters) during the first 15 min after birth.
Outcome assessment tools
All-cause mortality will be recorded. Cerebral injury will be assessed by cerebral ultrasound at 2–24 h (optional), 2–5 days, 6–8 days, 12–16 days, before discharge/37–42 weeks and optionally cerebral injury will be assessed by cerebral MRI, if performed routinely—mainly without sedation during postprandial sleep—before discharge or term age (37–42 weeks of gestation). Case history will be assessed until discharge or term age, depending on what comes first.
Blinding
Owing to the nature of the trial, the intervention cannot be blinded for the clinical staff and the parents. However, blinding will be used in some other aspect of the trial. The allocation sequence will be concealed.
Data management
Source data will be registered in the participant’s medical records/case report form (CRF) and the electronic CRF (eCRF). A common web-based eCRF will be used and entered in a central database (Medical Informatics, Statistics and Documentation, Medical University of Graz). Data entry into the central database is the responsibility of the investigators. After establishment of a “clean file”, the database will be locked, and data will be stored for statistical analysis at the Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz.
Trial data will be handled in accordance with regulations of the data protection agency in the respective countries. After completion of statistical data analysis, data will be pseudo-anonymized and deposited at the Medical University of Graz. After the end of trial, the data will be archived for 15 years in accordance with GCP guidelines. At each trial site, the data flow will be monitored in accordance with the GCP principles by a locally appointed external monitoring committee.
Safety
The preterm patient population is a very vulnerable and often seriously ill group. Most adverse events may be of a serious nature with or without the COSGOD trial intervention, and both groups are expected to have a very high proportion of serious adverse events (SAEs). It is therefore not possible, or meaningful, to record and report all adverse events. The SAE to be reported is mortality. Serious adverse reaction and suspected unexpected serious adverse reactions (SUSARs) will be reported to ethic committees and authorities.
An independent safety committee will perform a first monitoring after the inclusion of 20% of the neonates to evaluate the risk of SAEs and efficacy (primary and secondary outcome parameters) of the intervention with “certain” or “probably/likely” relationship with the cerebral NIRS oximeter or the application of the treatment guideline. The participants will be insured in accordance with existing legislation of their respective country.
Ethical considerations
The COSGOD trial will start at the different centers randomly assigning participants after approvals from the relevant ethics committees and authorities have been received. All parents will receive written and oral information about the trial before they are asked for their written consent. They will enroll their newborn infant in the trial only by their own free will and can withdraw their consent for participation at any time. If a parent wishes to withdraw the consent for participation, the patient will receive treatment in accordance with the respective hospital’s standard procedures. The trial will be conducted in compliance with the guidelines of the Declaration of Helsinki in its latest form and the ICH GCP guidelines.
In case of modifications in the study protocol that are not merely of a formal nature but contain changes pertinent to the study participants, a renewed vote of the ethics committee will be obtained. If applicable, the patients/parents will be informed in the patient information and consent form about changes in the terms and conditions of the trial.
Discussion
The primary aim of the present clinical trial is to increase survival without cerebral injury in preterm neonates by monitoring crSO2 in addition to routine monitoring of SpO2 and HR to guide supplemental oxygen delivery, respiratory support, or circulatory support (fluid bolus) (or a combination of these) in preterm neonates during the first 15 min after birth. In the pilot study [23], we demonstrated feasibility of NIRS measurements even in extremely-low-birth-weight infants. Burden of cerebral hypoxia was reduced without any SUSARs; in addition, a trend toward reduction of cerebral injury was observed. The SafeBoosC phase II randomized clinical trial recently demonstrated that the combination of crSO2 measurement and a treatment guideline could reduce the burden of cerebral hypoxia in preterm neonates with less than 28 weeks of gestation during the first three days after birth [32]. In this study, a trend toward reduction of cerebral injury was also observed. Both, COSGOD phase I/II and SafeBoosC phase II had trends to lower mortality or cerebral injury (or both), suggesting a positive effect on short-term outcome; however, neither study was powered for cerebral injury [23, 32].
Since pulse oximetry and ECG are recommended during immediate transition and resuscitation when monitoring is performed [28, 29], both monitoring methods will be performed in all neonates. SpO2 will be kept within recommended/local limits in all neonates (at least between the 10th and 90th centiles of published reference ranges) [30]. Therefore, in the study group and in the control group, hypoxia defined as SpO2 below the lower limit and hyperoxia defined as SpO2 above the upper limit should be avoided.
Monitoring of crSO2 would suggest increased respiratory support and increased use of supplemental oxygen in case of low cerebral oxygenation. In the COSGOD phase I/II trial, the supplemental oxygen support was lower in the NIRS-visible group in the first minutes [23]. For COSGOD III, according to ICH GCP, the research and clinical team involved in conducting this trial should be qualified by education, training, and experience to perform NIRS measurements and interventions.
Owing to the short intervention period, irritation of the skin caused by NIRS sensors will be unlikely. Moreover, this has not been observed in other studies using NIRS during immediate transition [2, 11]. Risks related to the manipulation of the patient during positioning and re-positioning of the NIRS sensors will be minimized by experience in use of NIRS. Neonates will always be continuously monitored and observed by the resuscitation team.
In conclusion, additional monitoring of crSO2 and dedicated interventions are feasible during immediate transition and resuscitation and reduce the burden of cerebral hypoxia. The present trial will examine whether monitoring of crSO2 and dedicated interventions will improve survival without cerebral injury.
Trial status
Recruitment started in September 2018 and is expected to be completed at the end of 2020.
Abbreviations
- CBF:
-
Cerebral blood flow
- CRF:
-
Case report form
- crSO2 :
-
Cerebral tissue oxygen saturation
- ECG:
-
Electrocardiogram
- eCRF:
-
Electronic case report form
- FiO2 :
-
Fraction of inspired oxygen
- GCP:
-
Good Clinical Practice
- HR:
-
Heart rate
- IVH:
-
Intra-ventricular hemorrhage
- MRI :
-
Magnetic resonance imaging
- NIRS:
-
Near-infrared spectroscopy
- PVL:
-
Periventricular leukomalacia
- SAE:
-
Serious adverse event
- SpO2 :
-
Arterial oxygen saturation
- SUSAR:
-
Suspected unexpected serious adverse reaction
References
van Vonderen JJ, Roest AA, Siew ML, Walther FJ, Hooper SB, Te Pas AB. Measuring physiological changes during the transition to life after birth. Neonatology. 2014;105:230–42. https://doi.org/10.1159/000356704 Epub 2014 Feb 6.
Pichler G, Cheung PY, Aziz K, Urlesberger B, Schmölzer GM. How to monitor the brain during immediate neonatal transition and resuscitation? A systematic qualitative review of the literature. Neonatology. 2014;105:205–10. https://doi.org/10.1159/000357162 Epub 2014 Jan 25.
Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–63. https://doi.org/10.1016/j.ijdevneu.2011.04.004 Epub 2011 Apr 15.
Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–61.
Linsell L, Johnson S, Wolke D, O’Reilly H, Morris JK, Kurinczuk JJ, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population-based cohort study. Arch Dis Child. 2018;103:363–70. https://doi.org/10.1136/archdischild-2017-313414 [Epub ahead of print].
Kamlin CO, O’Donnell CP, Davis PG, Morley CJ. Oxygen saturation in healthy infants immediately after birth. J Pediatr. 2006;148:585–9.
Rabi Y, Yee W, Chen SY, Singhal N. Oxygen saturation trends immediately after birth. J Pediatr. 2006;148:590–4.
Finer N, Leoni T. Oxygen saturation monitoring for the preterm infant: the evidence basis for the current practice. Pediatr Res. 2009;65:375–80.
Dawson JA, Davis PG, O’Donnell CPF, Kamlin COF, Morley CJ. Pulse oximetry for monitoring infants in the delivery room: a review. Arch Dis Child Fetal Neonatal Ed. 2007;92:F4–7.
Dawson JA, Morley CJ. Monitoring oxygen saturation and HR in the early neonatal period. Semin Fetal Neonatal Med. 2010;15:203–7.
Pichler G, Schmölzer GM, Urlesberger B. Cerebral tissue oxygenation during immediate neonatal transition and resuscitation. Front Pediatr. 2017;5:29. https://doi.org/10.3389/fped.2017.00029 eCollection 2017.
Peebles DM, Edwards AD, Wyatt JS, Cope M, Delpy DT, Reynold EO. Changes in human fetal cerebral oxygenation and blood volume during delivery. Am J Obstet Gynecol. 1992;167:1916–7.
Isobe K, Kusaka T, Fujikawa Y, Kondo M, Kawada K, Yasuda S, et al. Changes in cerebral hemoglobin concentration and oxygen saturation immediately after birth in the human neonate using full-spectrum near infrared spectroscopy. J Biomed Opt. 2000;5:283–6.
Urlesberger B, Kratky E, Rehak T, Pocivalnik M, Avian A, Czihak J, et al. Regional oxygen saturation of the brain during birth transition of term infants: comparison between elective cesarean and vaginal deliveries. J Pediatr. 2011;159:404–8.
Almaazmi M, Schmid MB, Havers S, Reister F, Lindner W, Mayer B, et al. Cerebral near-infrared spectroscopy during transition of healthy term newborns. Neonatology. 2013;103:246–51.
Noori S, Wlodaver A, Gottipati V, McCoy M, Schultz D, Escobedo M. Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr. 2012;160:943–8.
Urlesberger B, Brandner A, Pocivalnik M, Koestenberger M, Morris N, Pichler G. A left-to-right shunt via the ductus arteriosus is associated with increased regional cerebral oxygen saturation during neonatal transition. Neonatology. 2013;103:259–63.
Fuchs H, Lindner W, Buschko A, Trischberger T, Schmid M, Hummler HD. Cerebral oxygenation in very low birth weight infants supported with sustained lung inflations after birth. Pediatr Res. 2011;70:176–80.
Fuchs H, Lindner W, Buschko A, Almazam M, Hummler HD, Schmid MB. Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatol. 2012;32:356–62.
Binder C, Urlesberger B, Avian A, Pocivalnik M, Müller W, Pichler G. Cerebral and peripheral regional oxygen saturation during postnatal transition in preterm neonates. J Pediatr. 2013;163:394–9. https://doi.org/10.1016/j.jpeds.2013.01.026 [Epub ahead of print].
Pichler G, Avian A, Binder C, Zotter H, Schmölzer GM, Morris N, et al. aEEG and NIRS during transition and resuscitation after birth: promising additional tools; an observational study. Resuscitation. 2013;84:974–8.
Baik N, Urlesberger B, Schwaberger B, Schmölzer GM, Avian A, Pichler G. Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Arch Dis Child Fetal Neonatal Ed. 2015;100:F422–7.
Pichler G, Urlesberger B, Baik N, Schwaberger B, Binder-Heschl C, Avian A, et al. Cerebral oxygen saturation to guide oxygen delivery in preterm neonates for the immediate transition after birth: a 2-center randomized controlled pilot feasibility trial. J Pediatr. 2016;170:73–8.e1–4. https://doi.org/10.1016/j.jpeds.2015.11.053.
Maller VV, Cohen HL. Neurosonography: assessing the premature infant. Pediatr Radiol. 2017;47:1031–45. https://doi.org/10.1007/s00247-017-3884-z Epub 2017 Aug 4.
Volpe JJ. Neurology of the newborn. 5th ed. Saunders: Elesvier; 2008.
Whyte HEA, Blasé S. Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology. 2013;55(Suppl 2):S3–S11. https://doi.org/10.1007/s00234-013-1238-6.
O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662–9. https://doi.org/10.1542/peds.2008-0594.
Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European resuscitation council guidelines for resuscitation 2015: section 7. Resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249–63. https://doi.org/10.1016/j.resuscitation.2015.07.029 Epub 2015 Oct 15.
Wyllie J, Perlman JM, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Neonatal resuscitation chapter collaborators. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2015;95:e169–201. https://doi.org/10.1016/j.resuscitation.2015.07.045 Epub 2015 Oct 15.
Dawson JA, Kamlin COF, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340–7.
Pichler G, Binder C, Avian A, Beckenbach E, Schmölzer GM, Urlesberger B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr. 2013;163:1558–63. https://doi.org/10.1016/j.jpeds.2013.07.007.
Hyttel-Sorensen S, Pellicer A, Alderliesten T, Austin T, van Bel F, Benders M, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ. 2015;350:g7635. https://doi.org/10.1136/bmj.g7635.
Acknowledgments
Not applicable.
Funding
We would like to thank the public for donating money to our funding agencies: The “Fonds zur Förderung der wissenschaftlichen Forschung” FWF Austria supports this trial through an unconditional and unrestricted grant (KLI 586-B31). The study sponsor and funders have no influence on study design; collection, management, analysis, or interpretation of data; or writing of the report. GMS is a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation, a National New Investigator of the Heart and Stroke Foundation Canada, and an Alberta New Investigator of the Heart and Stroke Foundation Alberta. This research has been facilitated by the Women and Children’s Health Research Institute through the generous support of the Stollery Children’s Hospital Foundation.
Availability of data and materials
The datasets generated or analyzed (or both) during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
GP and GMS wrote the first draft of the protocol and the manuscript. AA drafted the statistical analysis, revised the manuscript critically for important intellectual content, and gave approval of the final manuscript. GP, SB, MB, ED, HF, TGG, GL, LL, LK, SM, LKC, AA, BU, and GMS contributed to the conception and design of the protocol, revised the manuscript critically for important intellectual content, and gave approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Medical University of Graz, Austria: Approved 06.06.2016; 28-456 ex15/16
University of Alberta, Edmonton, Canada: Approved 13.07.2016; Pro00065767
Medical University of Vienna, Austria: Approved 17.11.2071; EK Nr: 1823/2017
Medical University of Innsbruck, Austria: Approved 03.10.2017; EK Nr: 1048/2017
University Children’s Hospital of Tübingen, Germany: Approved 27.10.2017; 56/2017BO1
University Medical Centre Ljubljana, Slovenia: Approved 2.11.2017; 0120-529/2017/4
Medical Center-University of Freiburg, Faculty of Medicine, Germany: Approved 18.01.2018; 612/17
Cork University Maternity Hospital, Cork, Ireland: Approved 31.05.2018; ECM 4 17/01/18
Erasmus MC-Sophia Children’s Hospital, Rotterdam, the Netherlands: submitted to ethical committee
Ospedale dei Bambini “Vittore Buzzi” in Milano, Italy: submitted to ethical committee
Poznan University of Medical Sciences, Poznan, Poland: submitted to ethical committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pichler, G., Baumgartner, S., Biermayr, M. et al. Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial. Trials 20, 178 (2019). https://doi.org/10.1186/s13063-019-3258-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-019-3258-y