Abstract
Background
Extremely low birth weight (ELBW) infants are at risk for end-organ hypoxia and ischemia. Regional tissue oxygenation of the brain and gut as monitored with near-infrared spectroscopy (NIRS) may change with postnatal age, but normal ranges are not well defined.
Methods
A prospective study of ELBW preterm infants utilized NIRS monitoring to assess changes in cerebral and mesenteric saturation (Csat and Msat) over the first week after birth. This secondary study of a multicenter trial comparing hemoglobin transfusion thresholds assessed cerebral and mesenteric fractional tissue oxygen extraction (cFTOE and mFTOE) and relationships with perinatal variables.
Results
In 124 infants, both Csat and Msat declined over the first week, with a corresponding increase in oxygen extraction. With lower gestational age, lower birth weight, and 5-min Apgar score ≤5, there was a greater increase in oxygen extraction in the brain compared to the gut. Infants managed with a lower hemoglobin transfusion threshold receiving ≥2 transfusions in the first week had the lowest Csat and highest cFTOE (p < 0.001).
Conclusion
Brain oxygen extraction preferentially increased in more immature and anemic preterm infants. NIRS monitoring may enhance understanding of cerebral and mesenteric oxygenation patterns and inform future protective strategies in the preterm ELBW population.
Impact
-
Simultaneous monitoring of cerebral and mesenteric tissue saturation demonstrates the balance of oxygenation between preterm brain and gut and may inform protective strategies.
-
Over the first week, oxygen saturation of the brain and gut declines as oxygen extraction increases.
-
A low hemoglobin transfusion threshold is associated with lower cerebral saturation and higher cerebral oxygen extraction compared to a high hemoglobin transfusion threshold, although this did not translate into clinically relevant differences in the TOP trial primary outcome.
-
Greater oxygen extraction by the brain compared to the gut occurs with lower gestational age, lower birth weight, and 5-min Apgar score ≤5.
Similar content being viewed by others
Introduction
After birth, extremely low birth weight (ELBW) preterm infants are at risk for impaired end-organ perfusion and oxygenation. Factors including tenuous systemic oxygenation, compromised cardiac output, and anemia, may result in inadequate oxygen delivery to the brain and the intestines. Non-invasive monitoring of regional tissue saturation with near-infrared spectroscopy (NIRS) is feasible in preterm infants; however, normal ranges of cerebral (Csat) and mesenteric (Msat) oxygen saturation in preterms remain unclear in the first week after birth.1 Changes in perfusion and tissue oxygen extraction for brain and gut may vary depending on gestational and postnatal age and be influenced by physiologic changes occurring with the postnatal transition.
Single-center NIRS studies focusing on the first 72 h after birth show an increase in Csat levels in preterm infants with gestational age between 24 and 32 weeks and a decrease with chronologic age,2,3,4,5 as well as higher Csat levels in small for gestational age males.6 Less is known about preterm Msat ranges in preterm infants; with only single-center observational studies,3,7,8,9 suggesting that Msat decreases during the first week.
The Transfusion of Prematures (TOP) trial (NCT 01702805) allowed for the recruitment of a large number of preterm, ELBW infants to undergo NIRS monitoring of the brain and intestines at participating centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) and in collaboration with the National Heart, Lung, and Blood Institute.10 This current study was designed to examine how oxygen saturation and oxygen extraction of the brain and gut change over the first week of age in relation to other perinatal variables. We hypothesized that in the transitional period during the first week after birth, cerebral fractional tissue oxygen extraction (cFTOE) would be higher than mesenteric fractional tissue oxygen extraction (mFTOE), but both would increase over time.
Methods
Patient population and study design
Infants were eligible for this secondary study if they were enrolled in the NRN TOP trial with birth weight ≤1000 g and with gestational age between 22 weeks 0 days and 28 weeks 6 days, inclusive and postnatal age ≤48 h.10 Subjects were approached for parental informed consent, unless skin integrity was deemed inadequate to allow for NIRS sensor placement for the duration of the infant’s enrollment in the trial; or if any TOP exclusion criteria were present (cyanotic congenital heart disease, non-viability as deemed by attending neonatologist, in utero fetal transfusion, twin-to-twin transfusion syndrome, isoimmune hemolytic disease, congenital condition other than premature birth that adversely affects life expectancy or neurodevelopment, parents opposed to the transfusion of blood, parents with hemoglobinopathy or congenital anemia, prior blood transfusion beyond the first 6 h of life, or high probability that the family would not be able to return for follow-up at 22–26 months).10 The primary outcome was the pattern of Csat and Msat over the first week of age, with an estimated sample size of 61 having power over 99% to detect a minimum 8% decline in NIRS measures over the first week based on pilot data and two-sided Type I error of 0.05. Approval of the institutional review board at each site was obtained, and written informed consent was required for participation.
Intervention
Within 6 h of enrollment, a cerebral neonatal sensor connected to a NIRS device (INVOS 5100, Medtronic, Minneapolis, MN) was applied to the infant’s forehead to monitor Csat continuously until 7 days of age. A second sensor was applied to monitor Msat in the infant’s left-lower abdominal quadrant, a location commonly utilized in neonatal intestinal oxygenation studies.11,12 A Mepitel (Molnlycke, Gothenburg, Sweden) dressing was used for skin protection under each sensor and was previously confirmed not to alter NIRS measures.13 The NIRS monitor screen was obscured to mask clinicians to Csat and Msat measures. A Vital Sync device (Medtronic) captured and time-synchronized NIRS data with patient pulse oximetry data (Nellcor, Medtronic). NIRS sensors were assessed daily to evaluate surrounding skin integrity and replaced on day 4 of age and as needed. All data were downloaded to electronic media and securely transferred to the NRN/TOP data coordinating center at RTI.
Demographic and perinatal variables including birth weight, gestational age, sex, antenatal steroid exposure, maternal race, Apgar scores at 1 and 5 min, small for gestational age status, SNAPPE-II score, and mode of delivery were recorded as part of the TOP trial. Other clinical variables in the first week of life were collected including PaCO2 levels during the time of NIRS monitoring, concomitant medications, mode of ventilation, feeding details, and red blood cell (RBC) transfusions given. Data regarding the presence of a hemodynamically significant patent ductus arteriosus (PDA) was unavailable as not all centers perform echocardiograms in the first week.
Data processing
NIRS values of Csat, Msat, and peripheral oxygen saturation (SpO2) data were acquired every 30 s. Measures were averaged on an hourly basis for an individual infant if at least 10 min of consecutive data for the hour were available. Total time with missing data was recorded. cFTOE was calculated with the following formula: cFTOE = (SpO2 – Csat)/SpO2. mFTOE was similarly calculated. The splanchnic to cerebral oxygenation ratio (SCOR) was calculated as Msat/Csat. Outlying data were excluded as determined by the elimination of negative cFTOE or mFTOE values.
Statistical analyses
Descriptive trends in NIRS measures (Csat, cFTOE, Msat, mFTOE, Msat/Csat ratio (SCOR)) and SpO2 over 6-h time periods across the first week of life were summarized with plots of mean values with 95% confidence intervals and additional exploratory analyses for interactions with perinatal features performed. Validity checks were conducted by comparing the baseline characteristics of NIRS infants included in the analysis versus those who were missing data. Relationships of key baseline perinatal characteristics (birth weight, gestational age, sex, growth restriction status, 5-min Apgar score, number of transfusions received, and hemoglobin threshold group) with each NIRS measure over time were explored using cubic regression lines with 95% confidence intervals. Cubic regression was used to allow for non-linear changes in these associations over time that were suggested by the exploratory plots. Infant averages of NIRS measures and hemoglobin over the first week were estimated from models adjusting for the random effect of infant. Changes in infant mean NIRS measures over 1-h periods were analyzed using mixed modeling, testing the relationship of each baseline perinatal characteristic with each NIRS measure over time, and adjusting for infant within the center as a random effect. Multiplicative interactive effects of each characteristic with time were also tested. Least squares means of each NIRS measure were reported at key timepoints (48, 96, and 144 h of life). Final multivariable models of each NIRS measure were adjusted for critical baseline and the first week of life characteristics. Interactions among these characteristics were tested, and if significant, estimates from stratified analyses were reported. All analyses utilized SAS software version 9.4.
Results
Population
Of 179 enrolled subjects from 17 centers of the NICHD NRN between July 2015 and April 2017, 124 (72%) had adequate NIRS monitoring data from the first week (Supplementary Fig. S1). A total of 12,363 h of NIRS monitoring data were available for analysis. During the first week, 3.7% of NIRS data were excluded as outliers, and 2.5% of NIRS data were missing, mostly from the first 2 days as enrollment was permissible up to 48 h of age. Mesenteric NIRS data were more likely to be missing than cerebral NIRS data, with Msat (0.85%) and mFTOE (1%) missing of overall NIRS data. Available subject data for specific NIRS measures was accordingly variable, ranging from 22–37 at 24 h, to 55–76 at 48 h, and 58–92 at 72 h.
Patient characteristics including perinatal and neonatal variables are shown in Table 1. Additional selected factors known to potentially affect cerebral saturation occurred either transiently or infrequently; these included hypoglycemia and other events leading to prolonged systemic oxygen desaturation or subject agitation (tracheal intubations, extubations, or surfactant administration events in the first week). Hypocarbia with any PaCO2 reading of <40 mmHg occurred in over 50% of subjects; however, this was infrequent with a median of 1 episode (IQR 1–2) over the first week with mean (±SD) PaCO2 value of 34.9 ± 3.2 mmHg (range 24–39). In the study population, 92% had blood gases measured from either arterial, venous, or capillary samples. Prolonged or multiple episodes (>5/day) of hypocarbia occurred in the first 2 days in only three infants.
Primary outcome—pattern of NIRS measures over the first week
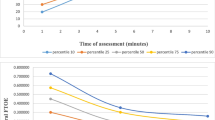
Plots of mean Csat, Msat, cFTOE, and mFTOE with 95% confidence intervals (Fig. 1) demonstrate the distribution of these NIRS measures over the first week of life. Both Csat and Msat levels decreased significantly over time, while cFTOE and mFTOE increased significantly over time. There was minimal change in SpO2 and SCOR. Mean (95% CI) Csat decreased by 6.1% (5.5–6.7) and mean Msat decreased by 5.2% (4.0–6.4) from day 3 to day 7 after adjusting for random effect of infant. Across the entire monitoring period of the first week, the estimated infant mean (±SE) Csat was 65 ± 1% and Msat 45 ± 2%, while estimated infant mean cFTOE was 0.31 ± 0.01 and mFTOE 0.54 ± 0.02. Estimated mean hemoglobin levels (±SE), when available, decreased from 16.2 ± 0.4 g/dl on the day of birth to 13.9 ± 0.2 g/dl on day of life 1 with a slower decline to 13.4 ± 0.3 g/dl on day of life 4 (Supplementary Fig. S2).
Effect of perinatal variables on NIRS measures
Variables of interest including gestational age, SGA status, and 5-min Apgar score were dichotomized, and select cubic regression lines of predicted NIRS measures over time with 95% confidence intervals are shown in Fig. 2. Estimates of mean NIRS measures by gestational age in the first week are included in Supplementary Table S1.
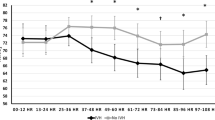
All analyses were considered exploratory and hypothesis-generating. Relationships are summarized in Supplementary Table S2, with p values for test of interaction of each variable with hour of age across the entire first week. As the test of interaction was significant (p < 0.05) for many variables, indicating changing relationships of variables with NIRS measures over time, we provided estimated NIRS measures at 96 h of age as a single time point midway through the first week. At this time point, lower birth weight, lower gestational age, and two or more RBC transfusions in the low hemoglobin threshold group in the first week appeared to be associated with decreased Csat and increased cFTOE. In contrast, Msat and mFTOE showed opposite directionality for these conditions. Figure 2 also suggests a similar relationship for those with 5-min Apgar score ≤5. No differences were seen based on sex. Infants with small for gestational age status had decreased Csat and increased cFTOE, but notably also had decreased Msat and increased mFTOE. Analyses at early (48 h) and later (144 h) timepoints demonstrated slightly different NIRS estimates, but similar relationships between NIRS measures, confirming the complex interactions over time (data not shown).
Multivariable models
Multivariable modeling revealed a complex interaction between the transfusion characteristics of hemoglobin threshold level, number of transfusions, and postnatal age for all NIRS measures (p < 0.001). Models for changes in NIRS values over the first week adjusting for the covariates of gestational age, sex, SGA status, 5-min Apgar score ≤5, and the three-way interaction between time, number of RBC transfusions, and transfusion threshold group are presented in Table 2. Gestational age effects on NIRS measures were of borderline significance, with an increase in Csat but a decrease in Msat with increasing gestational age. The effects of hemoglobin threshold and number of transfusions were reduced after models were adjusted for gestational age, SGA status, and 5-min Apgar score. SNAPPE-II score, a measure of illness severity, was also associated with lower Csat in a bivariate analysis, but the relationship was no longer significant after adjusting the model for gestational age and number of transfusions in the first week.
Transfusion characteristics and NIRS measures
Neonates received an average of 1.1 ± 1.3 RBC transfusions in the first week of life (Table 1). Infant mean (±SE) pre-transfusion hemoglobin was 10.2 ± 0.05 g/dl. Figure 3 depicts changes in mean Csat with transfusion parameters and time in the first week of age. The accompanying table in Fig. 3 reports estimated Csat and 95% confidence intervals for combinations of high/low threshold level and number of transfusions at selected timepoints of 48, 96, and 144 h after birth (2, 4, and 6 days of age). Modeling revealed that 13 subjects in the low hemoglobin threshold transfusion arm with two or more transfusions in the first week (low threshold/2+ transfusions) had the lowest adjusted mean Csat measures throughout the first week and the highest cFTOE measures. Moreover, these infants did not exhibit the pattern of decline in Csat over the first week as was demonstrated by the overall population. Cerebral NIRS differences were most significant at 2 days of age compared to 4 and 6 days of age. There were minimal differences between transfusion groupings for mesenteric NIRS measures (data not shown). There was no significant difference in timing of transfusions, availability of NIRS data, or SpO2 between transfusion threshold groups. However, the low threshold/2+ transfusions group also had a lower mean birth weight compared to all other groups (679 ± 123 versus 811 ± 135 g). Supplementary Fig. S3 demonstrates unadjusted NIRS changes in the first week by the hemoglobin threshold group alone, suggesting increased Csat and Msat, but decreased cFTOE and mFTOE in the high hemoglobin threshold group compared to the low threshold group.
Discussion
This study is the largest to date in the ELBW, preterm population to describe developmental changes in simultaneously recorded cerebral and mesenteric oxygenation and oxygen extraction over the first week of life, and the first to directly examine the effect of anemia and RBC transfusions on these physiologic measures. We found that despite minimal change in SpO2, both Csat and Msat decreased over the first week, whereas cFTOE and mFTOE increased. However, risk factors for impaired end-organ perfusion (lower gestational age, lower birth weight, 5-min Apgar ≤5) affected cerebral and mesenteric NIRS measures differently, with greater oxygen extraction by the brain compared to the gut. These findings suggest unique developmental regulation of oxygen use for different end organs.
Existing literature shows a decline in Csat and increase in cFTOE in the first month,3,14,15 potentially reflecting changes in brain blood flow and improved ability of the brain to extract oxygen with maturation. The largest single-center NIRS monitoring study demonstrated a parabolic change in Csat in the first 72 h, with a peak at 36 h of age.2 However, we could not replicate these findings as infants were enrolled for up to 48 h. We confirmed studies showing an increase in Csat and decrease in cFTOE with increasing gestational age,2,3 although one study demonstrated the opposite.15 Findings may differ by monitoring device, sensor type, sampling periods, gestational age or postnatal age range, and clinical management of early SpO2, blood pressure, and hemoglobin goals. However in our population, mean Csat was 65 ± 16%, consistent with previously published ranges in preterm infants and the 55–85% range used for current interventional studies.2,4,16
Our study is the first multicenter prospective investigation of mesenteric oxygenation in the ELBW infant. It confirms both the feasibility of mesenteric NIRS monitoring in this population and the simultaneous decline in Msat and increase in mFTOE over the first week as described in other studies.3,7,8,9,17 Blood flow to the preterm infant intestine slowly increases in the first week of age as documented by ultrasound Doppler studies.18,19,20 However, despite this increase in intestinal blood flow, several studies, including ours, documented a decrease in mesenteric oxygenation measurements using NIRS in the first week of life.3,7,8,9 It is hypothesized that the preterm intestine extracts more oxygen to meet metabolic demands during this transitional early period of life; this study confirms increased mFTOE in the first week. Van der Heide et al, in a large (N = 220) single-center study, found a nadir in splanchnic oxygen saturation occurred on day 4 at 38.7 ± 16.6% and then slightly increased to 44.2 ± 16.6% by day 7.9 Comparable values of Msat in our study showed a similar pattern with a nadir of 38.2 ± 22% by day 5 and a plateau at 45.4 ± 21% by day 7. No clear association between a specific Msat threshold and gastrointestinal morbidity such as necrotizing enterocolitis (NEC) or spontaneous intestinal perforation has yet been established.
Limited research into variables affecting mesenteric oxygenation has been performed. We describe increased Msat and decreased mFTOE in infants with lower gestational ages. Other investigators have demonstrated contradictory findings with lower gestational age resulting in decreased Msat.3,9,21 However, these investigators did not measure corresponding changes in oxygen extraction over time, and they examined more mature and less anemic preterm infants than we studied. Differences could also be attributable to use of imputed values, different transfusion or feeding guidelines, and monitoring only at selected timepoints in other studies. Similar to our findings, lower splanchnic oxygenation in SGA compared to appropriate for gestational age preterm infants has been described.9,22 We further supplement this association with evidence of corresponding higher mFTOE in SGA infants, potentially reflecting the response to fetal ischemia and growth restriction by increasing intestinal oxygen extraction. This process is similar to that which occurs in the SGA infant brain.6 While 41% of monitored infants were enterally fed in the first week, we did not specifically measure changes in Msat with response to feeds. One study investigating the effect of feeding on Msat8 suggests that postprandial Msat increases only in more mature preterm infants (postmenstrual age >32 weeks), which may confirm the limited ability of preterm infants to meet metabolic demands associated with early feeding.
Combined cerebral and mesenteric NIRS monitoring suggests different end-organ effects in response to perinatal stressors, with preferential oxygen extraction by the brain. Our study demonstrated greater cFTOE but lower mFTOE in infants with lower gestational age, lower birth weight, 5-min Apgar ≤5, and need for ≥2 transfusions. These infants may be at risk for ongoing hypoxia and ischemia, with adaptive efforts by the brain to increase oxygen extraction at the expense of other end organs like the gut. Indeed, research in preterm fetal sheep demonstrates a preferential increase in cerebral oxygen extraction rather than cerebral blood flow during non-injurious hypoxia.23,24 However, potentially longer-standing conditions like in utero growth restriction may have allowed time for both brain and gut to adapt with increased cFTOE and mFTOE. Lack of a clear pattern of SCOR over the first week suggests not only differential organ blood flow, but also distinct changes in oxygen extraction by brain and gut. In a small study, Bozzetti et al. monitored SGA infants and found higher cerebral than splanchnic oxygenation measures, suggesting a brain-sparing effect in the first 24 h after birth, with greater FTOE in the brain but with reperfusion of the splanchnic region by 72 h.25 The complex relationship between brain and gut oxygenation is also evident from literature suggesting lower Csat in infants who developed NEC26 and a possible explanation for worse neurodevelopmental outcome in infants with NEC.27
Complex interactions exist between hemoglobin level, number of transfusions, and postnatal age with regard to tissue oxygenation. Studies have examined transient increases in Csat and Msat and decreases in cFTOE and mFTOE with blood transfusions.14,22,28,29 Literature supports decreased Csat30 and increased cFTOE in the most anemic infants29,31,32 but limited compensatory increase in gut oxygen extraction.29,33 Our study provides further evidence of this prioritization of oxygen use by the brain over the gut under conditions of anemia. Others have also found that the association between cerebral NIRS measures and hemoglobin is mitigated with repeated exposures to red-cell transfusions,30 possibly related to adaptive changes over time including increased cardiac output and cerebral blood flow.34,35 As hemoglobin levels slightly decrease over the first week (Supplementary Fig. S2) while SpO2 remains the same, increases in cFTOE and mFTOE may also be due in part to postnatal reduction in hemoglobin.
The TOP trial found that a higher hemoglobin threshold for red-cell transfusion in ELBW infants did not improve survival without neurodevelopmental impairment.10 This secondary study demonstrated that within the confines of the TOP transfusion algorithm, infants with a greater degree of anemia in the first week (those in the low hemoglobin threshold group requiring two or more transfusions) had the lowest Csat and highest cFTOE measures, especially in the first few days. Whether NIRS measures, including cerebral oxygenation, may be more predictive of neurodevelopmental outcomes compared to traditional measures of anemia severity such as hemoglobin level is unknown.
Limitations of this study include lack of correlation between NIRS data and outcomes such as death, neurodevelopmental impairment, or NEC. However, clinical outcome data in relation to NIRS measures will be forthcoming in future analyses. Monitoring data available over 7 days from 124 ELBW infants from multiple centers using the same type of NIRS device and neonatal sensor is a significant strength of this investigation. However, data were analyzed as hourly averages and not continuous waveforms, potentially not accounting for subtle changes. While we investigated other factors contributing to Csat or Msat, additional variables were not accounted for, including degree of hemodynamic significance of a PDA and feeding intervals, both of which could significantly impact mesenteric perfusion. Moreover, hypercarbia or hypocarbia, hypotension, PDA, and severe intraventricular hemorrhage are not infrequent in the ELBW population and may impact Csat measures. Adjustment for all these potential confounders was not done given the focus on patterns of NIRS measures in a representative ELBW population over time but must be considered as a limitation of the investigation. This study also did not capture the immediate transition period after birth, as monitoring did not occur in the delivery room, and early missing data were noted given variable enrollment until 48 h of age. Although Msat has been characterized as an unreliable measure due to the potential effects of peristalsis, underlying intestinal air or stool, and variability of blood supply,36,37 we found only minimal missing Msat or mFTOE data overall, confirming the feasibility of abdominal NIRS monitoring in the preterm population.
Conclusion
Tissue oxygenation of the brain and gut declines over the first week with a corresponding increase in oxygen extraction by these organs and a preferential increase in brain oxygen extraction for more immature and anemic preterm ELBW infants. These distinct changes in end-organ oxygen balance may clarify the risks of impaired cerebral and mesenteric oxygenation inherent to the preterm population. Knowledge of these early changes in tissue saturation and oxygen extraction may better inform brain-protective strategies and feeding approaches for the ELBW infant in the first week of age.
References
Bruckner, M. et al. Normal regional tissue oxygen saturation in neonates: a systematic qualitative review. Pediatr. Res. https://doi.org/10.1038/s41390-021-01786-y (2021).
Alderliesten, T. et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 79, 55–64 (2016).
McNeill, S., Gatenby, J. C., McElroy, S. & Engelhardt, B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 31, 51–57 (2011).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 350, g7635 (2015).
Hoeller, N. et al. Cerebral and peripheral muscle oxygenation and perfusion: course in moderate and late preterm neonates during the first day after birth. Physiol. Int. https://doi.org/10.1556/2060.2020.00028 (2020).
Cohen, E. et al. Growth restriction and gender influence cerebral oxygenation in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 101, F156–F161 (2016).
Cortez, J. et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern. Fetal Neonatal Med. 24, 574–582 (2011).
Kuik, S. J. et al. The effect of enteral bolus feeding on regional intestinal oxygen saturation in preterm infants is age-dependent: a longitudinal observational study. BMC Pediatr. 19, 404 (2019).
van der Heide, M. et al. Regional splanchnic oxygen saturation for preterm infants in the first week after birth: reference values. Pediatr. Res. https://doi.org/10.1038/s41390-020-01323-3 (2021).
Kirpalani, H. et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N. Engl. J. Med. 383, 2639–2651 (2020).
Metcalfe, K. H. M., Stienstra, R. & McHoney, M. NIRS as a biomarker of bowel ischaemia & surgical pathology: a meta-analysis of studies in newborns. Early Hum. Dev. 161, 105437 (2021).
Gillam-Krakauer, M. et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J. Perinatol. 33, 609–612 (2013).
Cerbo, R. M. et al. Global perfusion assessment and tissue oxygen saturation in preterm infants: where are we? Early Hum. Dev. 89(Suppl 1), S44–S46 (2013).
Banerjee, J., Leung, T. S. & Aladangady, N. Cerebral blood flow and oximetry response to blood transfusion in relation to chronological age in preterm infants. Early Hum. Dev. 97, 1–8 (2016).
Mohamed, M. A. et al. Changes in cerebral tissue oxygenation and fractional oxygen extraction with gestational age and postnatal maturation in preterm infants. J. Perinatol. https://doi.org/10.1038/s41372-020-00794-w (2020).
Chock, V. Y. et al. Cerebral oxygenation and autoregulation in preterm infants (Early NIRS Study). J. Pediatr. 227, 94–100.e1 (2020).
Patel, A. K. et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr. Crit. Care Med. 15, 735–741 (2014).
Havranek, T., Miladinovic, B., Wadhawan, R. & Carver, J. D. Factors that affect the postnatal increase in superior mesenteric artery blood flow velocity in very low birth weight preterm infants. J. Perinat. Med. 40, 565–570 (2012).
Maruyama, K., Koizumi, T., Tomomasa, T. & Morikawa, A. Intestinal blood-flow velocity in uncomplicated preterm infants during the early neonatal period. Pediatr. Radiol. 29, 472–477 (1999).
Martinussen, M., Brubakk, A. M., Vik, T. & Yao, A. C. Mesenteric blood flow velocity and its relation to transitional circulatory adaptation in appropriate for gestational age preterm infants. Pediatr. Res. 39, 275–280 (1996).
Thompson, A., Silva, C. T., Gork, A. S., Wang, D. & Ehrenkranz, R. A. Intestinal blood flow by Doppler ultrasound: the impact of gestational age and time from first enteral feeding in preterm neonates. Am. J. Perinatol. 31, 261–268 (2014).
Dani, C., Pratesi, S., Fontanelli, G., Barp, J. & Bertini, G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Bennet, L., Rossenrode, S., Gunning, M. I., Gluckman, P. D. & Gunn, A. J. The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion. J. Physiol. 517(Pt 1), 247–257 (1999).
Andersen, C. C. et al. The cerebral critical oxygen threshold of ventilated preterm lambs and the influence of antenatal inflammation. J. Appl. Physiol. (1985) 111, 775–781 (2011).
Bozzetti, V. et al. Cerebral and somatic NIRS-determined oxygenation in IUGR preterm infants during transition. J. Matern. Fetal Neonatal Med. 29, 443–446 (2016).
Howarth, C. et al. Cerebral oxygenation in preterm infants with necrotizing enterocolitis. Pediatrics 146, e20200337 (2020).
Hintz, S. R. et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115, 696–703 (2005).
Bailey, S. M., Hendricks-Muñoz, K. D., Wells, J. T. & Mally, P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am. J. Perinatol. 27, 445–453 (2010).
Mintzer, J. P., Parvez, B. & La Gamma, E. F. Regional tissue oxygen extraction and severity of anemia in very low birth weight neonates: a pilot NIRS analysis. Am. J. Perinatol. 35, 1411–1418 (2018).
Whitehead, H. V., Vesoulis, Z. A., Maheshwari, A., Rao, R. & Mathur, A. M. Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised? J. Perinatol. 38, 1022–1029 (2018).
Whitehead, H. V., Vesoulis, Z. A., Maheshwari, A., Rambhia, A. & Mathur, A. M. Progressive anemia of prematurity is associated with a critical increase in cerebral oxygen extraction. Early Hum. Dev. 140, 104891 (2019).
van Hoften, J. C. R., Verhagen, E. A., Keating, P., ter Horst, H. J. & Bos, A. F. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch. Dis. Child. Fetal Neonatal Ed. 95, F352–F358 (2010).
Sandal, G. et al. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion 54, 1100–1105 (2014).
Jain, D., D’Ugard, C., Bancalari, E. & Claure, N. Cerebral oxygenation in preterm infants receiving transfusion. Pediatr. Res. 85, 786–789 (2019).
Jani, P. et al. Liberal hemoglobin threshold affects cerebral arterial pulsed Doppler and cardiac output, not cerebral tissue oxygenation: a prospective cohort study in anemic preterm infants. Transfusion 59, 3093–3101 (2019).
Akotia, D. H., Durham, J. T., Arnell, K. M., Petruzzelli, D. L. & Katheria, A. C. Relationship between near-infrared spectroscopy and transabdominal ultrasonography: noninvasive monitoring of intestinal function in neonates. Med. Sci. Monit. 22, 61–68 (2016).
Thompson, A., Benni, P., Seyhan, S. & Ehrenkranz, R. Meconium and transitional stools may cause interference with near-infrared spectroscopy measurements of intestinal oxygen saturation in preterm infants. Adv. Exp. Med. Biol. 765, 287–292 (2013).
Acknowledgements
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Heart, Lung, and Blood Institute (NHLBI), the National Center for Research Resources (NCRR), and the National Center for Advancing Translational Sciences (NCATS) provided grant support for the Neonatal Research Network’s Transfusion of Preemies (TOP) trial through cooperative agreements. While NICHD and NHLBI staff had input into the trial design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data included in this trial. On behalf of the NRN, RTI International had full access to all the data in the trial and take responsibility for the integrity of the data and accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this trial. The investigators listed in Supplementary Table S3, in addition to those listed as authors, participated in this trial.
Funding
The National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL12216701A1, U01 HL112776, U01 HL112748), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880, UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68270, UG1 HD68278, UG1 HD68263, UG1 HD68284; UG1 HD87226, UG1 HD87229) and the National Center for Advancing Translational Sciences (NCATS) (UL1 TR6, UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR105, UL1 TR442, UL1 TR454, UL1 TR1117) provided grant support for the Neonatal Research Network.
Author information
Authors and Affiliations
Consortia
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: VYC, ES, ST, MBB, AD, SRH, HK, EFB, and KPVM. Drafting the article or revising it critically for important intellectual content: VYC, ES, ST, AD, SRH, HK, EFB, RMP, and KPVM. Final approval of the version to be published: VYC, ES, ST, MBB, AD, SRH, HK, EFB, LFC, WAC, CMC, JAW, KAK, RKO, RBS, RMP, ARL, TM, GMS, MCW, BAY, BBP, SC, CTD, RDH, and KPVM.
Corresponding author
Ethics declarations
Competing interests
This study was supported by loan of equipment from Medtronic (Minneapolis, MN). The authors have no additional conflicts of interest to disclose.
Consent to participate
Informed parental consent was obtained for study participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chock, V.Y., Smith, E., Tan, S. et al. Early brain and abdominal oxygenation in extremely low birth weight infants. Pediatr Res 92, 1034–1041 (2022). https://doi.org/10.1038/s41390-022-02082-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02082-z
- Springer Nature America, Inc.
This article is cited by
-
Neonatal somatic oxygenation and perfusion assessment using near-infrared spectroscopy
Pediatric Research (2024)
-
Imperative to accelerate research aligning real-time clinical demand with mental health supply
Pediatric Research (2022)