Abstract
Background
The incidence, mortality, and prevalence of stroke are high in China. Stroke is commonly associated with insomnia; both insomnia and stroke have been effectively treated with acupuncture for a long time. The aim of this proposed trial is to assess the therapeutic effect of acupuncture on insomnia following stroke.
Methods
This proposed study is a single-center, single-blinded (patient-assessor-blinded), parallel-group randomized controlled trial. We will randomly assign 60 participants with insomnia following stroke into two groups in a 1:1 ratio. The intervention group will undergo traditional acupuncture that achieves the De-qi sensation, and the control group will receive sham acupuncture without needle insertion. The same acupoints (DU20, DU24, EX-HN3, EX-HN22, HT7, and SP6) will be used in both groups. Treatments will be given to all participants three times a week for the subsequent 4 weeks. The primary outcome will be the Pittsburgh Sleep Quality Index. The secondary outcomes will be: the Insomnia Severity Index; sleep efficacy, sleep awakenings, and total sleep time recorded via actigraphy; the National Institutes of Health Stroke Scale; the Stroke-Specific Quality of Life score; the Hospital Anxiety and Depression Scale. The use of estazolam will be permitted and regulated under certain conditions. Outcomes will be assessed at baseline, 2 weeks after treatment commencement, 4 weeks after treatment commencement, and at the 8-week follow-up.
Discussion
This proposed study will contribute to expanding knowledge about acupuncture treatment for insomnia following stroke. This will be a high-quality randomized controlled trial with strict methodology and few design deficits. It will investigate the effectiveness of acupuncture as an alternative treatment for insomnia following stroke.
Trial registration
Chinese Clinical Trial Registry identifier: ChiCTR-IIC-16008382. Registered on 28 April 2016.
Similar content being viewed by others
Background
The Global Burden of Disease Study (2013) found that cerebrovascular disease was the third leading cause of disability-adjusted life years worldwide [1]. Cerebrovascular disease is a major chronic disease in China, ranked third among the most common causes of death for urban residents and first for rural residents [2]. Stroke morbidity in China is 89.6–314 out of every 100,000 in men and 76.7–212.2 out of every 100,000 in women [3]. Stroke can be divided into two major types based on the type of pathological process: hemorrhagic stroke and ischemic stroke. The incidence of hemorrhagic stroke in China declined by 1.7% over a recent 21-year period, but that of ischemic stroke increased by 8.7% annually [4]. Common post-stroke complications include sleep disorders (SD), such as insomnia, sleep apnea, and daytime sleepiness [5], and SD continues to be the most unrecognized modifiable risk factor for stroke [6]. Untreated SD causes cognitive dysfunction, altered mood, sleepiness, and fatigue, and may impede stroke rehabilitation, lengthen hospital stay, influence stroke outcomes, and increase the possibility of stroke recurrence [7].
One of the most common SD associated with stroke is insomnia [5]. Insomnia is characterized by difficulty in falling asleep and staying asleep, early morning awakening, and nonrestorative sleep. The primary pharmacological treatments for insomnia are benzodiazepines, nonbenzodiazepine sedatives, and melatonin agonists. Although effective pharmacologic treatments for insomnia are available, these medications often cause side effects such as residual daytime sedation, drowsiness, dizziness, lightheadedness, cognitive impairment, motor incoordination, and dependence [8]. The mainstay of nonpharmacologic intervention for insomnia is cognitive behavioral therapy (CBT). However, CBT often causes an acute reduction in total sleep time (TST) during the first few weeks of treatment, which means that SD improvements with CBT require long-term implementation [9].
Faced with the limitations of currently available insomnia treatments, more people are seeking complementary and alternative medicine options. According to the theory of traditional Chinese medicine, acupuncture provides overall coordination to help the body achieve a state of relative equilibrium of yin and yang, restoring its physiological function and regulating the sleep-awake cycle. Recently, the frequency of visits to acupuncturists has markedly increased in the United States [10]; however, there has been little focus on research into acupuncture for insomnia following stroke. Two recent randomized controlled trials (RCTs) had shown that insomnia following stroke was positively affected by intradermal acupuncture and low-frequency electric stimulation at acupoints [11, 12]. However, these RCTs had methodological deficits, including problems with randomization, lack of sample size estimation, blinding issues, no mention of acupuncturist certification, and no use of the intention-to-treat (ITT) analysis [13]. These design deficits make their findings far from conclusive. More high-quality clinical RCTs are required to investigate the effectiveness of acupuncture treatment for insomnia following stroke. In this proposed RCT, we aim to investigate the effectiveness of acupuncture for insomnia following stroke.
Methods/design
Hypotheses
-
1.
Participants with insomnia following stroke treated with real acupuncture will have greater improvement in sleep quality and other related symptoms than those treated with sham acupuncture
-
2.
The sham acupuncture procedure can be successfully implemented in this RCT in which participants will be unaware of the group assignment
Objectives
-
1.
To compare improvement in sleep quality recorded using the Pittsburgh Sleep Quality Index (PSQI) between the intervention group and control group
-
2.
To compare changes between the intervention group and control group in the Insomnia Severity Index (ISI), actigraphy, the National Institutes of Health Stroke Scale (NIHSS), the Stroke-Specific Quality of Life (SS-QOL), and the Hospital Anxiety and Depression Scale (HADS)
-
3.
To evaluate the design of the sham acupuncture procedure
Design
This is a single-center, patient-assessor-blinded, parallel-group RCT that conforms to the Consolidated Standards of Reporting Trials [14] and Standards for Reporting Interventions in Clinical Trials of Acupuncture [15] guidelines for acupuncture studies. Eligible patients will be randomly divided into the intervention group and the control group in a 1:1 allocation ratio.
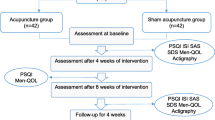
The flowchart of the study process is detailed in Fig. 1. The timing of treatment visits and data collection is detailed in Table 1.
Recruitment
All participants will be recruited through advertisements on micromessage (Wechat) and hospital bulletin boards in Shanghai Municipal Hospital of Traditional Chinese Medicine in Shanghai, China. If they show interest in participating, they can call the researchers with the phone number reserved. The researcher in charge of recruitment will explain the study in detail, and outline the potential benefits of this RCT to patients with insomnia following stroke. Patients who show interest in participating will be assessed according to the inclusion and exclusion criteria on their first visit. Once they agree to participate in this RCT, they will be asked to sign written informed consent before undergoing acupuncture intervention.
Participants
Inclusion criteria: men or women aged 18–75 years who (1) meet the diagnostic criteria of ischemic stroke according to the guidelines for the diagnosis and treatment of acute ischemic stroke in China (2014) [16] in the past 2 weeks to 2 years, (2) meet the diagnostic criteria of insomnia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [17] for at least 1 month, (3) PSQI score ≥ 6, (4) ISI score ≥ 8, (5) demonstrate no problems with communication, both visual and auditory (reading, writing, hearing, speaking, and seeing), and (6) sign a written informed consent for participation in the RCT.
Exclusion criteria: (1) those who have experienced insomnia before their stroke, (2) a history of sleep apnea syndrome, (3) a history of severe neural or psychiatric disorder or major neuropsychiatric disorder (such as autism, learning disorder, or mental retardation), (4) severe primary disease of the cardiovascular, hepatic, renal, or hematopoietic system, (5) infection or inflammation at the acupoints, (6) hemorrhagic disease or anticoagulant intake, (7) those who have used Western and/or Oriental medicine in the past 2 weeks for depression, or anxiety, (8) those who have received acupuncture for insomnia in the past month, (9) those who have participated in any other clinical trial in the past 3 months, (10) those who are pregnant or breastfeeding, and women of childbearing age who are not using proper birth control, and (11) refusal to wear a wristwatch-like actigraph while sleeping.
Dropout criteria: (1) development of serious disease (such as heart disease, pneumonia, or stroke) during the observation period, (2) receiving other treatments for insomnia (such as other Western medicine, Oriental medicine, moxibustion, or massage) during the observation period, (3) participants who present with serious adverse events, (4) participants who refuse to continue to participate in the RCT, and (5) those lost to follow-up.
Intervention
The participants from outpatient departments will receive acupuncture treatments in the outpatient clinic of the acupuncture department three times per week for 4 weeks. The inpatients will receive acupuncture treatment three times per week at the time of hospitalization, and will then receive outpatient treatment three times per week for 2 weeks. In each treatment session, every patient will be placed in a separate quiet space and asked to wear an eye-patch. Each treatment session will be 20 min. The treatment course will last for 4 weeks, and the follow-up period will be 4 weeks. All participants will receive standard Western medicine therapy for stroke, limb function rehabilitation therapy, and clinical nursing in Shanghai Municipal Hospital of Traditional Chinese Medicine. To improve adherence to intervention protocols, all participants will receive intervention and assessments by telephone reservations, and be given some financial subsidies after follow-up.
Patients in both groups will be treated with stainless steel needles (0.25 × 25 mm and 0.30 × 40 mm; Wuxi Jiajian Medical Material Co., Ltd., Wuxi, China) at the same acupoints, including Baihui (DU20), Shenting (DU24), Yintang (EX-HN3), bilateral Anmian (EX-HN22), bilateral Shenmen (HT7), and bilateral Sanyinjiao (SP6). The acupuncturist will use 0.25 × 25-mm needles for acupoints DU20, DU24, EX-HN3 and HT7, and 0.30 × 40-mm needles for acupoints EX-HN22 and SP6 (Table 2).
The intervention group
The intervention group will be treated with the real tube-needling method, while lying in the supine position and wearing an eye-patch. After routine skin sterilizing, DU20, DU24, and EX-HN3 will be punctured obliquely 10 mm deep into the skin; EX-HN22 and SP6 will be punctured 15 mm deep in a direction perpendicular to the skin, and HT7 will be punctured perpendicularly to a depth of 5 mm. Licensed and experienced acupuncturists will conduct needle manipulation including lifting, thrusting, and rotating to obtain the proper needling sensation (De-qi sensation). Then they will stick a 2 × 2-cm piece of tape on the skin beside each needle. After a 20-min retention period, the needles and tapes will be removed simultaneously.
The control group
The control group will receive sham acupuncture treatment. Before each session of sham acupuncture, we will prepare plastic tubes with a blunt needle inside each one. The acupuncturist will put the tube needle-end-up on the skin, and knock the tube slightly. The needles with blunt tips will not be inserted into the skin, but will create a pricking sensation similar to real acupuncture. After removing the tubes, the blunt needles will be stuck at the acupoints with 2 × 2-cm pieces of tape. After a 20-min retention period, the needles and tapes will be removed simultaneously.
Outcomes
Primary outcomes
The primary outcomes of this study will be the changes in the PSQI between baseline, 4 weeks after treatment commencement, and the 8-week follow-up. All questionnaires will be in Chinese.
-
Pittsburgh Sleep Quality Index
-
The PSQI is a self-rated questionnaire used to assess sleep quality and disturbances over a 1-month period. It comprises 19 self-rated items and five other-rated items [18]. The scores for the 19 self-rated items and five other-rated items are not included in the total score. Except for the first item, the remaining 18 items include the seven following subscales: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficacy (SE), sleep disturbances, use of sleeping medication, and daytime dysfunction. Each subscale is rated from 0 to 3, and the accumulated scores of the seven subscales constitute the total score of the PSQI (0–21). A higher score indicates a more severe SD.
-
Secondary outcomes
-
Secondary outcomes refer to the ISI score, the NIHSS score, the SS-QOL, the HADS, and the dose of estazolam during the 8-week trial at baseline, 2 weeks after treatment commencement, 4 weeks after treatment commencement, and the 8-week follow-up. All questionnaires will be presented in Chinese
-
Insomnia Severity Index
-
The ISI is a Likert 5-point self-rated questionnaire designed to assess the nature, severity, and impact of insomnia. It includes seven items, with the sum score ranging from 0 to 28 [19]. A higher score suggests more severe sleep disturbance. ISI categories are: clinically significant insomnia (score 0–7), subthreshold insomnia (score 8–14), moderate clinical insomnia (score 15–21), and severe clinical insomnia (score 22–28)
-
-
Actigraphy
-
The wActiSleep-BT actigraph (Actigraph LLC, Pensacola, FL, USA) is a noninvasive, small, wristwatch-like device that will be worn on the patient’s nondominant wrist. Patients will be instructed to wear the actigraph for two consecutive nights before each assessment time point. The patients’ motion will be recorded in 1-min epochs during sleep and stored continuously as data of SE, sleep awakenings, and TST. The analysis of sleep quality will be performed by dedicated ActiLife 6 data analysis software (ActiGraph LLC)
-
-
National Institutes of Health Stroke Scale
-
The NIHSS is a stroke-specific quantitative scale used to assess neurological deficits in terms of consciousness, eye movements, visual fields, facial palsy, motor and sensory impairments, ataxia, language function, and neglect. A higher score indicates a more severe neurological deficit [20]. Each participant will be assessed by a trained, certified investigator at baseline, 2 weeks after treatment commencement, 4 weeks after treatment commencement, and at the 8-week follow-up
-
-
Stroke-Specific Quality of Life
-
The SS-QOL is used to assess the health-related quality of life of stroke patients in the past week [21]. The SS-QOL consists of 49 items, with 12 domains of energy, family roles, language, mobility, mood, personality, self-care, social roles, thinking, upper extremity function, vision, and work/productivity. The total score is the sum of the 12 domain scores. A higher score indicates better function
-
-
Hospital Anxiety and Depression Scale
-
The HADS is a validated, widely-used, 14-item self-report scale designed to briefly measure current anxiety and depressive symptoms in nonpsychiatric hospital patients [22]. It excludes somatic symptoms, therefore avoiding potential confounding factors. The HADS comprises two independent seven-item subscales for anxiety and depression
-
-
Estazolam dose
-
Throughout this RCT, participants will be allowed to take 0.5–2 mg estazolam when they experience difficulty falling asleep for more than two consecutive days, or when they feel seriously ill because of insomnia symptoms. The dose should be recorded in the paper
-
Sample size
The sample size calculation for our RCT is based on the change in the PSQI score. According to previous research [23], a sample size of 24 participants should be recruited in each group. Assuming about a 20% dropout rate, we estimate that a total of 60 participants should be recruited for this RCT.
Randomization
We plan to use the random block method. An independent researcher, who has no contact with any participant, will use SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA) to generate a random number table to divide the eligible participants in a 1:1 ratio into the intervention group and the control group. The random allocation sequence will be generated in a block of 3. The researcher will then make random allocation cards and seal each card in an opaque envelope whose number is the same as the number on the card. According to the time order in which patients are asked to go to the hospital, another researcher will arrange the patients into different groups and inform the acupuncturists of the group assignment.
Blinding method
This is a single-blinded (patient-assessor-blinded) study. Participants will be asked to wear an eye-patch in a separate quiet space before treatment, and will remain blinded during the whole treatment. Except for the acupuncturists, all other researchers (including the statisticians, outcome assessors, and data analysts) will be blinded to the group assignment. We will attempt to validate the successful implementation of the blinding method. At first treatment, all patients will be told that the needling sensation that they are experiencing is the real sensation of acupuncture. In addition, all researchers will undergo training on the specifications of this RCT before commencement, and will strictly adhere to the separation principle of each department. The primary blinding method was successfully implemented by Lao et al. [24] in a trial to evaluate the efficacy of acupuncture treatment for postoperative oral surgery pain. We used this report as a reference to improve the design of our RCT and to ensure successful completion.
Safety assessment
Participants will be asked to report any adverse event or related information after acupuncture treatment. The detail of every adverse event will be measured by Dr. Francisca Soto-Aguilar and reported in the Case Report Form. Adverse events include any unfavorable or unintended signs, symptoms, or diseases occurring during the whole RCT.
Statistical analysis
All data will be analyzed according to the ITT principle to reduce deviation. The Student’s t test will be used to compare differences in measurement data. The rank sum test will be used to deal with ranked data, and the chi-squared test will be used to analyze categorical data. Analyses will be performed with SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA).
Monitoring
To guarantee the quality of this RCT, it will be carried out by Shanghai Municipal Hospital of Traditional Chinese Medicine. We will promptly input data from this RCT on the ResMan website at http://www.chictr.org.cn/index.aspx. As the Data Monitoring Team to identify problems in the project, examine collected data, and control bias, The Clinical Research Center of Drugs of the Shanghai University of Traditional Chinese Medicine will have access to these interim results and make the final decision to terminate the trial. A qualified clinical trial expert will be invited to monitor this RCT.
Clinical trial registration
We applied for registration of this RCT in the Chinese Clinical Trial Registry. This will reduce all kinds of bias in clinical research, increase the transparency of trials, ensure the high quality of clinical trials and test processes, and the credibility of results.
Discussion
Acupuncture treatment has been widely used to reduce the symptoms of insomnia [25]. There is minimal theoretical research or clinical RCTs on acupuncture for insomnia following stroke. This RCT will investigate the effectiveness of acupuncture in reducing insomnia following stroke. We are attempting to conduct a clinical RCT with adequate design and few deficits. We designed the sham acupuncture method without needle insertion to assess the specific effects of acupuncture rather than its placebo effect for treating insomnia following stroke. Furthermore, in contrast to the subjective assessments made in most trials, we will use actigraphy in our RCT to objectively assess the physiological changes in patients with insomnia. We will use the ITT principle to handle statistical data to reduce deviation of the RCT.
There are some inevitable limitations in this RCT. For example, it is hard to make the RCT double-blinded. The participants, the assessor, and the statistical expert will be blinded to the group assignment in this RCT; however, it will be impossible to blind the acupuncturist. This RCT is also restricted to a single-center hospital. Furthermore, although actigraphy is suitable for measuring nocturnal restlessness, it cannot differentiate between sleep stages or score rapid eye movement sleep, which may have helped to explain the mechanism of acupuncture for treating insomnia following stroke. In further studies, we may consider a multicenter clinical trial, using polysomnography as an assessment method.
Trial status
We started recruiting participants in July 2016.
Abbreviations
- CBT:
-
Cognitive behavioral therapy
- HADS:
-
Hospital Anxiety and Depression Scale
- ISI:
-
Insomnia Severity Index
- ITT:
-
Intention-to-treat
- NIHSS:
-
National Institutes of Health Stroke Scale
- PSQI:
-
Pittsburgh Sleep Quality Index
- RCT:
-
Randomized controlled trial
- SD:
-
Sleep disorders
- SE:
-
Sleep efficacy
- SS-QOL:
-
Stroke-Specific Quality of Life
- TST:
-
Total sleep time
References
DALYs, Global Burden of Disease Study, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91.
Li GQ, et al. Impact of cerebrovascular disease mortality on life expectancy in China. Biomed Environ Sci. 2014;27(3):169–75.
Liu M, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6(5):456–64.
Zhao D, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39(6):1668–74.
Pasic Z, et al. Incidence and types of sleep disorders in patients with stroke. Med Arh. 2011;65(4):225–7.
Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73(16):1313–22.
Culebras A. Sleep and stroke. Semin Neurol. 2009;29(4):438–45.
Buscemi N, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335–50.
Mitchell MD, et al. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40.
Nahin RL, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1–14.
Lee SY, et al. Intradermal acupuncture on shen-men and nei-kuan acupoints improves insomnia in stroke patients by reducing the sympathetic nervous activity: a randomized clinical trial. Am J Chin Med. 2009;37(6):1013–21.
Tang L, Ma C, You F, Ding L. Impacts of the low-frequency electric stimulation at the acupoints on the content of plasma 5-HT and NE in the patients with insomnia following stroke. Zhongguo Zhen Jiu. 2015;35(8):763–7.
Sun P, Du YH, Xiong J, Lin XM, Chen YW, Gao X, et al. Assessment of the reporting quality of randomized controlled trials on acupuncture for simple obesity with the CONSORT Statement and STRICTA. Lishizhen Medicine and Materia Medica Research. 2010;21(4):943–5.
Schulz KF, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
MacPherson H, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT Statement. J Altern Complement Med. 2010;16(10):ST1–14.
Neurology Branch of Chinese Medical Association. Neurology cerebrovascular disease study group of the Chinese Medical Association. Guidelines for the diagnosis and treatment of acute ischemic stroke in 2014 in China. Chin J Neurol. 2015;48(4):246–57.
Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. 2013;25(2):191–2.
Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
Brott T, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70.
Williams LS, et al. Development of a stroke-specific quality of life scale. Stroke. 1999;30(7):1362–9.
Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Sun YZ, Xia KP. Acupuncture plus auricular point sticking for treating post-stroke insomnia. Shanghai J Acu-mox. 2011;30(6):363–5. doi:10.3969/j.issn.1005-0957.2011.06.363.
Lao L, et al. Evaluation of acupuncture for pain control after oral surgery: a placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 1999;125(5):567–72.
Huang W, Kutner N, Bliwise DL. A systematic review of the effects of acupuncture in treating insomnia. Sleep Med Rev. 2009;13(1):73–104.
Acknowledgements
Shifen Xu provided general support as the head of the Acupuncture Department in Shanghai Municipal Hospital of Traditional Chinese Medicine, and was responsible for the design of this RCT.
Funding
This RCT is funded by the Shanghai Committee of Science and Technology, China (Grant No. 14401930900), the State Administration of Traditional Chinese Medicine (Grant No. JDZX2015024), and the Shanghai Municipal Commission of Health and Family Planning (Grant No. 20154Y0107).
Availability of supporting data
All data are fully available without restriction.
Authors’ contributions
The trial design was developed by LXL, SFX, and WTL. YC drafted the manuscript, which was carefully revised, edited, and discussed by XY, FSA, YPL, PY, JYW, and BCZ. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors read and approved the final manuscript.
Ethics approval and consent to participate
This RCT was approved by the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine on 27 April 2016 (certificate number 2016SHL-KY-05). The purpose, procedures, and potential risks of the RCT will be explained clearly to the participants. All participants shall give their written informed consent before joining the RCT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cao, Y., Yin, X., Soto-Aguilar, F. et al. Effect of acupuncture on insomnia following stroke: study protocol for a randomized controlled trial. Trials 17, 546 (2016). https://doi.org/10.1186/s13063-016-1670-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-016-1670-0