Abstract
Background
Radiation therapy (RT) of bone metastases provides an important treatment approach in palliative care treatment concepts. As a consequence of treatment, the extent of radiation-induced toxicity is a crucial feature with consequences to a patient’s quality of life. In this context this study aims at reducing the extent of radiation-induced side effects and toxicity by assuming a better sparing of normal tissue with the use of intensity-modulated instead of conventionally delivered external beam radiotherapy.
Methods/design
In this prospective, randomized, single-center trial for patients with spinal bone metastases, RT is performed as either image-guided intensity-modulated radiotherapy (10x3Gy) or conventionally fractionated external beam radiotherapy (10x3Gy). Afterwards radiation-induced toxicity will be assessed and compared 3 and 6 months after the end of radiation.
Discussion
The aim of this pilot study is the evaluation of achievable benefits, with reduced radiation toxicity being the primary endpoint in the comparison of intensity-modulated radiotherapy versus conventional radiotherapy for patients with spinal bone metastases. Secondarily, bone re-calcification, quality of life, pain relief, spinal instability, and local control will be measured and compared between the two treatment groups.
Trial registration
ClinicalTrials.gov, NCT02832830. Registered on 12 July 2016.
Similar content being viewed by others
Background
Secondary bone metastases in patients with cancer are most often located in the vertebral spine [1–4], leading to complications such as bone fractures, neurological impairment, and especially pain [5–10]. Radiation therapy of bone metastases as a palliative oncological treatment concept is supposed to prevent and treat symptoms such as fatigue and pain. As a consequence of treatment, the extent of radiation-induced toxicity should be kept as low as possible, as it may also immensely reduce the patients’ quality of life.
Conventionally fractionated external beam radiotherapy is one of the most important radiotherapeutic treatment options for spinal bone metastases, delivering common doses of 30 Gy and resulting in a significant decrease in pain [11]. Nevertheless, the use of this technique is limited due to unsatisfactory sparing of surrounding normal tissue as well as application of high doses to organs at risk such as the spinal cord. As the spinal cord is centrally localized within the vertebral body and thus in the target volume, even modern techniques will never be able to completely spare this at-risk organ. However, every reduction of the dose delivered to nearby tissue still can lead to a decrease of side effects and thus a higher quality of life. The use of intensity-modulated radiotherapy aims to better spare the surrounding tissue, leading to a reduction of possible radiation-induced side effects [12]. Image,guidance additionally matches the patient’s positioning to the treatment field and thus improves the accuracy of dose distribution [13].
Considering the extent of radiation-induced acute and late side effects as well as toxicity with its contribution to reduced quality of life, this randomized study compares the quantitative amount of toxicity by using the two above-mentioned radiotherapeutic treatment concepts.
Methods/design
Recruitment and study design
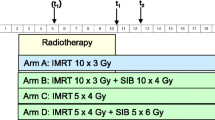
This prospective, single-center pilot study is performed at the Department of Radiation Oncology at the University Hospital of Heidelberg for patients with spinal bone metastases with indication for radiotherapy. As shown in Fig. 1, participants will be randomized into two groups: one group receiving intensity-modulated radiotherapy treatment (group A) and the other conventional external beam radiotherapy (group B).
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 checklist shows the study guidelines in more detail (see Additional file 1).
Before the start of radiotherapy, the recruitment of eligible participants firstly includes the explanation, to each potential study participant, of the purpose, implementation, design, aims, requirements, and timeline of this study as well as the description of the two different radiation techniques and common ethical issues.
Inclusion criteria
Patients will be included who:
-
Have metastases in the vertebral spine, regardless of number of metastases
-
Have an indication for radiation therapy, such as bone pain, spinal instability, and neurological deficit
-
Are aged 18 to 85
-
Have Karnofsky performance status ≥50
-
Have signed a Declaration of Informed Consent
Exclusion criteria
Patients will be excluded who:
-
Have bony lesions caused by multiple myeloma or lymphoma
-
Have severe neurological and psychiatric impairment
-
Have undergone previous radiation therapy with overlapping areas
Drop-out criteria
Criteria that will lead to the drop-out of a patient before completion of this study may be any kind of treatment for medical reasons or impairment that will result in an interruption or early completion of radiotherapy. Furthermore, the patient’s desire to exit the study or withdrawal of consent as well as any unexpected serious adverse event (SAE) occurring during treatment or in follow-up that leads to early completion of the study will result in drop-out.
Radiotherapeutic planning and treatment implementation
All patients receive a planning computed tomography (CT) scan with a slice thickness of 3–5 mm. Patient positioning depends on the area being treated and includes the use of various fixation devices, such as Wingstep® and Prostep® (Elekta, Stockholm, Sweden) for lesions in the thoracic or lumbar spine as well as head holders such as Aquaplast masks (Aquaplast Corporation, Wyckoff, NJ, USA) for cervical spine immobilization. In addition to skin tattoo marks, this guarantees the achievement of a high reproducibility of patient positioning and accuracy of treatment delivery.
Afterwards these CT scans are used to outline organs at risk (OARs) and to contour the clinical target volume (CTV), which encompasses the metastatic vertebral body and, with a margin of 1 cm, results in the planning target volume (PTV). The target volume (=PTV) in each treatment group consists of the entire metastatic vertebral body in a craniocaudal direction including the Proc. costales et transversi to the right and left lateral sides.
In group A, treatment is being performed as intensity-modulated radiotherapy (IMRT) and planned as either tomotherapy, volumetric arc therapy (VMAT), or step-and-shoot IMRT with daily cone-beam CT imaging for rigid patient deformation during radiation. In group B radiation is delivered by conventional, three-dimensional (3D) planned, external beam radiotherapy in two to three fields. Each group uses 6 MV photon energy plans with multileaf collimator shaped radiation fields. A palliative treatment regimen with a total prescribed dose of 30 Gy in 3 Gy fraction doses and daily treatment sessions five times per week is performed, with the 90% isodose surrounding the PTV. OARs such as the skin, heart, or the esophagus are delineated, and dose limit constraints are prescribed to each of them following common considerations according to QUANTEC parameters.
Data collection and follow-up period
During study observation, patient data are collected before the start of radiotherapy treatment (t0), at the end of treatment (t1), and in the follow-up period at scheduled visits in our department at time points of 3 months (t2) as well as 6 months (t3) after completion of treatment (Figs. 1 and 2).
Patient clinical information is carefully documented in case report forms (CRFs) using questionnaires, filled out by each patient, that measure and quantify clinical symptoms and factors such as pain, radiation-induced side effects and toxicity, as well as quality of life (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with Bone Metastases and for measure of Fatigue (EORTC BM22, FA13) and visual analog scale (VAS)). Furthermore, the follow-up requirements include a CT scan 3 and 6 months after completion of radiotherapy for evaluation of treatment response, bone sclerosis, spinal stability, and possible complications such as bone fractures.
Assessment of the primary and secondary endpoints
In this trial, radiation-induced toxicity 3 months after completion ofpalliative radiotherapy will be evaluated as the primary endpoint. Its extent is measured according to criteria delineated by NCI Common Toxicity Criteria for Adverse Events (CTCAE v 4.0).
Secondary endpoints will be bone re-calcification, quality of life, spinal instability and fractures, local control, and clinical symptoms such as fatigue, pain relief, or neurological deficits.
Statistical analysis and randomization
This trial is being performed as a pilot study; in the calculation for statistical analysis a total of about 30 patients in each treatment group is considered an appropriate sample size. Due to the explorative character of this study, it is not possible to estimate the total number of cases. However, with a scheduled number of 30 patients per group, it will be possible to detect a standardized mean-value effect (Cohen’s d) of 0.8 with a power of 80% and an alpha significance level of 5%. A blocked randomization is used to allocate each participant into group A or B before start of treatment. All variables were analyzed descriptively by tabulation of the measures of the empirical distributions. According to the scale level of the variables, means, standard deviations, and medians as well as minimum and maximum or absolute and relative frequencies, respectively, will be reported. Additionally, for variables with longitudinal measurements, the time courses of individual patients will be analyzed and summarized by treatment groups. Descriptive p values of the corresponding statistical tests comparing the treatment groups will be given. The Wilcoxon signed rank test will be used to compare changes in group difference. The Cohen’s effect size (ES) will be assessed for clinically relevant change in questionnaire measures (<0.3 low, 0.3–0.7 moderate, >0.7 strong difference). Moreover patient data are collected in table form at the above-mentioned time points for all completed questionnaires. All statistical analyses will be performed with SAS software v 9.3 or higher (SAS Institute, Cary, NC, USA).
Discussion
This prospective, single-center pilot trial performed at the Department of Radiation Oncology at the University Hospital of Heidelberg assesses radiation-induced toxicity 3 months after radiotherapy in patients with spinal bone metastases by comparing intensity-modulated radiotherapy versus conventional external beam radiotherapy. Especially in this palliative treatment setting a technical approach to a limited extent of side effects and a higher relief of symptoms is needed to result in higher quality of life for palliative patients. Furthermore, this explorative study analyzes the differences seen in bone sclerosis, quality of life, spinal stability, and local control as well as clinical symptoms such as fatigue, pain relief, or neurological deficits between the two treatment groups.
Trial status
Patient recruitment has not yet been completed.
Abbreviations
- CRF:
-
Case report form
- CT:
-
Computed tomography
- CTCAE:
-
Common Toxicity Criteria for Adverse Events
- CTV:
-
Clinical target volume
- GCP:
-
Good Clinical Practice
- Gy:
-
Gray
- IMRT:
-
Intensity-modulated radiotherapy
- OAR:
-
Organ at risk
- PTV:
-
Planning target volume
- RT:
-
Radiotherapy
- SAE:
-
Serious adverse event
- VAS:
-
visual analog scale
- VMAT:
-
Volumetric arc therapy
References
Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15(1):1–4.
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Gasser TC, Mihatsch MJWN. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–83.
Krempien B. Die Entstehung von Knochenschmerzen bei Knochenmetastasen und ihre Behandlung durch Bisphosphonate. In: Bartsch HH, Hornstein W, editors. Interdisziplinäre Schmerztherapie bei Tumorpatienten. Basel: Karger Publishers; 1998.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s.
Bostel T, Förster R, Schlampp I, Wolf R, Serras AF, Mayer A, Bruckner T, Welzel T, Schmidberger H, Debus J, Rief H. Stability, prognostic factors and survival of spinal bone metastases in malignant melanoma patients after palliative radiotherapy. Tumori. 2016;102(2):156–61.
Schlampp I, Lang H, Förster R, Wolf R, Bostel T, Bruckner T, Debus J, Rief H. Stability of spinal bone metastases and survival analysis in renal cancer after radiotherapy. Tumori. 2015;101(6):614–20.
Foerster R, Habermehl D, Bruckner T, Bostel T, Schlampp I, Welzel T, Debus J, Rief H. Spinal bone metastases in gynecologic malignancies: a retrospective analysis of stability, prognostic factors and survival. Radiat Oncol. 2014;9:194.
Schlampp I, Rieken S, Habermehl D, Bruckner T, Förster R, Debus J, Rief H. Stability of spinal bone metastases in breast cancer after radiotherapy: a retrospective analysis of 157 cases. Strahlenther Onkol. 2014;190(9):792–7.
Rief H, Muley T, Bruckner T, Welzel T, Rieken S, Bischof M, Lindel K, Combs SE, Debus J. Survival and prognostic factors in non-small cell lung cancer patients with spinal bone metastases: a retrospective analysis of 303 patients. Strahlenther Onkol. 2014;190(1):59–63.
Rief H, Heinhold M, Bruckner T, Schlampp I, Förster R, Welzel T, Bostel T, Debus J, Rieken S. German Bone Research Group. Quality of life, fatigue and local response of patients with unstable spinal bone metastases under radiation therapy—a prospective trial. Radiat Oncol. 2014;9:133.
Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–36.
Chung Y, Yoon HI, Kim JH, Nam KC, Koom WS. Is helical tomotherapy accurate and safe enough for spine stereotactic body radiotherapy? J Cancer Res Clin Ocol. 2013;139(2):243–8.
Sterzing F, Kalz J, Sroka-Perez G, Schubert K, Bischof M, Roder F, Debus J, Herfarth K. Megavoltage CT in helical tomotherapy — clinical advantages and limitations of special physical characteristics. Technol Cancer Res Treat. 2009;8(5):343–52.
Acknowledgements
We thank our German Bone Cancer Research Group Members for their great efforts.
Funding
Not applicable.
Availability of data and materials
The data used in this analysis are from publications available in the public domain.
Authors’ contributions
EM and HR developed and planned this trial. TB is responsible for the statistical considerations/basis of the analysis. EM, SEW, RF, TBo, IS, NHN, TS, JD, and HR estimated the treatment of bone metastases. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Heidelberg Ethics Committee approved this study on 6 June 2016 (S-238/2016).
Inclusion of a patient with spinal bone metastasis into this trial requires meeting the above-mentioned inclusion and exclusion criteria as well as the patients’ written informed consent. Additionally the above-listed drop-out criteria will be considered. Patients are being recruited over a period of 1 year starting in November 2016.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (PDF 130 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Meyerhof, E., Sprave, T., Welte, S.E. et al. Radiation-induced toxicity after image-guided and intensity-modulated radiotherapy versus external beam radiotherapy for patients with spinal bone metastases (IRON-1): a study protocol for a randomized controlled pilot trial. Trials 18, 98 (2017). https://doi.org/10.1186/s13063-017-1847-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-017-1847-1